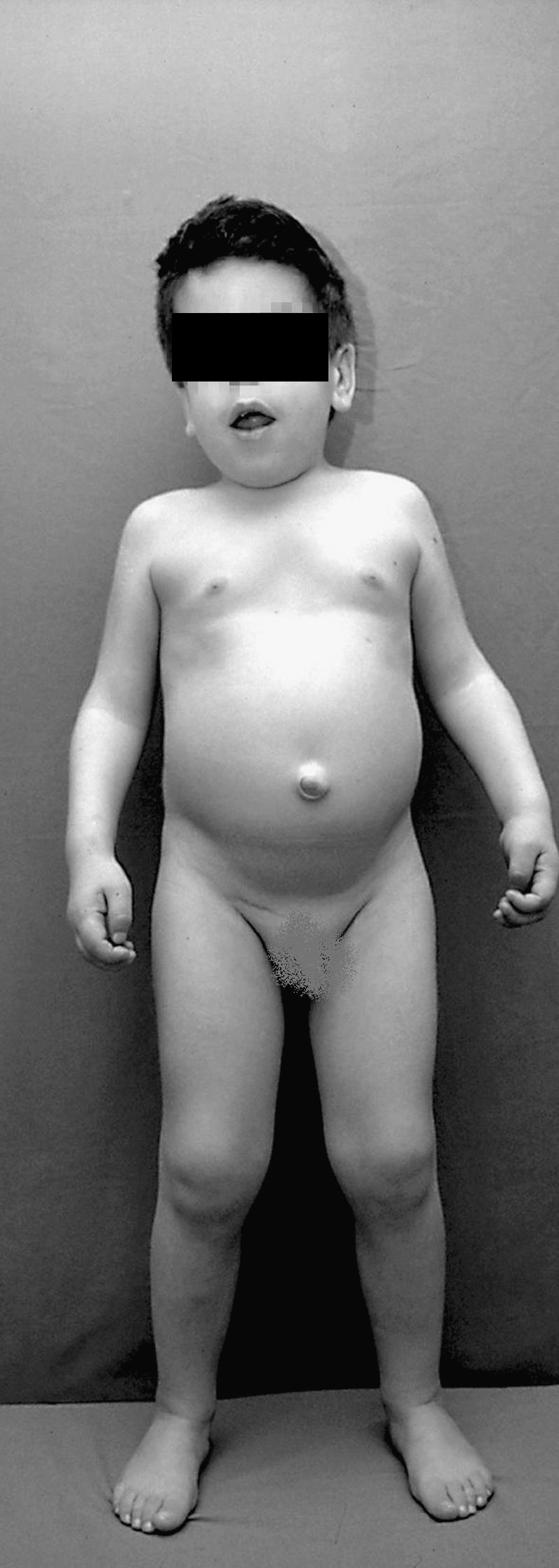
Mucopolysaccharidoses (MPS) are a group of systemic diseases characterised by a genetic deficiency of lysosomal enzymes that cause the accumulation of glycosaminoglycans in different tissues. The onset of symptoms usually occurs in early childhood, causing problems of otitis media, hearing loss and airway obstruction in the ENT area.
ObjectiveDescribing the audiological findings and airway pathology found in 9 children diagnosed as having MPS.
MethodsA retrospective review was performed of the clinical and audiological findings, exploratory results and therapeutic ENT procedures for 9 children diagnosed with MPS in an ENT service at a tertiary paediatric public centre in the period 2007–2010.
