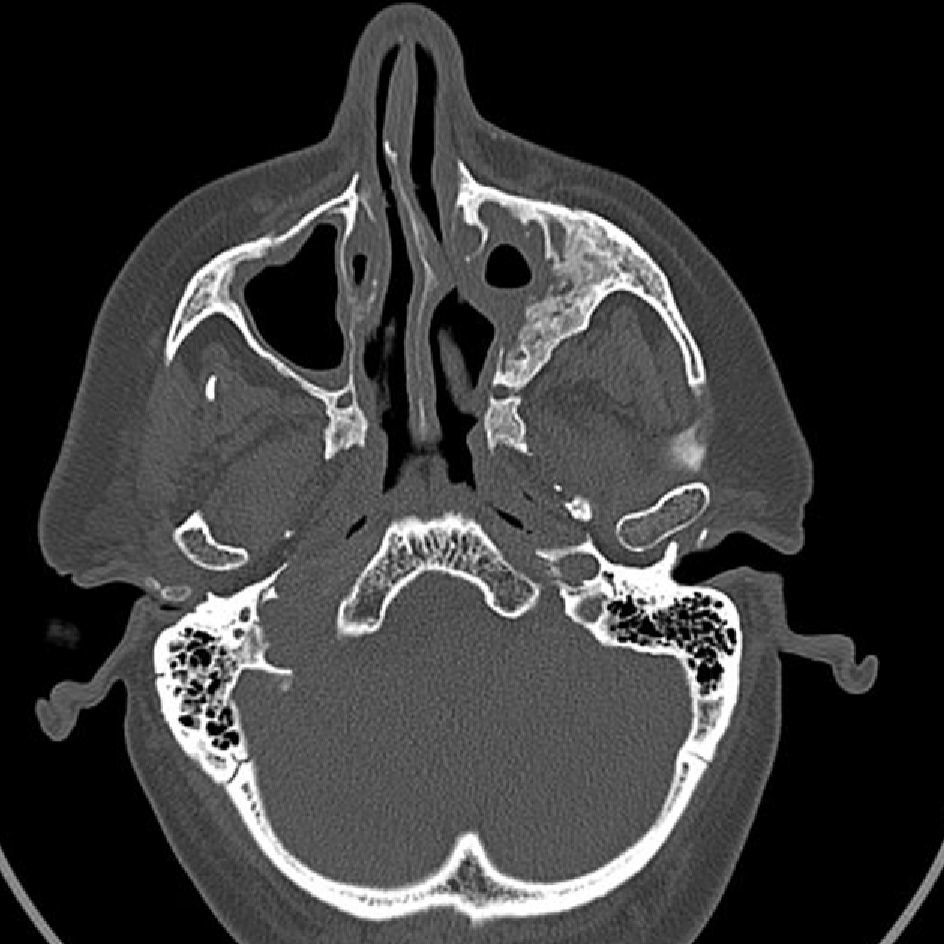
Granulomatosis with polyangiitis (GPA), previously called Wegener's granulomatosis, is a small vessel vasculitis often associated with clinical head and neck manifestations, which are sometimes the presenting symptoms of the disease. The aim of our study was to identify ear, nose and throat (ENT) manifestations associated with GPA and propose a work-up for the management and diagnosis for patients with suspicion or confirmed diagnosis of this ENT pathology.
Patients and methodsRetrospective review of the medical records of all patients diagnosed with GPA who were seen at the Department of Otolaryngology from a tertiary public hospital in Cantabria (Spain) over a 20-year period. Clinical and laboratory data, in particular those concerning ENT manifestations, were retrieved from the patients’ medical records.
