Primary non-response and secondary loss of response to anti-TNF agents are common in inflammatory bowel disease. Increasing drug concentrations are correlated to better clinical response and remission rates. Combination of granulocyte–monocyte apheresis (GMA) with anti-tumor necrosis factor (TNF) agents could be an option in these patients. The objective of our study was to perform an in vitro assay to determine if the GMA device can lead to infliximab (IFX) adsorption.
Patients and methodsA blood sample was obtained from a healthy control. It was incubated with three concentrations of IFX (3, 6, and 9μg/ml) at room temperature for 10min. At that time, 1ml was collected to determine the IFX concentration. Then, 10ml of each drug concentration was incubated with 5ml of cellulose acetate (CA) beads from the GMA device at 200rpm for 1h at 37°C to simulate physiological human conditions. A second sample of each concentration was collected and IFX levels were determined.
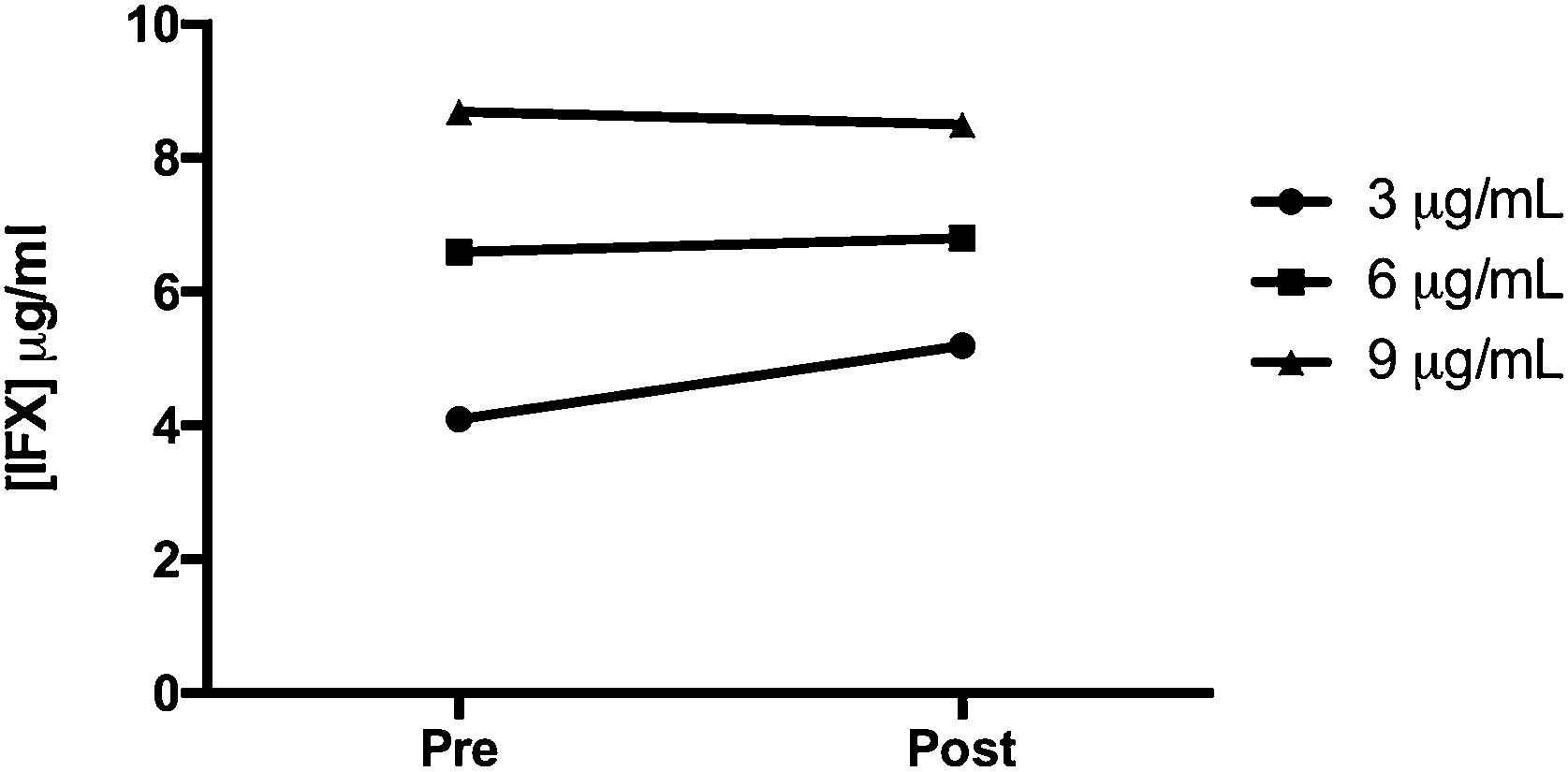
ResultsNo statistically significant differences were observed in the IFX levels in the blood samples before and after incubation with the CA beads (p=0.41) and after repeated measurements (p=0.31). Mean change was 3.8μg/ml.
