The main objective of this study is to analyze the efficacy of combined axillary marking (lymph node clipping and sentinel lymph node biopsy (SLNB)) for axillary staging in patients with primary systemic treatment (PST) and pathologically confirmed node-positive breast cancer at diagnosis. The secondary objective is to determine the impact of lymph node marking in the suppression of axillary lymph node dissection (ALND) in the study group.
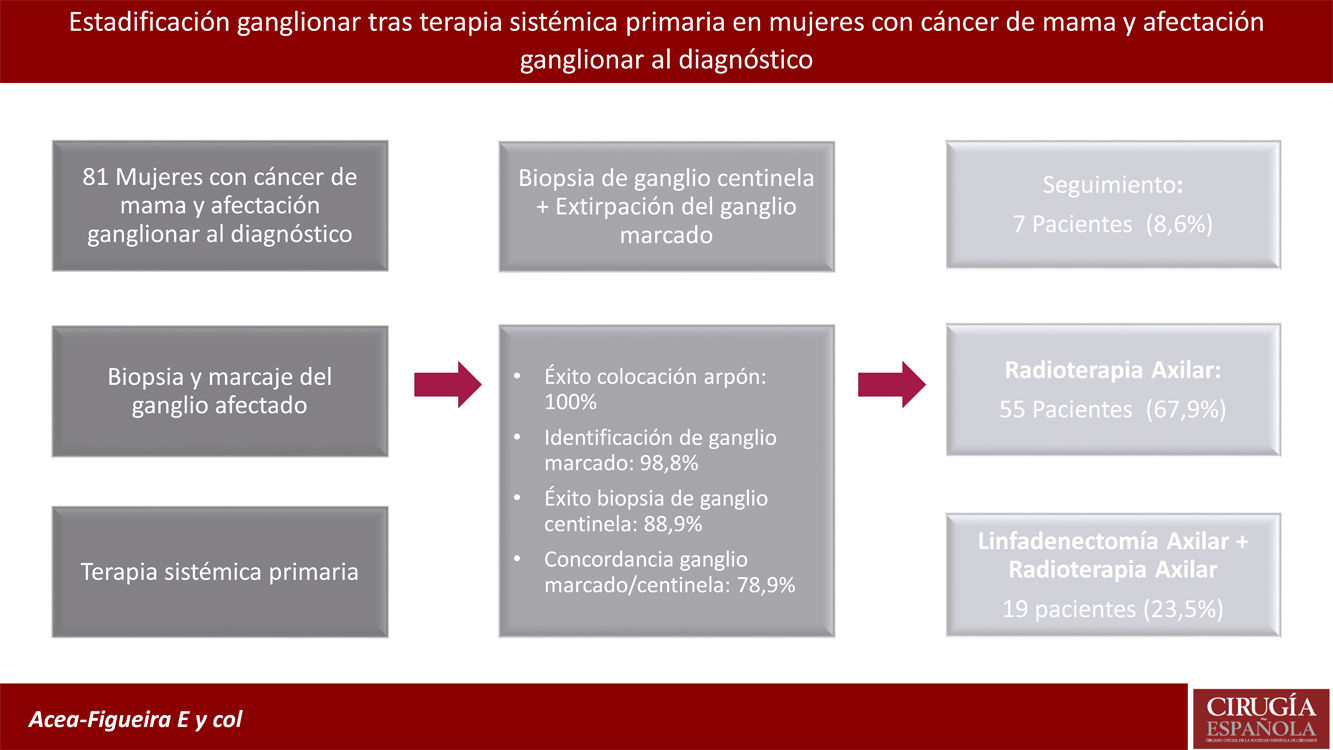
MethodsWe conducted a prospective study in which lymph node staging was performed using wire localization of positive lymph nodes and a SLNB with dual tracer. All patients who presented no metastatic involvement of the sentinel lymph node (SLN) or clip/wire-marked lymph node were spared an ALND. The multidisciplinary committee agreed on axillary treatment for patients with lymph node involvement. Results: Eighty one patients met the inclusion criteria. We identified and extirpated the clip/ wire-marked node in 80 of 81 patients (98.8%), with SLNB performed successfully in 88,9% of patients. The SLN and wire-marked node matched in 78.9% of patients; 76.2% of patients did not undergo ALND.
