To describe the age, signs and clinical symptoms of children with scarlet fever at the present time, and to check whether they are equivalent to those with traditional streptococcal pharyngotonsillitis.
Study designAn observational, retrospective study was conducted on the clinical records of 5500 children aged from 0 to 15 years attending a primary health care center. A record was made of the percentage of the cases in which signs and symptoms appear and the Centor score was calculated. Microbiological diagnosis of the disease was made using the rapid antigen-detection test or traditional culture.
ResultsA total of 171 out of 252 scarlet fever diagnoses were microbiologically verified in 158 patients. The median age was 3.8 years (interquartile range: 2.91–4.78), with the majority (57%) under the age of 4 years. There was fever in 89% of the processes (95% CI: 84–94%), with a temperature of >38°C in 73% (95% CI: 65–80%), enlarged lymph nodes in 70% (95% CI: 58–82%), absence of cough in 73% (95% CI: 65–80%), and tonsillar exudate in only 24% (95% CI: 17–31%). The Centor score (n=105) was ≤2 points in 86% (95% CI: 79–92%). The only difference regarding age is that episodes in patients under the age of 4 years old have significantly higher fever (>38°C) than the older ones (80% versus 63%. OR 3.13; 95% CI: 1.46–6.71).
ConclusionScarlet fever pharyngotonsillitis differs from the traditional streptococcal pharyngotonsillitis, and its evaluation using clinical prediction rules such as Centor or McIsaac is questionable. The main diagnostic key must certainly be rash, regardless of patient age.
Describir la edad, signos y síntomas clínicos de niños con escarlatina en la actualidad y comprobar si corresponden a los de la clásica faringoamigdalitis estreptocócica.
Diseño del estudioEstudio observacional, retrospectivo, sobre registros clínicos (5.500 niños de 0 a 15 años pertenecientes a un centro de atención primaria). Porcentaje de casos en los que aparecen los signos y síntomas y cálculo del escore de Centor. Diagnóstico microbiológico realizado mediante test rápido de detección de antígeno o cultivo tradicional.
ResultadosDe 252 diagnósticos de escarlatina se confirmaron microbiológicamente 171, en 158 pacientes. La mediana de la edad fue de 3,8 años (rango intercuartílico: 2,9-4,8), la mayoría (57%) menores de 4 años. Hubo fiebre en un 89% de episodios (IC 95: 84 a 94%), mayor de 38°C en el 73% (IC 95: 65 a 80%), adenopatías en un 70% (IC 95%: 58 a 82%), ausencia de tos en un 73% (IC 95: 65 a 80%), y exudado amigdalar sólo en un 24% (IC 95: 17 a 31%). El escore de Centor (n=105) fue ≤ 2 puntos en un 86% (IC 95: 79 a 92%). Los niños <4 años tienen significativamente más fiebre (> de 38°C) que los mayores (80% frente a 63%. OR 3,13; IC 95: 1,46 a 6,71).
ConclusiónLa faringoamigdalitis de la escarlatina difiere de la clásica estreptocócica y ha de ser cuestionada su valoración a través de reglas de predicción como las de Centor o McIsaac. La clave diagnóstica principal continúa siendo la erupción cutánea independientemente de la edad del paciente.
Among the beta-hemolytic Group A Streptococcus (GAS) infections in children, scarlet fever is known for its peculiarity and incidence. It consists mainly of a pharyngitis by GAS strains which synthesize a pyrogenic exotoxin (erythrogenic toxin types A, B or C) which determines the typical papular erythematous rash of the disease by non-immediate hypersensitivity reaction.
The pharyngotonsillar GAS (PAS) infection in childhood is described as an exudative tonsillitis with acute start, febrile, occasionally with feeling seriously sick and cervical lymphadenopathy, usually with no coryza symptoms but which particularly affects children aged from 4 to 18 years old.
