Zika fever is an arboviral systemic disease that has recently become a public health challenge of global concern after its spread through the Americas. This review highlights the current understanding on Zika virus epidemiology, its routes of transmission, clinical manifestations, diagnostic tests, and the current management, prevention and control strategies. It also delves the association between zika infection and complications, such as microencephaly or Guillem-Barré syndrome.
La fiebre del Zika es una enfermedad sistémica causada por un arbovirus que se ha convertido recientemente en un problema de salud pública de importancia mundial después de su propagación a través de las Américas. Esta revisión describe el conocimiento actual sobre la epidemiología del virus Zika, sus vías de transmisión, manifestaciones clínicas, las técnicas de diagnóstico y las estrategias actuales de gestión, prevención y control. También profundiza en la asociación entre la infección y las complicaciones atribuidas al virus Zika, como la microcefalia o el síndrome de Guillem-Barré.
Zika virus (ZIKV) is a mosquito-borne flavivirus of the family Flaviviridae included in the Spondweni serocomplex.1 The name was given after the isolation of the virus in 1947 from a sentinel rhesus monkey in the Zika forest, located near Entebbe, Uganda.2
ZIKV is thought to be originated in forested regions of Sub-Saharan Africa where is considered to be maintained in enzootic sylvatic cycles between non-human primates and mosquitoes.3 Cyclic epizootics in monkeys have been consistently reported.4 Interestingly antibodies against ZIKV have been detected in a numerous variety of mammal species other than non-human primates including lions, buffalos, elephants, zebras, hippos, impalas, sheep, goats and rodents.5,6 While in the sylvatic transmission cycle humans might only serve as incident hosts, in transmission areas without non-human primates, humans serve as the primary amplification hosts and potentially as reservoir hosts as in other arboviral transmission cycles.7,8
ZIKV presents an enveloped and icosahedral structure with a single-stranded positive-sense, RNA genome of approximately 11 kilobases in length. The genome includes a complete open reading frame (ORF) encoding a polyprotein constituted by three structural proteins: (the capsid (C), premembrane/membrane (prM), and envelope (E), and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, 2K, NS4B, and NS5).9 Phylogenetic analysis based on the conserved region NS5 has revealed the existence of two ZIKV lineages, with basal divergences between the African and the Asian lineage.10
EpidemiologyThe first isolate of ZIKV was obtained from the brain of mice inoculated with the serum of Rhesus 766 the monkey which had been placed in a cage in a tree platform as part of the yellow fever program of the Rockefeller foundation.2 One year later, a second isolation of the virus was obtained from the mosquito Aedes africanus in the same forest.11 Further serological studies confirmed that humans could be infected by ZIKV12 and transmission of the virus by artificially fed Aedes aegypti mosquitoes to mice was reported.13 Sporadic isolation of the virus from humans was obtained in studies in Nigeria in the next decades.14–16 Several studies in Africa (Uganda, Tanzania, Egypt, Central African Republic, Sierra Leone, and Gabon) and Asia (India, Malaysia, the Philippines, Thailand, Vietnam, and Indonesia) reported serological evidence of the spread and distribution of the virus.10 Sporadic isolation of the virus was latterly reported from humans in Senegal and Central African Republic as well as from mosquitoes in Ivory Coast, Burkina Faso and Malaysia.17–19 In 2007 an outbreak in the Yap Islands7,20 initiated the spread of ZIKV among the Pacific region. In 2013 a major epidemic broke out in French Polynesia8,21 and first autochthonous cases were reported in New Caledonia22 by the beginning of 2014 and later from the Cook Islands,23 Vanuatu and Solomon Islands. At the same time, in January 2014 a case of Zika fever was confirmed in the Eastern Island (Chile) with forty more cases suspected.24 During 2014, imported cases from the Pacific region were reported in travelers in Norway, Germany, Australia, France, Canada, Italy and Japan.25–31
In May 2015 the first cases of the ZIKV epidemic in the continental Americas were reported when 17 cases of Zika fever were confirmed from three states in Brazil: Bahia (8 cases), Rio Grande do Norte (8 cases) and São Paulo (1 case). Since then, the virus has spread in an explosive pandemic through South and Central America and the Caribbean. From October 2015 to February 2016, autochthonous transmission of ZIKV has been reported with more than 125.000 suspected cases in 28 countries (Aruba, Barbados, Bolivia, Bonaire, Brazil, Colombia, Curaçao, Costa Rica, Dominican Republic, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Nicaragua, Panama, Paraguay, Puerto Rico, Saint Martin, Suriname, United States Virgin Island and Venezuela).32 Outside of the Americas, the Atlantic island nation of Cape Verde announced its first ZIKV epidemic in October 2015. First sequencing studies suggest that the pandemic is due to the Asian lineage as happened in the epidemic at the pacific region.33,34 Worldwide, 39 countries have reported locally acquired circulation of the virus since 2007 to date (see Figs. 1 and 2).