ConclusionsThe combined axillary marking (clip and SLNB) in patients with metastatic lymph node at diagnosis and PST offers a high identification rate (98.8%) and a high correlation between the wire-marked lymph node and the SLN (78.9%). This procedure has enabled the suppression of ALND in 76.2% of patients.
El objetivo principal de este estudio es analizar la eficacia del marcaje ganglionar combinado (clip y biopsia de ganglio centinela (BGC)) para la estadificación axilar en pacientes con tratamiento sistémico primario (TSP) y cáncer de mama con ganglios positivos confirmados patológicamente en el momento del diagnóstico. El objetivo secundario es determinar el impacto del marcaje ganglionar en la supresión de la linfadenectomía axilar (LA) en el grupo a estudio.
MétodosEstudio prospectivo en el que se realizó la estadificación ganglionar mediante la localización con alambre metálico (arpón) de los ganglios afectados y una BGC con doble trazador. Todas las pacientes sin afectación metastásica del ganglio centinela (GC) o del ganglio marcado con clip/alambre no realizaron una LA. El comité multidisciplinar acordó el tratamiento axilar de las pacientes con afectación ganglionar.
ResultadosOchenta y un pacientes cumplieron los criterios de inclusión. Identificamos y extirpamos el ganglio marcado con clip/alambre en 80 de 81 pacientes (98,8%), y la BGC se realizó con éxito en el 88,9% de los pacientes. El GC y el nódulo marcado con arpón coincidieron en el 78,9% de las pacientes. El 76,2% de las pacientes no se sometieron a LA.
Conclusionesel marcaje axilar combinado (clip y BGC) en pacientes con ganglios metastásicos al diagnóstico y TSP ofrece una alta tasa de identificación (98,8%) y una alta correlación entre el ganglio marcado con arpón y el GC (78,8%). Este procedimiento ha permitido la supresión de la LA en el 76,2% de las pacientes incluidas en el estudio.
Sentinel node biopsy (SNB) is the procedure of choice for axillary staging in patients with breast cancer and primary surgery, as several studies have shown that overall survival (OS) and disease-free survival (DFS) is similar in patients undergoing SNB and cambiar axillary lymphadenectomy por axillary lymph node dissection (ALND) when the sentinel node (SN) is negative.1,2 The ACOSOG-Z00113 and AMAROS4 studies later demonstrated that omitting AL in patients with SN involvement offered similar survival to AL using whole breast and/or axillary irradiation. In contrast, after primary systemic treatment (PST), AL has been the technique of choice in patients with lymph node involvement at diagnosis or lymph node metastasis after PST.
There is currently controversy regarding the ideal axillary staging technique after PST in women with lymph node involvement at diagnosis. Several groups5,6 propose assessing nodal response after PST to avoid AL in patients with pathological complete response (pCR). In recent years, a new staging technique called targeted axillary dissection (TAD)7 has been proposed to reduce the indication for AL in this group of patients.
The primary objective of this study is to analyse the efficacy of combined lymph node marking (clip and SNB) for axillary staging in patients with PST and breast cancer with metastatic lymph node involvement at diagnosis. The secondary objective is to determine the impact of lymph node marking on avoiding AL in the study group.
MethodologyA prospective non-randomised study from our centre’s breast unit between May 2016 and January 2022. The study included women over 18 years of age with breast cancer (all histological types and tumour subtypes, except sarcomas) and axillary node involvement at diagnosis (N1) who received PST on the recommendation of the multidisciplinary committee, based on international clinical guidelines.8–10 Patients with distant metastases at diagnosis (M1), clinical or radiological progression of disease during PST, four or more nodes suspicious for malignancy at diagnosis, and patients who chose not to participate in the study were excluded (Table 1). The study was approved by the Autonomous Research Ethics Committee (Promoter Code: SENTINA 00-14; Registration Code: 2014/140).