Traditionally, scarlet fever pharyngotonsillitis was described as indistinguishable from the one of the PAS.1,2 However, the clinical presentation of scarlet fever in the most recent and limited publications which describe it does not fall within the age described (it appears ever more frequently under the age of 4) and it seems not to show either all the PAS symptoms described or so developed.3–6
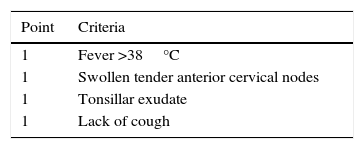
To assess the PAS clinical suspicion, two valid pediatric scores exist: the Centor clinical prediction score (Table 1)7 originally designed to be used in adults; and the McIsaac clinical prediction score which adds one more grade for ages age from 3 to 15 and considers tonsillar swelling as positive (despite no exudate).8 A study on 1848 children aged from 3 to 18 years old who suffered from pharyngitis at three primary health care pediatric centers showed how many pharyngitis were produced by GAS, according to McIsaac's grades, and even being graded with the maximum score, only 60% of them were PAS.9 This justifies the current recommendation of confirming them through the rapid antigen-detection test for GAS (RADT) or traditional culture before starting an antibiotic treatment. Today, most clinical practice guidelines10–20 accept that, in order to diagnose PAS, either previously explained scores or any other clinical item selection should be used to choose those patients to whom RADT or traditional cultures will be applied. Despite the limitations resulting from the assessment of just scores or items selection, in the case of absence of these tests, the scores should prove useful to select the patients who will be given antibiotic therapy.16
To verify whether scarlet fever pharyngitis is similar to PAS (that is, if it meets its criteria), the scarlet fever cases at our primary health care center in recent years have been reviewed, describing the frequency of its main signs and symptoms and assessing the Centor and McIsaac scores appropriateness for the scarlet fever pharyngotonsillitis diagnosis.
Patients and methodsA retrospective, observational cross-sectional study which arises from the digital clinical records from an urban primary health care center (Zaragoza, Spain) of 5500 children aged from 0 to 15 allocated to four pediatricians. From these records, the registers of the clinical episodes known as scarlet fever were drawn. Also reviewed were all the tonsillitis and pharyngotonsillitis diagnoses, in order to recover those in which rash, compatible with scarlet fever was present but which had not been appropriately classified when diagnosing. Traced dates start from the first diagnosis on digital record at the center (December, 2004) to the 31st of March, 2014.
Finally, all the episodes without microbiological confirmation were excluded (RADT, culture or both) as well as those on clinical record which had limited data. Since November, 2011, RADT has been available at our primary health care center (Alere TestPack Strep A, sensitivity 97.6% [95% CI: 93.1–99.5%], and specificity 98.4% [95% CI: 95.9–99.6%], information provided by Alere Medical Co.). Prior to that, pharyngeal swab was carried out and biological samples were transferred under adequate conditions and means of transport to carry out the traditional culture tests at the reference hospital (Microbiology laboratory, Hospital Universitario Miguel Servet, Zaragoza, Spain).
Patients with illnesses or treatments that might cause immunosuppression were also excluded.
From clinical records, the main variable acquired was the confirmed diagnosis of scarlet fever in a child with compatible rash. The presence or absence of the following were secondary variables: fever, cough, coryza symptoms, swollen tender anterior cervical nodes, tonsillar swelling, tonsillar exudate and the characteristic signs of scarlet fever (strawberry tongue, Filatow mask, Pastia lines, petechiae on the soft palate). Highest fever and epidemiological variables including age, sex, episode date and, if there were, any previous episodes of the disease were also registered. Data related to the use of antibiotic therapy were also obtained (antibiotic treatment or not, which one, and its duration). If there was any scarlet fever complication, it was also gathered.
The statistic management was based on a descriptive analysis of the items, in percentages, with their 95% confidence interval. In order to describe quantitative variables that do not follow a normal distribution (age, Kolmogorov–Smirnov test, Dmax: 0.133; p=0.005), the median and the interquartile range were calculated. In order to compare the pharyngotonsillar features that our patients showed with respect to the PAS classic, the Centor and McIsaac scores were calculated (Table 1). As a notable deviation from the disease at traditionally established younger ages was detected, the appearance of different events of the disease in children under the age of four in contrast to those of four years old or over was compared using the odds ratio (OR) estimation and its 95% confidence interval. The analysis was performed using statistics calculators (Centro de Medicina Basada en la Evidencia Tecnológico de Monterrey, http://cmbe.net/?page_id=296) and the SPSS statistical package software, and p value <0.05 was taken as the significance level.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee. The informed consent cannot be obtained a posteriori due to the fact that this is a retrospective study about clinical records. To compensate this limitation, our public health system (Servicio Aragonés de Salud) establishes that researchers are allowed to use clinical records for scientific studies only by guaranteeing the anonymisation of information and under the authorization of the Primary Health Care General Manager of the Servicio Aragonés de Salud.