Number of ZIKA cases (confirmed and suspected) reported during 2015–2016 in the Americas (source: PAHO [http://ais.paho.org/phip/viz./ed_zika_epicurve.asp]; last update 12 Feb 2016).
Map with all countries with ongoing transmission of zika virus (source: CDC Centers for Disease Control and Prevention [http://www.cdc.gov/zika/geo/active-countries.html]; last update 5 Feb 2016). Americas: Barbados, Bolivia, Brazil, Colombia, Commonwealth of Puerto Rico (US territory), Costa Rica, Curacao, Dominican Republic, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Nicaragua, Panama, Paraguay, Saint Martin, Suriname, U.S. Virgin Islands, Venezuela. Oceania/Pacific Islands: American Samoa, Samoa, Tonga. Africa: Cape Verde.
ZIKV is a considered to be transmitted mainly through mosquito bites. ZIKV was initially isolated from arboreal Aedes africanus, yet studies were unclear if the mosquito was the true vector of enzootic transmission in wild monkeys.3 Further isolation of the virus has been reported from Ae. apicoargenteus, Ae. luteocephalus, Ae. aegypti, Ae. vitattus, and Ae. furcifer mosquitoes, all belonging to the subgenus Stegomyia.10 During the Yap outbreak, Ae. henselii was the predominant mosquito in the island, though studies were unable to detect the virus among trapped mosquitoes.35Ae. aegypti is considered to be the main vector in Asia and the Americas and in some regions in the Pacific. Early studies showed the capacity of Ae. aegypti to transmit the virus with an extrinsic incubation period of around 10 days after artificial feeding.13 Mosquitoes could be infective up to 60 days after acquiring the virus. Ae. aegipti is recognized to be adapted to urban settings in the “tropical belt” and has been the main responsible of the transmission of other arbovirus such as Yellow Fever, dengue and chikungunya. The role of Ae. albopictus, the other major vector of in the transmission of arbovirus is still unclear. Ae. albopictus is a known competent vector for ZIKV transmission.36,37 The global spread of Ae. albopictus during the last decades, caused by human activities, such as intercontinental trade, could alter the transmission dynamics of arboviral diseases including ZIKV and increase the risks of humans to mosquito-borne viral infections in regions were Ae. aegypti is not able to survive.
There are other important routes of transmission independents to the vector. Of special relevance is the mother-to-child transmission of ZIKV, which has been reported in the past38 and consistently confirmed during the current outbreak. Vertical transmission can happen at any time of the pregnancy through the placental barrier, or intrapartum, causing congenital infection. ZIKV RNA has been detected in amniotic fluid, placenta and fetal tissues.39 ZIKV transmission through sexual intercourse has been suggested and ZIKV has been isolated from semen up to sixty days after onset of infection.17,40 The virus has also been detected in breast milk, although this way of transmission has not been reported.38 Potential transmission through blood transfusion is also possible, and the virus has been detected and isolated in blood of asymptomatic donors during the French Polynesia outbreak.41 Transmission of ZIKV to humans following a monkey bite has also been reported.42
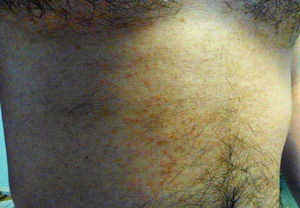
