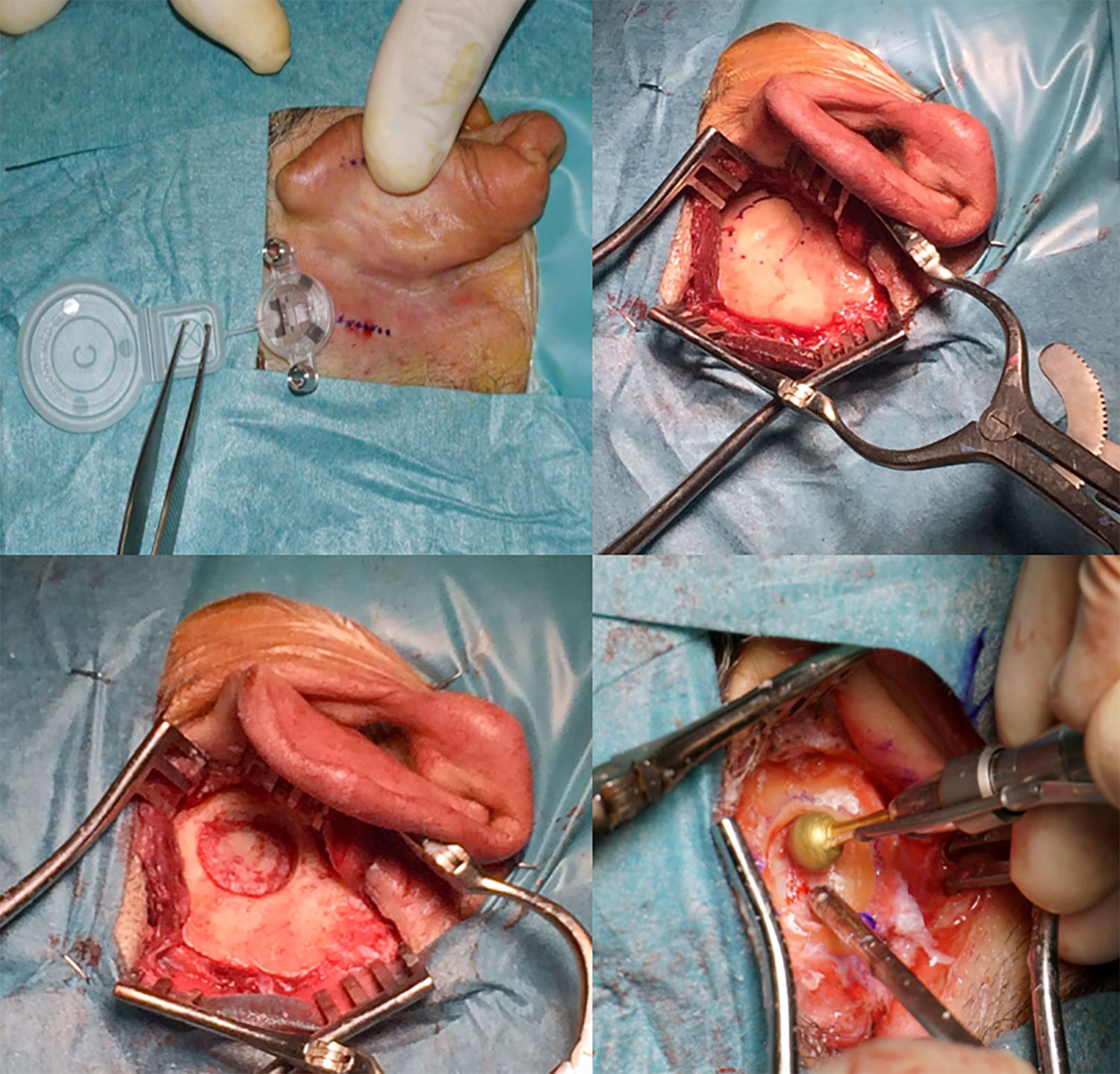
The active transcutaneous bone conduction implant Bonebridge®, is indicated for patients affected by bilateral conductive/mixed hearing loss or unilateral sensorineural hearing loss, showing hearing outcomes similar to other percutaneous bone conduction implants, but with a lower rate of complications. The aim of this study was to analyse the hearing outcomes in a series of 26 patients affected by conductive or mixed hearing loss and treated with Bonebridge®.