ConclusionsThe in vitro combination of GMA and IFX did not change the circulating levels of IFX at the three concentrations tested, suggesting that there is no interaction between the drug and the apheresis device in vitro and that they might be safely combined with each other.
La falta de respuesta primaria y la pérdida de respuesta secundaria a los agentes antifactor de necrosis tumoral (TNF) son comunes en la enfermedad inflamatoria intestinal. El aumento de los niveles de fármaco se correlaciona con una mejor respuesta clínica y de las tasas de remisión. La combinación de la aféresis selectiva de granulocitos y monocitos (GMA) con agentes anti-TNF podría ser una opción en estos pacientes. El objetivo de nuestro estudio fue realizar un ensayo in vitro para determinar si el dispositivo de GMA puede interaccionar con infliximab (IFX).
Pacientes y métodosSe obtuvo una muestra de sangre de un control sano. Se incubó con 3 concentraciones de IFX (3, 6 y 9μg/ml) a temperatura ambiente durante 10 minutos. En ese momento, se recogió 1ml para determinar la concentración de IFX. Luego, se incubaron 10ml de cada concentración de fármaco con 5ml de cuentas de acetato de celulosa del dispositivo GMA a 200rpm durante una hora a 37°C para simular las condiciones fisiológicas humanas. Se recogió una segunda muestra de cada concentración y se determinaron los niveles de IFX.
ResultadosNo se observaron diferencias estadísticamente significativas en los niveles de IFX en las muestras de sangre antes y después de la incubación con las cuentas de acetato de celulosa (p=0,41) ni tras mediciones repetidas (p=0,31). La media de cambio fue de 3,8μg/ml.
ConclusionesLa combinación in vitro de IFX y GMA no modificó los niveles circulantes del fármaco en las 3 concentraciones probadas, lo que indica que no existe interacción entre el fármaco y el dispositivo de aféresis in vitro y que podrían combinarse de forma segura.
Anti-TNF monoclonal antibodies as infliximab (IFX) are effective treatments for patients with inflammatory bowel disease (IBD) refractory to conventional therapies. Successful treatment leads to mucosal healing, less hospitalisations and surgeries, and improvement in quality of life.1–3 Nevertheless, primary non-response to anti-TNF treatment is common4,5; 24–46% of patients can develop secondary loss of response (LOR) during the first year,6 and approximately 10% show intolerance.7
In the largest prospective study of anti-TNF therapy in IBD to date, Kennedy et al. showed that the main modifiable factors associated to a reduced treatment effectiveness were low drug concentrations and immunogenicity.8 It has been reported that higher serum drug concentrations are associated with better therapeutic outcomes regarding both maintenance and induction therapy, when the inflammatory burden and drug clearance are high.9–13
Granulocyte–monocyte apheresis (GMA) can selectively deplete monocytes/macrophages by adsorption and has been associated with significant clinical efficacy in patients with IBD.14,15 The GMA device consists of a column of cellulose acetate (CA) beads immersed in physiological saline solution.16 Its mechanism of action is based on the interaction between cellular and humoral blood components and CA beads. Blood passes through the device and is further reinfused to the patient. The recommended infusion rate is 30ml/min and the duration of the sessions is 60min,17,18 though a duration of 90min is also common.19,20
The mechanism of action of GMA appears to be more than adsorption of excess neutrophils and TNF-producing CD14+ CD16+ monocytes. Adsorbed monocytes/macrophages release interleukin (IL)-1 receptor antagonist, hepatocyte growth factor and soluble TNF receptors, which are anti-inflammatory.21 Additionally, a sustained increase in lymphocytes including the regulatory CD4+ CD25+ T cells is observed after GMA.17
The objective of our study was to perform an in vitro assay to determine if the GMA device can lead to adsorption of anti-TNF agents as IFX when used in combination, thus preserving the therapeutic effects of the drug.