Clinical manifestationsUntil recently, the knowledge on the clinical manifestations of zika fever was limited to the description of isolated cases and case series in epidemics. The incubation period of the flavivirus is thought to be of around 3–7 days (range 3–14) after the mosquito bite.43 Symptomatic patients might represent around 1 in 5 of those infected with ZIKV.7 The illness might last for several days up to weeks and is usually of mild intensity. It is thought that permanent immunity is acquired after infection as up to date re-infections have not been reported. The common clinical symptoms of ZIKV are acute onset of fever with pruriginous maculopapular rash (exanthema), arthralgia, and/or non-purulent conjunctivitis (Fig. 3). Other commonly reported symptoms are muscle pain and headache. However, reporting clinical manifestations might vary within different regions and epidemics. From early studies, Zika fever had been generally reported as a self-limited mild disease with relatively unspecific symptoms such fever, malaise, headache, back pain and commonly accompanied with rash.44,45 Other less frequently reported symptoms included anorexia, abdominal pain, diarrhea, constipation, dizziness and conjunctivitis.46 The most frequently reported clinical picture during the Pacific outbreak was fever, rash, conjunctivitis, and arthralgia followed by myalgia, headache, retroorbital pain, edema, and vomiting.7,47 During the 2015 outbreak in Brazil, early reports described initially a “dengue-like” syndrome. Patients were reported presenting with arthralgia, edema of extremities, mild fever, maculopapular rash frequently pruritic, headache, retro-orbital pain, no purulent conjunctivitis, vertigo, myalgia and digestive disorder.48
Fever is generally of mild intensity and is accompanied with cephalea. A commonly pruriginous exanthema can appear within 48hours of fever onset and can affect face, trunk, and extremities including palms and soles which can persist up to 14 weeks (average 6 days). Fever is generally limited to 24–48hours after the appearance of the rash. Conjunctivitis has been consistently observed and it is characteristically non-purulent. Myalgia is also commonly reported, as well as lower-back and joint pain. In contrast with chikungunya fever, pain and inflammation are of low intensity and have not been reported to involve hands, knees or ankles. Information about hematological alteration is scarce in the literature though discrete leucopenia and thrombocytopenia has been reported.28
Zika fever is considered to present benign evolution. No hemorrhagic events associated to Zika fever, characteristic in other flavivirus infections, have been reported to date. Few cases reporting co-infection of ZIKV with dengue and chikungunya have been described, yet no synergic effect with regards to severity of disease has been observed.49,50 ZIKV infection has been recently associated with neurological complications. The main and most serious reported complication is Guillain-Barré Syndrome (GBS), but others such as encephalitis or meningitis have also been observed. During the outbreak in the French Polynesia in 2014, severe cases of Zika fever were reported for the first time: seventy-four cases of severe presentations with severe neurological symptoms were notified. Among these, forty GBS Zika-associated were diagnosed, though direct involvement of the occurrence of the severe presentations with the virus was unclear.51 The incidence of GBS associated to ZIKV in the Americas is still to be elucidated. Nevertheless, countries in Latin America have observed increase of GBS through surveillance. In July 2015, Brazil reported the detection of patients with neurological syndromes including confirmed GBS with consistent history of ZIKV infection.32 Other countries reporting an increase of GBS are El Salvador, Surinam, Venezuela and Colombia. Despite this temporal association between Zika fever and GBS, up to date there has not been reported analytical evidence of causality and the underlying mechanism, potentially of autoimmune origin, is still to be elucidated.
Dengue and chikungunya infection should be included in the differential diagnosis of Zika infection; while symptoms are very similar, laboratory testing is necessary for discrimination.