ResultsFrom the 252 scarlet fever clinical diagnoses, 171 episodes (158 patients) were microbiologically confirmed, of which 36 were by culture, 134 by RADT and one episode by RADT and culture. This is the sample to study; the 171 microbiologically verified episodes of pharyngotonsillar infection by GAS and rash (verified scarlet fever).
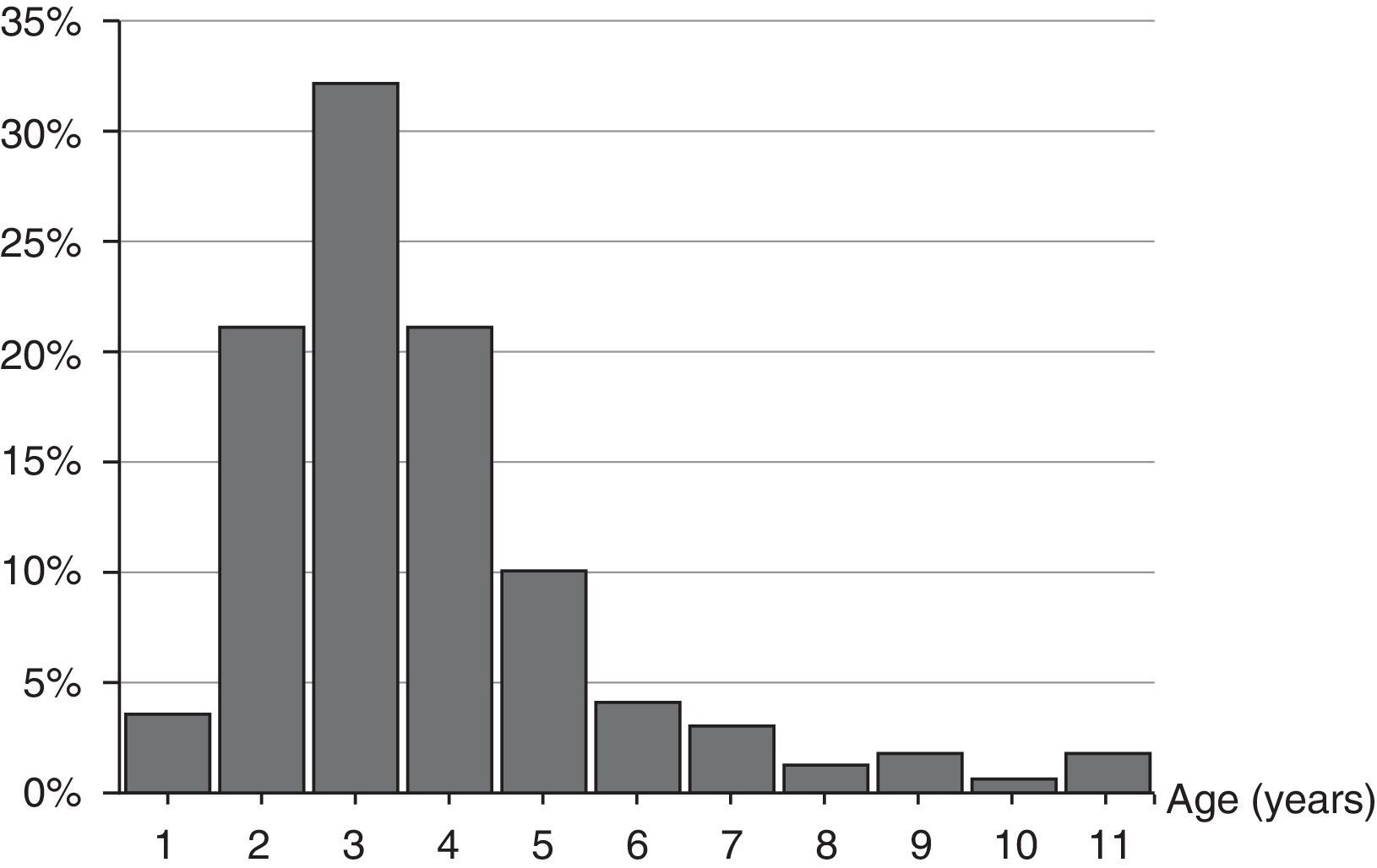
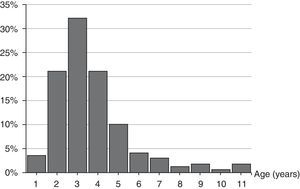
The age range was from 1 year and 8 months to 11 years and 7 months. Median age was 3.80 (interquartile range: 2.91–4.78) years, 84.2% of the episodes were from 2 to 5 years old, and 56.7% under the age of 4. The distribution by age is shown in Fig. 1.