Patients and methodsStudy designBlood sample was obtained from a healthy control (43-year-old woman; non-smoker; total leukocyte count 6.35×109/L) at Hospital Universitario de Galdakao, Spain. Sample contained 450ml of unprocessed full blood. The donor signed an informed consent before any study procedure.
The IFX biosimilar used (Inflectra®, Pfizer, Brussels) is a chimeric IgG1 monoclonal antibody that binds with high affinity to both the soluble and transmembrane forms of TNF.23 Blood sample (30ml) was incubated with three concentrations of IFX (3, 6, and 9μg/ml) at room temperature for 10min. We decided to use progressive dilutions that would be representative of three clinical scenarios of through levels in clinical practice. At that time, 1ml was collected to determine the IFX concentration (pre-incubation sample). Then, 10ml of each drug concentration was incubated with 5ml of CA beads from the GMA device (Adacolumn®, JIMRO, Takasaki, Japan) at 200rpm for 1h at 37°C to simulate physiological human conditions. After incubation, the second sample of each concentration was collected (post-incubation sample). Samples were stored at −80°C. IFX levels were determined using the Promonitor® kit (Progenika Biopharma, Derio, Vizcaya). Two additional separate measurements and with the same technique and under the same conditions were performed. Results obtained from the blood sample were compared using a Wilcoxon test for continuous variables and by one-way ANOVA.
ResultsIFX levels pre- and post-incubation are presented in Fig. 1. No statistically significant differences were observed in the IFX levels determined in the blood samples before incubation and after contacting the CA beads (p=0.41). Mean change was 3.8μg/ml. Mean infliximab concentrations at baseline were 10.39, 7.8 and 5.20μg/ml, at each of the prespecified target levels. In the second measurement, values were 7.75, 7.21 and 5.95μg/ml, respectively. Repeated measurements showed no statistically significant differences between both time points (p=0.31; Supplementary Fig. 1).
DiscussionOur study shows that the in vitro combination of GMA and IFX does not change the circulating levels of IFX at the three concentrations tested, suggesting that there is no interaction between the drug and the apheresis device.
Anti-TNF agents are effective for the management of IBD but primary non-response and secondary loss of response are common. Increasing drug concentrations are correlated to better clinical response and remission rates, higher endoscopic healing rates and lower C-reactive protein and fecal calprotectin levels. This correlation between exposure and response has been demonstrated across several anti-TNF agents approved for the treatment of ulcerative colitis (UC).9–13
Combination of GMA with anti-TNF agents could be an option in patients who do not respond adequately or lose response to biologics.22–25 In a case report of a female with Crohn's disease who lost response while being on maintenance with IFX, the patient remained in stable clinical and endoscopic remission after 5 GMA courses without experiencing any serious side effects.23 In 2017, we described our experience with this combined therapy in UC patients after loss of response to anti-TNF treatment. GMA was indicated in 4 patients with left-sided or extensive colitis because of partial response to biological therapy or secondary LOR to it. A decrease in the Mayo score was observed. The overall response rate was 50% with one patient demonstrating sustained response.24 Later, we described a retrospective study on 42 UC patients (23 receiving IFX), where GMA was combined after a primary non-response or secondary LOR to anti-TNF therapy. Fifteen patients (32%) responded to the combination therapy without anti-TNF intensification, switch, swap or colectomy.25 Furthermore, GMA seems to be well tolerated by the patients, with acceptance rates as high as 82% regardless of the response to the treatment.26
GMA has been shown to have clinical efficacy together with immunomodulatory effects in IBD patients. Hiraishi et al. investigated in 2003 the mechanisms underlying the adhesion of granulocyte and monocyte to CA beads following exposure of human blood to the carriers at 37°C for up to 60min under controlled conditions. Beads selectively adsorbed granulocytes, monocytes, CD19+ (B cells) and CD56+ (NK cells). The granulocyte and monocyte adsorption was inhibited by heat-inactivated plasma and EDTA, suggesting that the adsorption was plasma protein and calcium dependent. The results showed that IgG and active complement fragments mediated leukocyte adhesion to CA beads via the FcμR and/or leukocyte complement receptors like CR3. Additionally, CA beads induced loss of expression of TNF receptors on CD16+ granulocytes and CD14+ monocytes, but not on CD3+ lymphocytes.27
