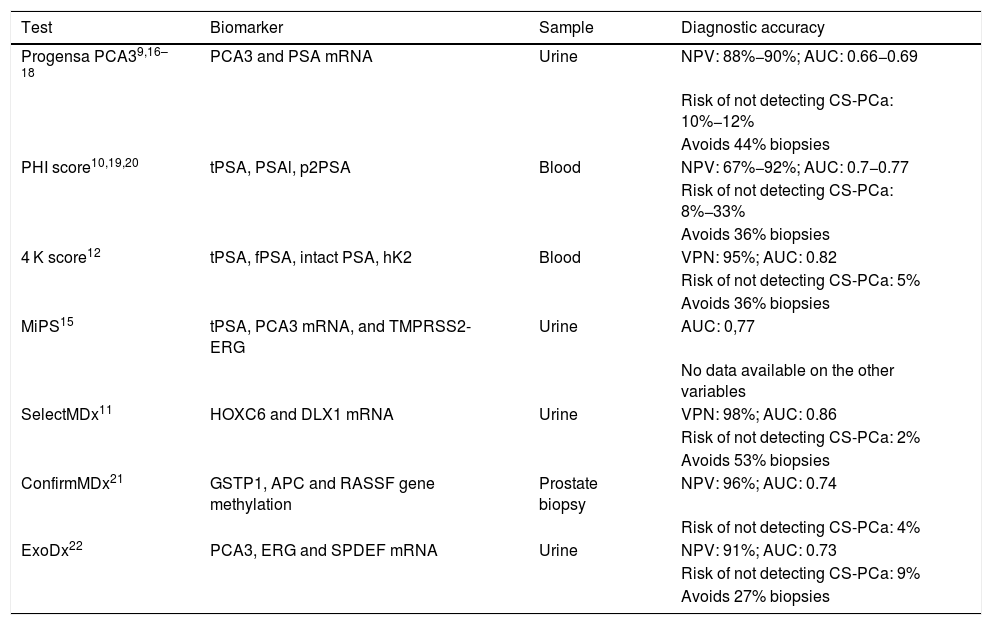
The use of biomarkers in the detection of prostate cancer (PC) can decrease overdiagnosis and overtreatment of non-significant PC. We analyze the usefulness and applicability of the SelectMDx® marker in a routine clinical practice setting.
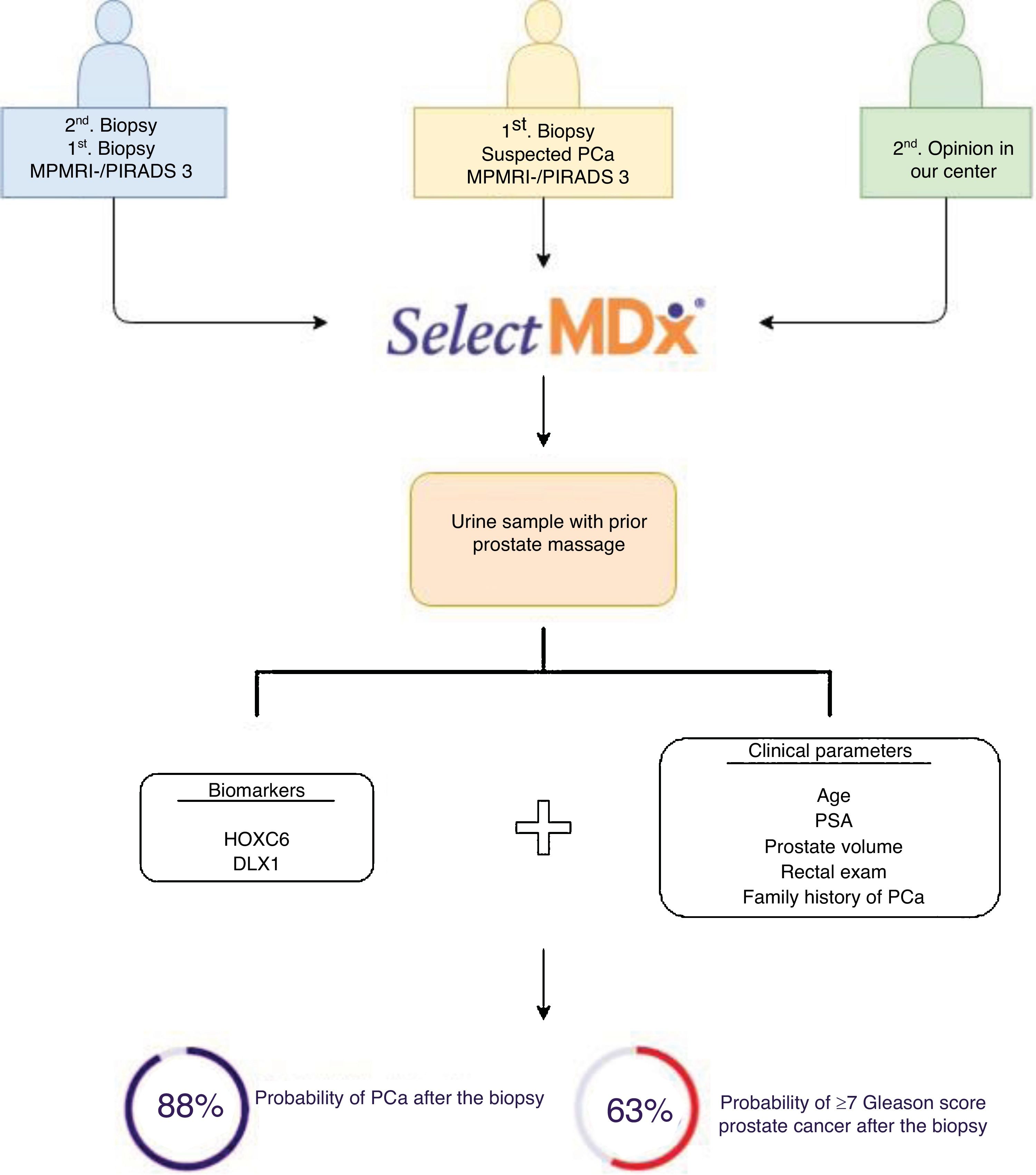
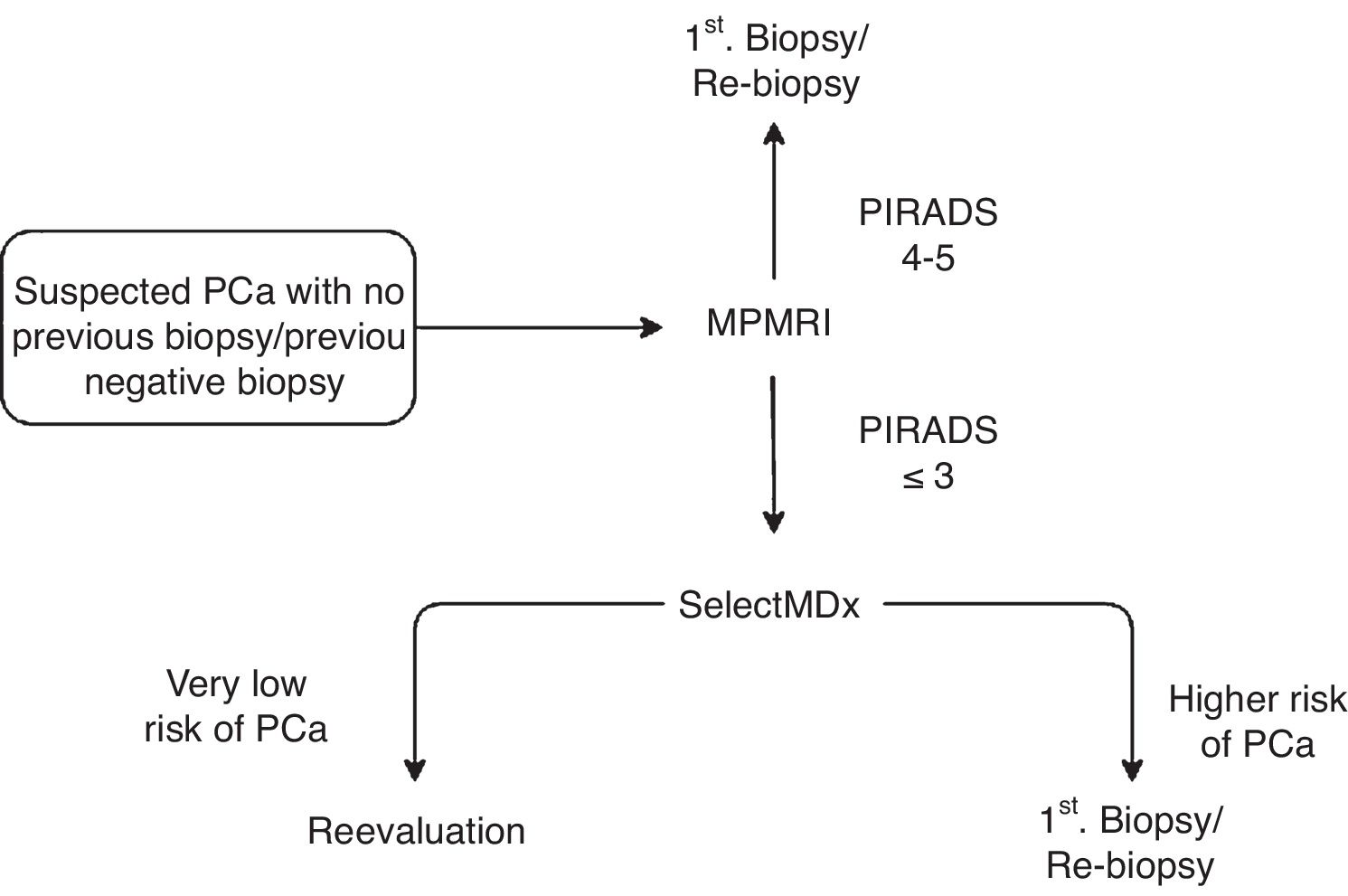
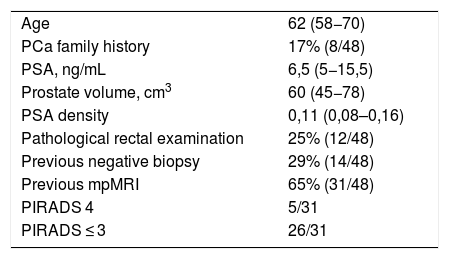
Material and methodsRetrospective study of 48 patients evaluated by the SelectMDx® test between July 2017 and April 2019. Patients were stratified into two groups according to the risk estimated by the clinically significant CP test (CS-PC): <2% or "very low risk", and >2%. Results were expressed based on previous prostate biopsy (PB) and MRI outcomes.