There are no differences in the distribution by genders: 84 episodes in males (49.1%) and 87 in females (50.9%), whose median ages (3.80 [interquartile range: 2.90–4.71] and 3.80 [interquartile range: 2.95–4.89] years old, respectively) bore no difference.
From December to April there was a major concentration of cases, representing 70.2%. As for seasonal distribution, 70 out of 171 appeared in winter (40.9%), 32.2% in spring, 25.7% in autumn and 1.2% in summer. The month with the most cases was March (17%) and no cases were reported in August.
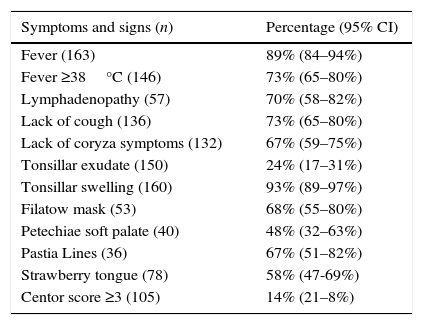
Data regarding symptomatology are summarized in Table 2. It is noteworthy to remark that in 27% of the episodes (95% CI: 20–35%) fever did not exceed 38°C and even there was no presence of fever in 11% (95% CI: 6–16%). Furthermore, although there was presence of tonsillar swelling in 93% of the episodes (95% CI: 89–97%), tonsillar exudate was just appreciated in 24% (95% CI: 17–31%).
Clinical findings on the total of 171 episodes.
| Symptoms and signs (n) | Percentage (95% CI) |
|---|---|
| Fever (163) | 89% (84–94%) |
| Fever ≥38°C (146) | 73% (65–80%) |
| Lymphadenopathy (57) | 70% (58–82%) |
| Lack of cough (136) | 73% (65–80%) |
| Lack of coryza symptoms (132) | 67% (59–75%) |
| Tonsillar exudate (150) | 24% (17–31%) |
| Tonsillar swelling (160) | 93% (89–97%) |
| Filatow mask (53) | 68% (55–80%) |
| Petechiae soft palate (40) | 48% (32–63%) |
| Pastia Lines (36) | 67% (51–82%) |
| Strawberry tongue (78) | 58% (47-69%) |
| Centor score ≥3 (105) | 14% (21–8%) |
Note: n=number of episodes in which the piece of data was registered.
It was possible to calculate the Centor score in 105 episodes (61%) with the information reflected on the clinical records, which was equal to or less than 2 in 86% of the patients (95% CI: 79–92%). McIsaac was equal to or less than 3 points in 65% (95% CI: 55–74%; n=98).