PregnancyPregnant women can be infected at any time during pregnancy; however, neither a higher susceptibility for ZIKV during pregnancy nor a more severe disease has been documented. Vertical transmission can occur at any time of the pregnancy through the placental barrier, or intrapartum, causing congenital infection.38
The temporal overlap between the twenty-fold increase in cases of microcephaly last year in Brazil with the current ZIKV outbreak, and the detected cases in French Polynesia during the past ZIKV outbreak between October 2013 and February 2014, suggest a possible cause-effect relationship.52,53 Furthermore, fetal losses and ophthalmic lesions have been reported in newborns with suspicion of congenital ZIKV infection54–56. Recently it has been reported a suspected case of vertical transmission of ZIKV in a woman who was probably infected in northeastern Brazil at the end of the first trimester of pregnancy. Ultrasonography in the second trimester revealed microcephaly and calcifications in the fetal brain and placenta. Anatomopathological findings of the fetus’ brain and virological results were consistent with a ZIKV infection by the same strain present in Brazil.39 Interestingly, increasing incidence of microcephaly in the rest of the countries affected by the current outbreak has not been reported to date. Taking in to account all the emerging data, there is not enough evidence yet to confirm the cause-effect relationship.57
Diagnostic testsZika Fever is diagnosed on the basis of clinical, epidemiological and laboratory findings. Laboratory diagnostic is needed for confirmation as the infection usually presents with unspecific signs and symptoms such as fever, headache or myalgia. Moreover, dengue and chikungunya have been usually epidemic in regions where ZIKV is circulating with similar clinical picture and clinical diagnosis may be confounded. Thus, definitive diagnostic relies on the detection of viral RNA through reverse-transcriptase protein-chain-reaction testing (rt-PCR) in acute-phase sera. The viraemic phase has not been well established though is thought to be relatively short (3–5 days after onset of symptoms). However, detection of viral RNA has been reported up to 10 days after symptoms onset.20 The suitability of other less invasive specimens such as urine and saliva has been confirmed. Urine samples seem to present higher loads of the viral RNA and for longer periods.26,27,58 The use of saliva sample could increase the rate of molecular detection of ZIKV at the acute phase of the disease but has not been demonstrated to enlarge the window of detection of ZIKV RNA.59 Detection of the virus in amniotic fluid as well as placenta and fetal tissues following congenital infection has been recently confirmed.39,60
Culture of ZIKV as any other flavivirus is possible in a variety of cells which allow further virological characterization. A biosafety level 3 of contention laboratory is considered mandatory for viral culture and does not add any value over rt-PCR in clinical practice, yet should not be performed routinely (Table 1).
Comparison of clinical manifestations for dengue, chikungunya and Zika fever.
| Dengue | Chikungunya | Zika | |
|---|---|---|---|
| Fever | ++++ | +++ | +++ |
| Myalgia/arthralgia | +++ | ++++ | ++ |
| Headache/retro-orbital pain | ++ | + | ++ |
| Maculopapular rash | +++ | ++ | +++ |
| Pruritus | + | − | + |
| Conjunctival hyperemia | + | + | +++ |
| Edema of extremities | + | − | ++ |
| Lymphadenopathies | ++ | ++ | + |
| Hepatomegaly | − | + | − |
| Hemorrhage | + | − | − |
| Leukopenia/thrombopenia | +++ | +++ | + |
| Elevation of transaminases | ++ | + | + |
| Neurological complication | + | + | + |
Detection of specific antibodies against ZIKV antigen through enzyme linked-immunoassay (ELISA) or immune fluorescence assay (IFA) can be also performed. Serum IgM is thought to rise and be detectable around 5 days after onset of symptoms. Seroconversion can also be detected as an increase in IgG by a factor of 4 or more between acute-phase and convalescent-phase serum samples. Cross-reactivity with other flavivirus such as dengue virus, yellow fever, Japanese encephalitis, Murray Valley encephalitis, or West Nile viruses is frequent. Cross-reactivity is more common in those cases with previous flavivirus infection.20 Neutralization assays, such as the plaque reduction neutralization assay generally has been reported to improve specificity over immunoassays, but may still yield cross-reactive results in secondary flavivirus infections. To date, no commercial tests have been developed and validated for serological diagnostics of ZIKV. As per usual in the diagnostic of flavivirus infection, testing should include at least an acute-phase serum collected as early as possible and a second convalescent-phase samples one to three weeks after the first.
ManagementZika fever is in most of the cases self-limited and usually resolves within days or weeks without any sequelae. Specific antiviral treatment for acute illness is not available. Treatment is generally supportive and might include rest, abundant fluids and the use of antipyretic and analgesic medication if needed, such as paracetamol or nonsteroidal anti-inflammatory drugs (NAIDs). The use of NAIDs, especially aspirin (salicylates) in patients that have not been confirmed by PCR is not recommended due to its association with hemorrhagic events in dengue. The pruritus accompanying the exanthema can produce serious discomfort in some patients. The efficacy of antihistaminic drugs is unclear. Corticosteroids should be avoided due to its unknown efficacy and adverse events. Conventional approach to GBS is recommended. Diagnostic can be done by observation of rapid development of muscle paralysis and areflexia. Suspected patients should be closely monitored due to the risk of respiratory muscle paralysis and potential need of intensive care and ventilation.