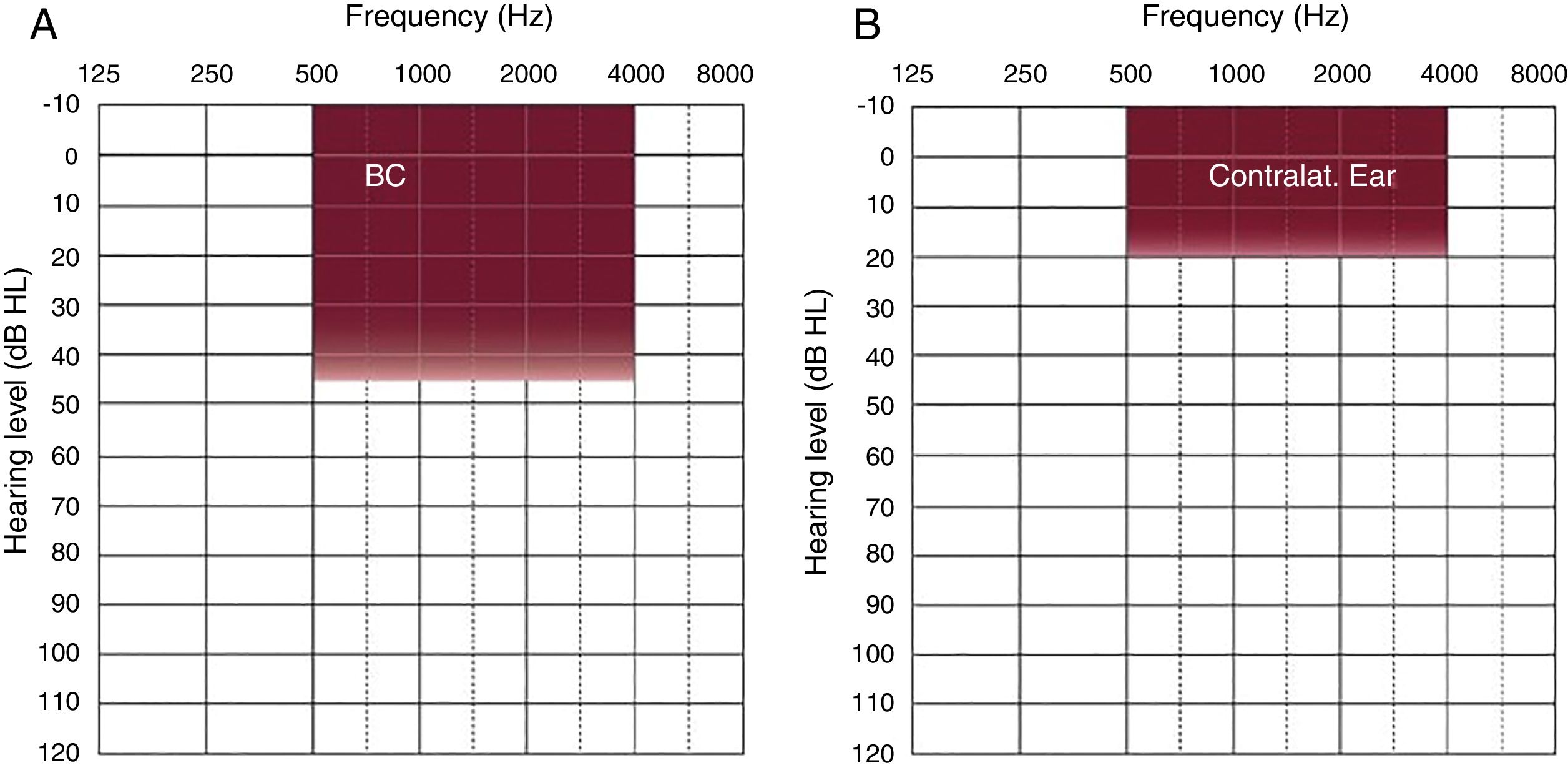
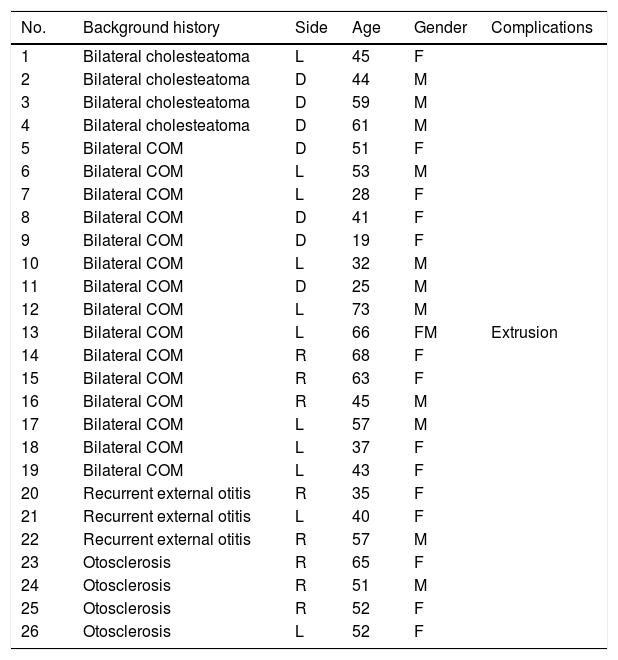
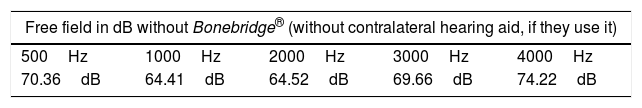
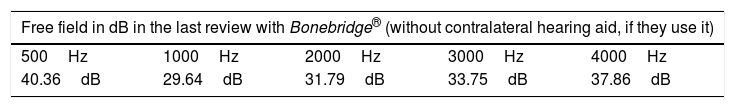
Methods26 of 30 patients implanted with Bonebridge® between October 2012 and May 2017, were included in the study. We compared the air conduction thresholds at the frequencies 500, 1000, 2000, 3000, 4000Hz, the SRT 50% and the percentage of correct answers at an intensity of 50dB with and without the implant.
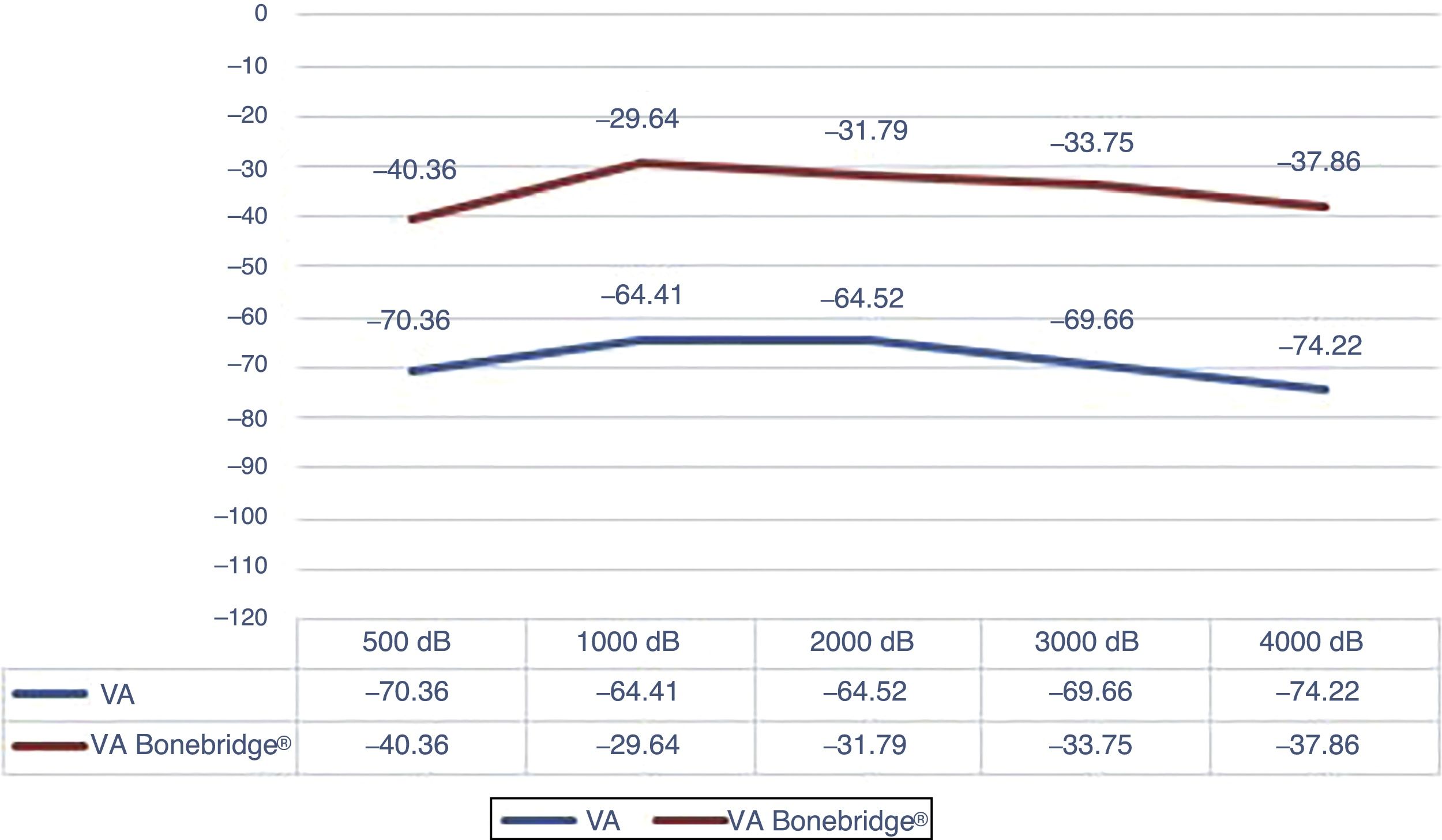
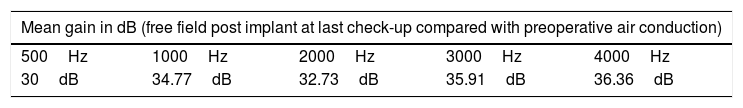
Results“Pure tone average” with the implant was 34.91dB showing an average gain of 33.46dB. Average SRT 50% with the implant was 34.33dB, whereas before the surgery no patient achieved 50% of correct answers at a sound intensity of 50dB. The percentage of correct answers at 50dB changed from 11% without the implant to 85% with it. We only observed one complication consisting of an extrusion of the implant in a patient with a history of 2 previous rhytidectomies.