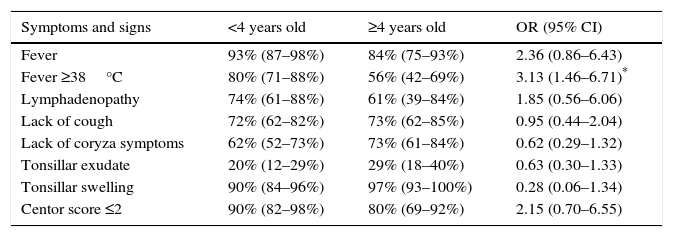
In Table 3, the presence of the different symptoms and clinical signs is analyzed according to whether patients were under or over 4 years old. There were no significant differences in the presence of the different symptoms analyzed, except for fever above 38°C which occurred more frequently in episodes of patients under the age of 4 (OR 3.13; 95% CI: 1.46–6.71; p=.0034).
Percentage and odds ratio (OR) with confidence intervals at 95% (CI 95), of different signs and symptoms among children under the age of 4 years old and equal or older than 4 (OR>1 means, in this case, that the event is more frequent in the group under 4 years old).
| Symptoms and signs | <4 years old | ≥4 years old | OR (95% CI) |
|---|---|---|---|
| Fever | 93% (87–98%) | 84% (75–93%) | 2.36 (0.86–6.43) |
| Fever ≥38°C | 80% (71–88%) | 56% (42–69%) | 3.13 (1.46–6.71)* |
| Lymphadenopathy | 74% (61–88%) | 61% (39–84%) | 1.85 (0.56–6.06) |
| Lack of cough | 72% (62–82%) | 73% (62–85%) | 0.95 (0.44–2.04) |
| Lack of coryza symptoms | 62% (52–73%) | 73% (61–84%) | 0.62 (0.29–1.32) |
| Tonsillar exudate | 20% (12–29%) | 29% (18–40%) | 0.63 (0.30–1.33) |
| Tonsillar swelling | 90% (84–96%) | 97% (93–100%) | 0.28 (0.06–1.34) |
| Centor score ≤2 | 90% (82–98%) | 80% (69–92%) | 2.15 (0.70–6.55) |
* Statistically significant difference (p=.0034).
All cases were treated with antibiotic therapy, most of them (71.4%) with amoxicillin, 25.2% penicillin V, 1.2% macrolides and 0.6% amoxicillin–clavulanate or cefuroxime axetil. The number of days under treatment was 10 days in 88.4%, 7 in 8.2%, 8 in 2.4% and 14 days in 0.4% (associated to perianal streptococcal infection).
There were nine patients with two scarlet fever episodes and two patients with three episodes (relapsed in 7% of our patients). There were no confirmed complications on clinical record, either suppurative or non-suppurative in any of the analyzed cases.
DiscussionThere are few national and international studies that give an accurate clinical description of the disease we are dealing with. In the main, it seems clear that in our environment, the most recent ones coincide in that the age at which scarlet fever appears is lower than that expected and in that it was previously described for GAS infections.3,4,21–23 Traditionally, the disease was described in a higher age-group (from 5 to 10 years old in the “Tratado de Pediatría” de M. Cruz2) and an in-force document can be consulted from the Center for Disease Control for the population in which it is stated that it is typical of ages between 5 and 12 years old.24 Regarding our series, 57% of the episodes are in patients under 4 years old (24% under the age of 3). In the series at the Hospital del Mar de Barcelona (118 verified scarlet fever cases between 2006 and 2008), 62% were under 4 years old and 31% under 3.3 In the study on patients that attended the emergency services at the Hospital Príncipe de Asturias in Alcalá de Henares in Madrid (165 verified scarlet fever between 1997 and 2000), 83% of the patients were under the age of 5 and 23% of them under the age of 3.4 With many few cases, other two outbreaks published in Spain21,23 showed similar findings. Regarding the outbreak in the United Kingdom between 2013 and 2014 (3752 cases including both children and adults), 87% were under the age of 10, and the median age were 4 years old.5 Another British publication states that, because of the early school enrolment and the major children's attendance to kindergarten, in the last two decades scarlet fever has moved most of its prevalence to preschool age.25 However, racial factors can be involved in the age of the scarlet fever. Two publications related to extensive outbreaks in Hong Kong and Shanghai do not coincide with what is being detected in Western countries. In the first one,6 the median age was 6 years old in the 2011 outbreak (including adults) whereas in the second one,26 including only children, 8.4% were under 4 years old and the group with the most prevalence was the one from 6 to 7 years old (38.4%).