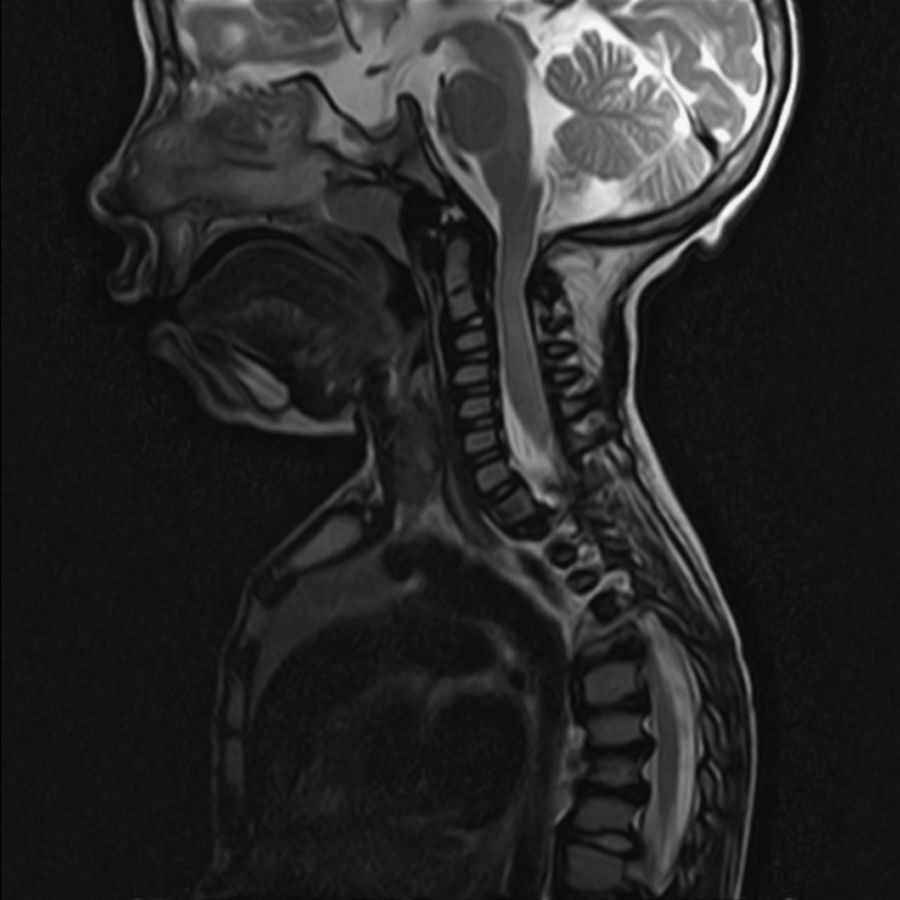
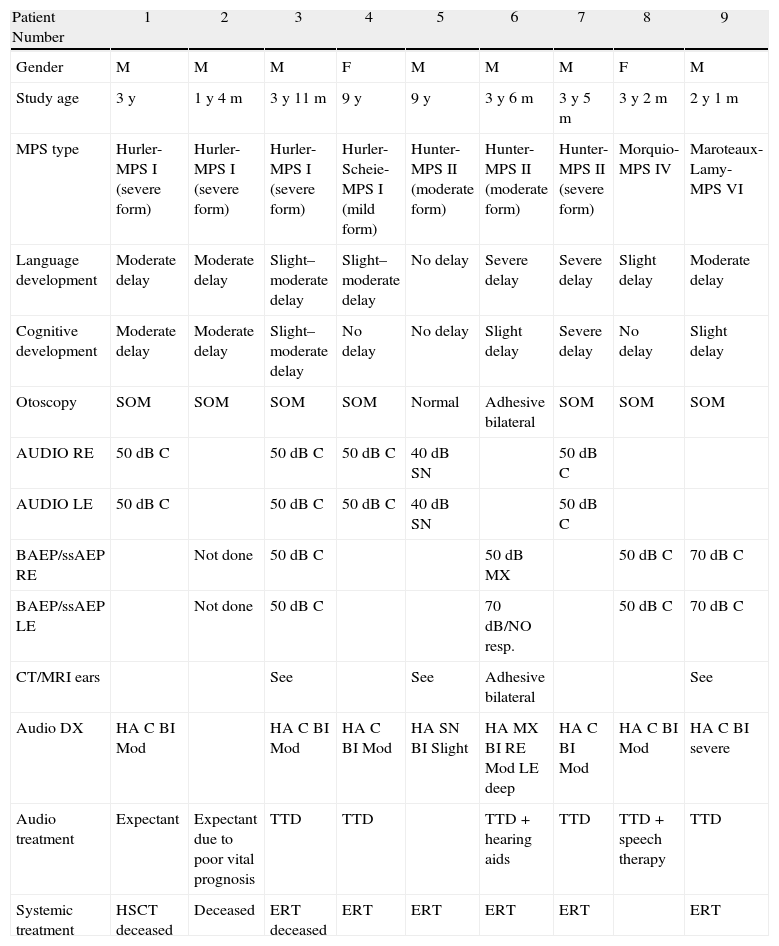
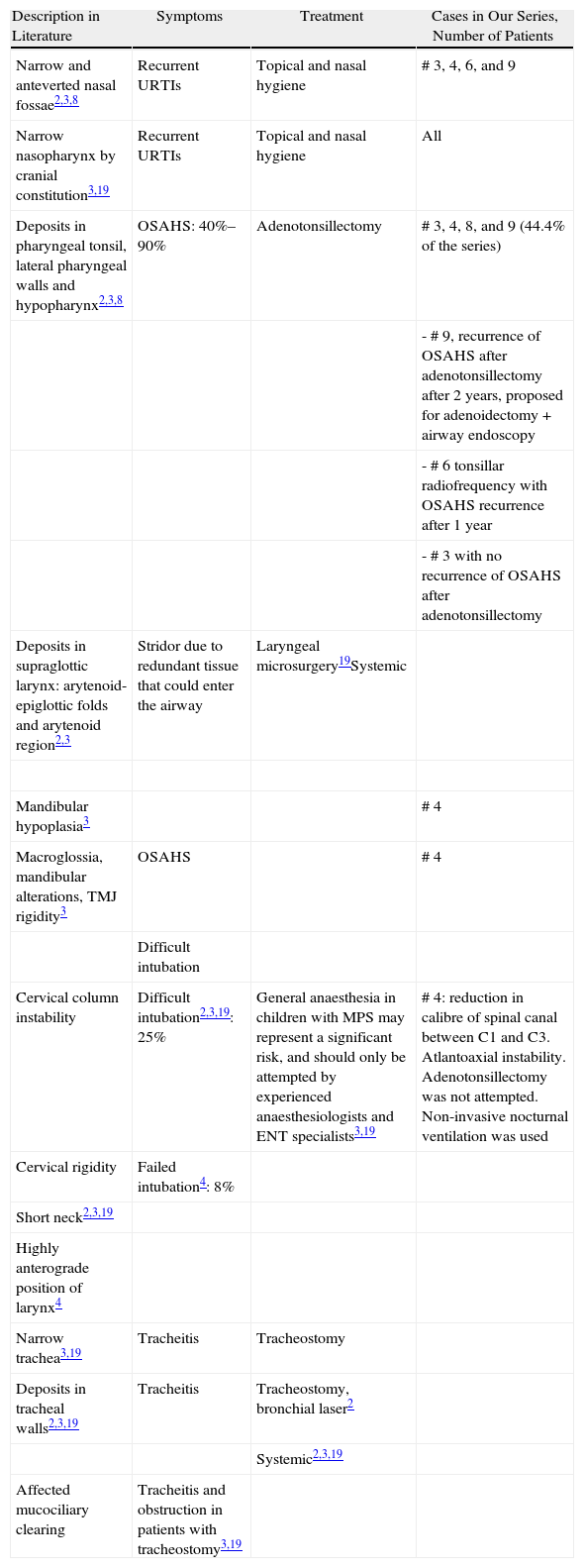
ResultsSubtypes found were 4 MPS type I, 2 moderate MPS type II, 1 severe MPS type II, 1 MPS type IV and 1 MPS type VI. All patients presented chronic middle ear effusions. A child developed mild bilateral sensorineural hearing loss; another case was diagnosed as mixed hearing loss. The remaining auditory pattern was moderate bilateral conductive hearing loss. Four patients showed secondary obstructive sleep apnoea/hypopnoea syndrome (OSAHS) due to Waldeyer ring hyperplasia; surgery could not be performed on one of them because of cervical spinal cord compression from mucopolysaccharide deposits. In 2 cases, there was OSAHS relapse.
ConclusionsChildren with MPS are at increased risk for developing sensorineural hearing loss. The OSAHS syndrome appears in greater proportion than in the general child population, and recurrences may occur more frequently after surgery. Such children can also be risk patients in airway management.
Las mucopolisacaridosis (MPS) son un grupo de enfermedades sistémicas caracterizadas por un déficit genético de enzimas lisosomales que ocasiona el acúmulo de glucosaminoglucanos en diferentes tejidos. El inicio de los síntomas suele presentarse en la primera infancia, ocasionando en el área ORL problemas de otitis media, hipoacusia y obstrucción de vía aérea.