ConclusionsThe hearing outcomes obtained in our study are similar to those published in the literature. Bonebridge® represents an excellent alternative in the treatment of conductive or mixed hearing loss, and with a lower rate of complications.
El implante activo de conducción ósea transcutáneo Bonebridge® está indicado en pacientes con hipoacusia conductiva/mixta bilateral o en casos de hipoacusia neurosensorial unilateral, mostrando resultados auditivos similares a otros dispositivos percutáneos de conducción ósea pero con menor tasa de complicaciones. El objetivo del siguiente trabajo ha sido analizar los resultados auditivos en una serie de 26 pacientes con hipoacusia conductiva/mixta tratados con Bonebridge®.
MétodosVeintiséis de un total de 30 pacientes implantados con Bonebridge® entre octubre 2012 y mayo 2017 fueron incluidos en el estudio. Se compararon los umbrales de vía aérea a las frecuencias 500, 1.000, 2.000, 3.000 y 4.000Hz, umbral de reconocimiento verbal 50% y el porcentaje de aciertos a 50dB sin y con el implante.
ResultadosEl umbral tonal medio en campo libre con el dispositivo en funcionamiento fue de 34,91dB, obteniendo unas ganancias medias de 33,46dB. La SRT 50% media con el implante fue de 34,33dB mientras que sin él nadie alcanzaba el 50% de aciertos a una intensidad de hasta 50dB. Con respecto al porcentaje de aciertos a 50dB, mejoró desde un 11% sin implante a un 85% con el mismo. Entre las complicaciones solo se observó un caso de extrusión del dispositivo en una paciente con antecedentes de 2 ritidoplastias previas.
ConclusionesLos resultados audiológicos obtenidos en nuestro estudio son similares a los publicados en la literatura. Bonebridge® representa una excelente alternativa en el tratamiento de la hipoacusia conductiva/mixta, pero con una tasa menor de complicaciones.