Also, the clinical descriptions of the disease probably need to be updated in some treatise on paedriatics.1,2 The most recent studies seem to demonstrate that it is a milder disease and with few complications. In developed countries it appears afebrile in a percentage of cases, and as a not so typical PAS, as in most cases it does not show exudates.3-5,21–23,27 Also, in one out of every four cases it is accompanied by a coryza syndrome including cough,3,4,27 and even without swollen tender anterior cervical nodes.4,21,27 In this regard, 11% of our patients had no fever and an overall of 27% of the episodes did not reach 38°C fever which provides a point in the Centor score. Only 5% of the patients at the Hospital del Mar in Barcelona did not reach 38°C fever (the sickest children probably go to emergency services rather than to primary health care)3 while 15% of the 13 children in Sagunto had no fever.21 Over 900 cases in Hong Kong, including children and adults, 7% suffered no fever.6 In Oxford, among the 25 verified cases, 28% did not exceed 37.5°C.27
Remarkable fact is also the absence of tonsillar exudate in most of the episodes. Tonsillar swelling is very frequent (93% in our series) but exudate only appeared in one out of every four episodes of our patients (Centor's criteria just evaluate the presence of exudate, whereas McIsaac's ones evaluate one or another interchangeably). These data are very similar to those obtained at the Hospital del Mar with only 25% of children showing exudate.3
The most striking fact regarding pharyngotonsillitis scarlet fever is that if the recommendation to carry out the diagnosis would have been followed, considering only those who achieved three or four Centor criteria, 11,12,19,28 86% of our episodes would have missed PAS diagnosis (McIsaac equal to or less than 3 in 65% of the cases). The diagnostics key was, without doubt, papular erythematous rash, because it has been proved that they failed to meet certain Centor criteria such as pharyngotonsillitis (mainly due to the lack of exudate, temperature >38°C and the presence of lymphadenopathy). This has important implications in carrying out diagnostic tests in the light of the suspect disease, independently of a clinical study not so defined as the classical type, and despite the patient being under 4 years old.
Finally, and due to the fact that the age in the episodes is lower than that traditionally described, we compare patients under or over the age of four. There was only statistically significant major likelihood of fever occurring in the youngest patients.
As for the presence of scarlet fever relapse, they are somewhat more circumstantial than in other series3,4,21 and an event that happens in up to 7% of the patients is by no means a rarity.29 Despite rising incidence of the invasive GAS infections described,30 no complications were detected.
As limitations to our study, they are the ones underlying a study carried out on clinical records retrospectively (in this case computerized). In general, the majority of the main data were recorded but as no specific detailed record for the disease is available, regarding the particular symptoms, a bias exists in the gathering of data when the consultant completing the clinical record tends to reflect the finding that does exist, but with more likelihood to forget the one that does not exist. For this reason, in order to accurately assess the symptoms, especially those more related to the disease (Pastia lines, Filatow mask, strawberry tongue, petechiae soft palate), prospective studies would need to be carried out with specific registers for these data when a patient meets the diagnostic criteria of the disease, even with medical supervision after the diagnosis which allows us to assess the evolution of each of these parameters.
As main strengths of this study we believe that it groups an important number of verified cases, 171, and that due to the fact that it deals with patients that attend the first level of assistance, they show a complete spectrum of how the disease presents itself (including the milder symptoms that might elude the field of the emergency services). Therefore, it offers a description in quite a modern way with regard to epidemiology, clinical and development of the disease today in a developed country. Thus, it would appear advisable that, in the presence of any scarlet fever indicator (typical rash, strawberry tongue, Filatow mask or Pastia Lines) a diagnostic test is carried out at any age, even though pharyngitis does not meet PAS criteria.
FundingThe authors availed technical support for computerized medical records of patients, rendered by the health center belonging to the Aragon Health Service, which is authorized by the Director of Primary Care, for the realization of this research. With generous cooperation of all authors, the rest of the work did not require any funding at all.
Conflict of interestThe authors declare that there are no conflicts of interest.