The aim of the combination of GMA and anti-TNF agents is to obtain a synergistic effect of both mechanisms of action to block the migration of leukocytes to inflamed tissue. However, both can contribute to the increase in some peripheral lymphocyte subpopulations (especially Treg) observed in the first weeks of treatment,28 and the changes induced by GMA in the profile of cytokines expressed in the colonic mucosa can increase the effect of anti-TNF drugs.25
Potential interaction between both treatments has also been postulated, either by increasing the blood trough levels or by reducing anti-drug antibodies,29,30 although there is no direct evidence. Yokoyama et al. first reported an improvement in anti-TNF biologic drug levels with this combination.29 As described, the presence of antibodies-to-IFX (ATI) in IBD patients is responsible for LOR and infusion reactions (IR) to IFX. This group measured ATI in patients receiving IFX (56 with sustained response, 76 with LOR and 6 with IR). Fourteen patients with LOR (6 with Crohn's disease and 7 with UC), showed significantly improved clinical activity, and decreased ATI and IL-6 at week 8 following initiation of GMA plus IFX. Nine patients achieved remission, which was maintained at week 24 with IFX alone. Pre- and post-IFX infusion ATI levels were similar. Patients with ATI>0.153μg/ml (cut-off value) were likely to experience LOR. The authors concluded that patients who received GMA in addition to IFX appeared to regain clinical response to IFX by a decrease in ATI level, and the concentration of IFX was associated with clinical response.30
Our study has also some limitations that should be considered. The use of blood obtained from a single donor could be a major concern. Also, samples from individuals without IBD may not be representative of the actual immunological and pro-inflammatory environment expected in patients with active disease. Another important methodological aspect could be the absence of information on TNF concentrations in these samples. Despite these relevant considerations, it should be noted that our aim was to extrapolate how IFX and GMA may interact in vivo from data obtained at ideal laboratory conditions. However, exploring this aspect in patients with IBD is advisable in order to provide even more appropriate data to our clinical practice.
The data reported in our study show that the in vitro combination of GMA with IFX does not modify the circulating levels of the biologic at the three concentrations tested, suggesting that there is no interaction between the drug and the apheresis device and that they might be safely combined and without risk of interaction with each other. It could be hypothesized that, by recovering clinical response without modifying the drug levels, the combination with GMA might reduce the inflammatory burden thus restoring anti-TNF efficacy.
Authors’ contributionsIR-L, LA and JLC: study design, data analysis and drafting the manuscript.
LA, IS and JA: performed the in vitro analysis.
JA and JLC: revised the manuscript for important intellectual content.
Ethical considerationsThis study was conducted under the Declaration of Helsinki. The study protocol was approved by the local Ethics Committee (reference 24/18).
Data availability statementThe data that support the findings of this study are available from the corresponding author upon reasonable request.
FundingThis work was supported by the Research Committee from Hospital Universitario de Galdakao. IR-L is supported by a research grant from Gobierno Vasco – Eusko Jaurlaritza (grant number 2020111061) and Biobizkaia (BCB/I/LIB/22/008). The apheresis column and Promonitor kit were kindly provided by Adacyte and Progenika Biopharma, neither of them were involved on study design nor interpretation of the results.
Conflicts of interestIR-L has received financial support for traveling and educational activities from or has served as an advisory board member for Abbvie, Adacyte, Celltrion, Chiesi, Danone, Ferring, Faes Farma, Janssen, Galapagos, MSD, Pfizer, Roche, Takeda, and Tillotts Pharma. Financial support for research: Tillotts Pharma.
JLC has received financial support for traveling and educational activities from or has served as an advisory board member for Abbvie, Adacyte, Chiesi, Ferring, Janssen, MSD, Pfizer, Takeda, and Tillotts Pharma.
The remaining authors have no conflicts of interest to declare.
We thank Miguel Ángel Pascual-Itoiz for his technical support.