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
An axillary ultrasound was performed for lymph node staging prior to PST. Each lymph node was classified based on morphological features of the cortical and hilum lymph nodes using the Bedi et al.11 classification and nodes with a Bedi category 4–6 were biopsied and clip marked. In patients with more than 1 suspicious node, the node with the highest suspicion of metastatic involvement was biopsied and marked. Once the PST had been completed, the clip-marked lymph node was marked with a metal guide (harpoon) during diagnosis. Likewise, the SN was marked by isotope mapping with technetium-99 in the periareolar region. If no radioisotope uptake was evident, the SN mapping was complemented with patent blue. All patients underwent a breast MRI prior to starting PST and another when PST had finished to determine the degree of response in the breast and axilla.
Primary systemic therapyAll the patients included in the study underwent PST according to the clinical guidelines for each period.9,10 All the patients were treated with four cycles of adriamycin and cyclophosphamide, and 12 cycles of paclitaxel. The medical oncologist decided the sequence of these therapies. All the women who were HER2 receptor positive were treated with monoclonal antibodies (trastuzumab with or without pertuzumab).
Surgical methodAll the patients included in the study underwent surgery after completing the selected neoadjuvant chemotherapy regimen. On the day of surgery, the clip-marked lymph node was removed. Once this node was removed, it was checked for isotopic load and/or patent blue staining. The presence of the clip in the marked lymph node was confirmed by X-ray of the sample. Finally, a complementary axillary examination was conducted to check for the presence of other radioisotope/dye-marked SN. We defined as an SN those with an isotope count ten times higher than baseline and/or those stained with patent blue or with a blue lymphatic duct. Non-sentinel nodes were defined as those suspicious on palpation, those that did not show blue dye, nodes without radiotracer activity. The surgery report specified whether the SN coincided with the clip-marked node.
In the patients who underwent mastectomy, an intraoperative study of the removed nodes was performed using the OSNA method. In case of macrometastatic lymph node involvement, AL was performed in the same surgical procedure. A delayed lymph node study was performed in the patients who underwent breast-conserving surgery. If this study showed metastatic lymph node involvement, the case was discussed in the multidisciplinary breast committee to assess the indication for AL. The multidisciplinary committee is made up of medical oncologists, radiation oncologists, breast surgeons, breast radiologists, and pathologists. The individual characteristics of each patient were assessed (number of affected nodes, tumour biology, response to PST in the breast, associated morbidities, etc.). The committee agreed to omit AL in women with any favourable characteristics such as complete pathological response in the breast, removal of more than one node and one without disease, and/or micrometastasis of the node. In patients undergoing AL, Berg levels I and II were removed, and only level III was removed in the patients with macroscopic involvement of this level. Radiotherapy of lymph node chains was indicated in cases where the committee recommended omitting AL.
Micrometastases were defined as tumour inclusions between 0.2 and 2 mm, equivalent in the OSNA method to a copy number between 250 and 5000. Macrometastases are metastases >2 mm or more than 5000 copies by OSNA. Finally, isolated tumour cells are metastases smaller than 0.2 mm and correspond to <250 copies by OSNA assay.
Indication and planning of radiotherapy treatmentAll the breast-conserving surgery patients included in the study received breast radiotherapy between the fourth and sixth week after surgery. Breast irradiation was performed using tangential fields at a dose of 50 Gy in 25 fractions of 2 Gy, 5 times per week. In case with over-impression of the tumour bed, additional doses of 8−10 Gy were given, spread over 4 or 5 sessions. In all the women undergoing mastectomy, chest wall radiotherapy was indicated at doses of 45−50 Gy in 25 fractions of 1.8−2 Gy/day to the chest wall. Patients with an indication for radiotherapy of lymph node chains received doses of 50 Gy in 25 sessions, with a depth of 3 cm.
