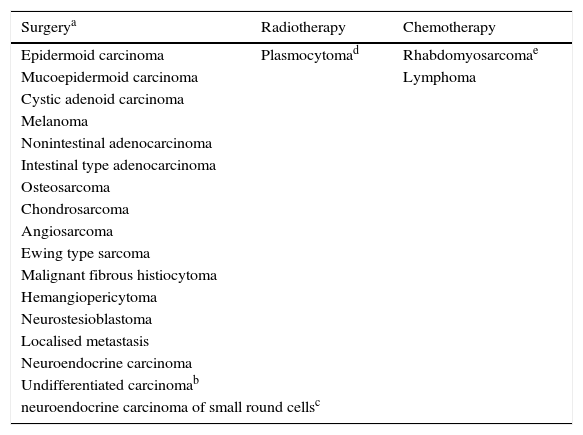
Sinonasal tumors are rare neoplasms with distinctive clinical, aetiological and pathological features. The diagnosis and treatment of these tumors is challenging because of their low incidence, histological diversity and production of nonspecific symptoms in the early stages. They have a variable prognosis depending on their histology, origin and staging. Their location, close to neurocritical structures, which are of special relevance to surgery and postoperative treatment, makes their treatment difficult and complex, leading to high morbidity and mortality. Surgery followed by radiotherapy is the mainstay of treatment. To provide the best possible care, patients with sinonasal cancer should be treated in clinical referral centres specialising in skull-base pathologies. Such centres should include a multidisciplinary team led by otolaryngologist surgeons. This article outlines a consensus protocol for the management of these tumors devised by the Spanish Society of Otolaryngology in collaboration with the Spanish Society of Medical Oncology and the Spanish Society for Radiation Oncology.
Los tumores nasosinusales son neoplasias poco frecuentes. Su epidemiología, histopatología y características clínicas son diferentes a las del resto de neoplasias malignas de cabeza y cuello. El diagnóstico y tratamiento de estos tumores plantea diversos desafíos debido a su escasa incidencia, su diversidad histológica, la producción de sintomatología inespecífica en los estadios precoces y por tener un pronóstico variable en función de su histología, lugar de origen y estadificación. Su localización centrofacial y la proximidad de estructuras como la órbita y la base del cráneo hacen que su tratamiento sea difícil y complejo, conllevando una elevada morbimortalidad. La cirugía seguida de radioterapia es el tratamiento de elección en la mayor parte de los casos. Para conseguir unos buenos resultados se requiere de equipos multidisciplinares altamente especializados. En este artículo se expone un protocolo de consenso para el tratamiento de los tumores nasosinusales realizado por la Sociedad Española de Otorrinolaringología en colaboración con la Sociedad Española de Oncología Médica y la Sociedad Española de Oncología Radioterápica.