Management in pregnancyClinical management in pregnant women does not differ from general population. Currently, breastfeeding is not contraindicated. Due to the uncertainties regarding the ZIKV infection in pregnant women, a close follow up is recommended with monthly ultrasounds to monitor fetal growth and morphology.52
Infection prevention and controlSuccessful vaccine development has been achieved for other flavivirus such as yellow fever or Japanese encephalitis. It has taken over 20 years for developing a dengue vaccine candidate available for clinical use and only in the past years Phase III studies have been performed.61 To date no vaccine against zika fever is available and it is anticipated that it will take several years for reaching full production. However, ZIKV vaccine might benefit from other flavivirus vaccine candidates and clinical testing could begin earlier than it is usual in vaccine development.62 Thus, management of the mosquito is the only current available method for controlling ZIKV epidemic.
Mosquito control relies on the use of insecticides and the removal of larval breeding sites. Mosquito control has been previously successful, though the persistence of pockets of mosquitos lead to rapid re-emergence of arboviral diseases. Due to the current widespread resistance to insecticide, including pyrethroids63,64 and the difficulties on eliminating breeding sites at a city-scale, the capacity for containing the disease is substantially limited.
New approaches targeting the vector have been developed and are currently under investigation. Release of genetically modified male mosquitoes that compete with wild-type males to mate females resulting in transmission of lethal genes (RIDL strategy) has shown promising results, with offspring incapable to reach adult stage and reducing 80–95% of population in the field.65 An alternative approach is the introduction of the endosymbiont bacterium Wolbachia into Ae. aegipti. Wolbachia-infected mosquitoes have shown relative resistance to flavivirus infection such as dengue or yellow fever through inhibition of replication and without impact on the mosquito fitness.66 Interestingly, infected mosquitoes can displace natural populations and could lead to a potential introduction of natural biological resistance to flavivirus infection.
Individual preventive measures include the use of DEET- and picaridin-based repellents and minimizing day-biting of Aedes mosquitos.
Future directionsThe threat of Zika virus and other arbovirus such as dengue and chikungunya represent a major public health challenge. Multidisciplinary and co-ordinated approaches are needed. Following the recognition of the burden of arboviral disease, adequate resources must be allocated in order to improve the knowledge of the disease diagnosis, pathogenesis and management especially with regards to congenital infections and severe neurological manifestations. Development of new antivirals and other therapeutic drugs as well as vaccine development should be fostered. Efforts on new innovative approaches for preventing transmission of the virus need to be maintained. Finally, deeper understanding of the epidemiology and spread of arboviruses is critical to assist in planned provision of resources in order to ensure preparedness and prevent future outbreaks and epidemics.
Funding sourceNone.
Conflict of interestThe authors declare no conflict of interest.








![Number of ZIKA cases (confirmed and suspected) reported during 2015–2016 in the Americas (source: PAHO [http://ais.paho.org/phip/viz./ed_zika_epicurve.asp]; last update 12 Feb 2016). Number of ZIKA cases (confirmed and suspected) reported during 2015–2016 in the Americas (source: PAHO [http://ais.paho.org/phip/viz./ed_zika_epicurve.asp]; last update 12 Feb 2016).](https://static.elsevier.es/multimedia/0213005X/0000003400000004/v1_201604090025/S0213005X16300015/v1_201604090025/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Map with all countries with ongoing transmission of zika virus (source: CDC Centers for Disease Control and Prevention [http://www.cdc.gov/zika/geo/active-countries.html]; last update 5 Feb 2016). Americas: Barbados, Bolivia, Brazil, Colombia, Commonwealth of Puerto Rico (US territory), Costa Rica, Curacao, Dominican Republic, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Nicaragua, Panama, Paraguay, Saint Martin, Suriname, U.S. Virgin Islands, Venezuela. Oceania/Pacific Islands: American Samoa, Samoa, Tonga. Africa: Cape Verde. Map with all countries with ongoing transmission of zika virus (source: CDC Centers for Disease Control and Prevention [http://www.cdc.gov/zika/geo/active-countries.html]; last update 5 Feb 2016). Americas: Barbados, Bolivia, Brazil, Colombia, Commonwealth of Puerto Rico (US territory), Costa Rica, Curacao, Dominican Republic, Ecuador, El Salvador, French Guiana, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Nicaragua, Panama, Paraguay, Saint Martin, Suriname, U.S. Virgin Islands, Venezuela. Oceania/Pacific Islands: American Samoa, Samoa, Tonga. Africa: Cape Verde.](https://static.elsevier.es/multimedia/0213005X/0000003400000004/v1_201604090025/S0213005X16300015/v1_201604090025/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)