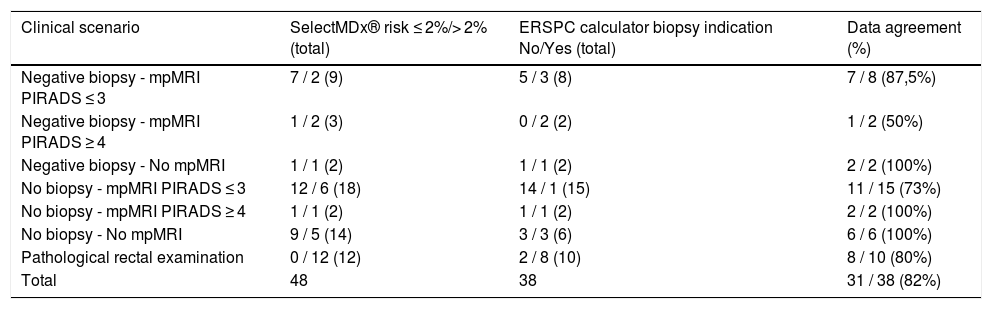
ResultsPatients with negative PB and normal/doubtful MRI had <2% risk in 7/9 cases. Patients without PB and normal/doubtful MRI had <2% risk in 12/18 cases, and 2/6 cases with a >2% risk presented CS-PC. Of the 14 patients with no previous PB or MRI, 9 had <2% risk, and 2 cases were diagnosed with PC from the group of patients (5) with risk >2%. The number of patients in the remaining subgroups is too small to draw any conclusions. In all cases with pathological digital rectal examination, the test showed a >2% PC risk.
ConclusionSelectMDx® is a promising test for detecting patients with a very low risk of CS-PC, especially in patients with suspected PC, with or without negative PB, with normal/doubtful MRI. The presence of a pathological digital rectal examination may condition the result of the test.
El uso de biomarcadores en la detección del cáncer de próstata (CP) puede disminuir el sobrediagnóstico y sobretratamiento de CP no significativos. Analizamos la utilidad y aplicabilidad del marcador SelectMDx® en un entorno de práctica clínica habitual.
Material y métodosEstudio retrospectivo de 48 pacientes evaluados mediante el test SelectMDx® entre julio de 2017 y abril de 2019. Los pacientes se estratificaron en dos grupos según el riesgo estimado por el test de CP clínicamente significativo (CP-CS): <2% o “muy bajo riesgo”, y >2%. Los resultados se expresaron en función de los antecedentes de biopsia prostática (BP) y RMmp.
ResultadosEn pacientes con BP negativa y RMmp normal/dudosa el riesgo fue <2% en 7/9 casos. En pacientes sin BP y RMmp normal/dudosa el riesgo fue <2% en 12/18 casos, y 2/6 casos con un riesgo >2% presentaron un CP-CS. De los 14 pacientes sin BP ni RMmp previas, 9 presentaron un riesgo <2%, con 2 casos diagnosticados de CP en los 5 pacientes con riesgo >2%. En el resto de subgrupos el número de pacientes es pequeño como para poder extraer conclusiones. En todos los casos con tacto rectal patológico el test demostraba un riesgo de padecer CP > 2%.
ConclusiónSelectMDx® es un test prometedor para detectar pacientes con un riesgo muy bajo de CP-CS, especialmente en pacientes con sospecha de CP con o sin BP negativas, en los que la RMmp muestre un resultado normal/dudoso. La presencia de un tacto rectal patológico puede condicionar el resultado del test.