ObjetivoDescripción de los hallazgos audiológicos y la patología de vía aérea encontrados en 9 niños diagnosticados de MPS.
MétodosRevisión retrospectiva de los hallazgos clínicos, audiológicos y procedimientos exploratorios y terapéuticos ORL realizados a 9 niños diagnosticados de MPS en un centro público pediátrico terciario en el período 2007-2010.
ResultadosLos subtipos encontrados fueron 4 MPS I, 3 MPS II, 1 MPS-IV y 1 MPS VI. Todos los pacientes presentaban otitis seromucosa. Un caso desarrolló hipoacusia neurosensorial bilateral leve, otro fue diagnosticado de hipoacusia mixta. El patrón auditivo restante fue hipoacusia conductiva bilateral moderada. Cuatro pacientes presentaban SAHOS (síndrome de apnea/hipopnea del sueño) secundario a hiperplasia del anillo linfático de Waldeyer, en uno de ellos no pudo realizarse cirugía por compresión medular cervical por depósitos de mucopolisacáridos. En 2 de los casos el SAHOS recidivó.
ConclusionesLos niños con MPS presentan mayor riesgo para desarrollar hipoacusia neurosensorial. El SAHOS se encuentra en mayor proporción que en la población general infantil, pudiendo recidivar más frecuentemente tras cirugía. Asimismo pueden ser pacientes de riesgo en el manejo de la vía aérea.