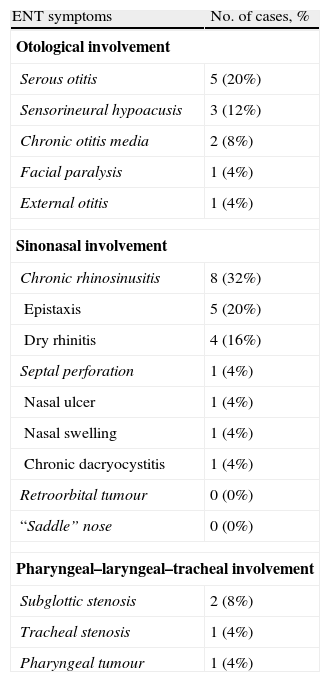
ResultsTwenty-five patients (age range: 30–81 years) were included in the study. Of these, 88% had ENT manifestations at some point in the course of the disease. In 28% of the cases, ENT features were the presenting manifestations. The most frequent ENT manifestations were sinonasal symptoms (52%), followed by otological manifestations (32%).
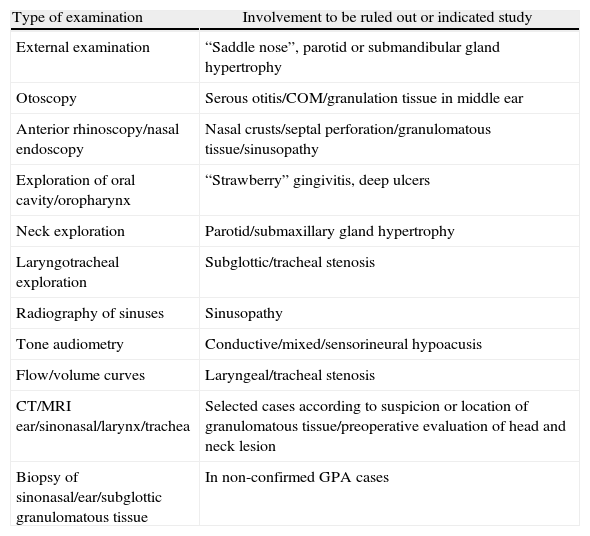
ConclusionsPatients with GPA often present with clinical ENT manifestations. Consequently, routine ENT physical examination must be performed in patients with suspected vasculitis to establish a diagnosis of GPA or to better determine the degree of organ system involvement in patients with GPA.
La granulomatosis con poliangeitis (GPA), previamente llamada granulomatosis de Wegener, es una vasculitis de pequeños vasos que con frecuencia se asocia a manifestaciones clínicas de cabeza y cuello, y en ocasiones constituyen los síntomas de presentación de la enfermedad. El objetivo de nuestro estudio fue identificar la afección otorrinolaringológica asociada a dicha enfermedad y proponer un protocolo diagnóstico de los pacientes con sospecha clínica o confirmada de la misma.
Pacientes y métodosSe realizó un estudio retrospectivo descriptivo que incluyó los pacientes diagnosticados de GPA que recibieron asistencia por el Servicio de Otorrinolaringología de un hospital público de tercer nivel de Cantabria. El período de inclusión fue de 20 años. Se recogieron de la historia clínica diferentes variables clínicas concernientes al área de cabeza y cuello.
ResultadosVeinticinco pacientes con edades comprendidas entre 30 y 81 años de edad fueron incluidos en nuestro estudio. El 88% de los mismos presentaron manifestaciones otorrinolaringológicas en algún momento de la evolución de la enfermedad, y en un 28% constituyeron la forma de presentación de la misma. Los síntomas nasosinusales fueron los más frecuentes (52%), seguido de los otológicos (32%). Un paciente desarrolló un carcinoma de nasofaringe 3 años después del tratamiento con ciclofosfamida.
ConclusionesLos pacientes con GPA presentan con frecuencia manifestaciones clínicas de cabeza y cuello. Es necesario realizar una exploración sistemática otorrinolaringológica en los pacientes con sospecha o diagnóstico confirmado de GPA, tanto para contribuir al diagnóstico de la enfermedad, si esta no estuviese confirmada, como para establecer el grado de afección sistémica de pacientes con esta vasculitis.