Statistical methodWe used IBP SPSS Statistics version 22 (IBM Corporation, Armonk, NY, USA) for data collection and analysis. Quantitative variables are listed with their mean and standard deviation and qualitative variables are listed with their proportions. Differences between qualitative variables will be found by Fisher's exact test or χ2 test. Kaplan-Meier curves were used to perform the OS study.
ResultsThe study group consisted of 81 patients with infiltrating breast carcinoma and metastatic involvement of the axilla at diagnosis treated with PST. The most frequent tumour subtype was luminal B Her2 negative (32.1%) followed by luminal B Her2 positive (27.2%).
Series characteristics. The mean initial tumour size was 3.6 cm (±1.5) and the mean final tumour size 0.7 cm (±1.1) (Table 2). The breast was conserved in 76.6% of the patients (lumpectomy 66.7% and oncoplasty 9.9%) (Table 3). Of the patients with a pCR in the breast, 85.7% also had a pCR in the axilla (ypN0). In contrast, only 34% of patients with a partial response in the breast were negative for lymph node disease. Axillary response was statistically superior in tumours with Her2 overexpression and response in the breast. Thus, 91.7% of pure Her2 tumours and 90.9% of Her2-positive luminal B tumours with pCR in the breast, were negative in the axilla (p = 0.001). Two patients with Her2-negative luminal B tumours that did not respond to PST had residual axillary disease.
Clinical and pathological characteristics of the patients.
| Patients n = 81 | |
|---|---|
| Age (years) | |
| Mean | 53.3 (±11.5) |
| Range | 26−81 |
| Tumour subtype | |
| Luminal A | 2 (2.5%) |
| Luminal B Her2- | 26 (32.1%) |
| Luminal B Her2+ | 22 (27.2%) |
| Her2+ | 13 (16.0%) |
| Triple negative | 18 (22.2%) |
| SN marking | |
| Technetium | 41 (50.6%) |
| Patent blue | 5 (6.2%) |
| Blue + Technetium | 35 (43.2%) |
| Initial tumour size (cm) | |
| Mean | 3.6 (±1.5) |
| Range | 1.2–8.4 |
| Initial tumour size (cTNM) | |
| T1c | 9 (11.1%) |
| T2 | 57 (70.4%) |
| T3 | 15 (18.5%) |
| Final tumour size (cm) | |
| Mean | 0.7 (±1.1) |
| Range | 0–6 |
| Surgical results | Patients n = 81 |
|---|---|
| Type of breast surgery | |
| Lumpectomy | 54 (66.7%) |
| Oncoplasty | 8 (9.9%) |
| Mastectomy | 19 (23.5%) |
| pCR in breast | 35 (43.2%) |
| pCR in breast according to tumour subtype | |
| Luminal A | 0/2 (0%) |
| Luminal B Her2− | 5/26 (19.2%) |
| Luminal B Her2+ | 11/22 (50%) |
| Her2+ | 12/18 (66.7%) |
| Triple negative | 7/13 (53.8%) |
| Excised SN | 2,6 (±1.5) |
| Patients with axillary involvement after chemotherapy | 36 (44.4%) |
| Axillary lymph node dissection | 19 (23.5%) |
| SN excised in the lymphadenectomy | 12.6 (±5.9) |
| 3−27 | |
| Type of axillary involvement | |
| ypN0 | 45 (55.6%) |
| ypN1mi | 14 (17.3%) |
| ypN1 | 22 (27.2%) |
| pCR in the marked SN/lymph node | |
| Luminal A | 0/2 (0.0%) |
| Luminal B Her2− | 6/26 (23.1%) |
| Luminal B Her2+ | 15/22 (68.2%) |
| Her2+ | 15/18 (83.3%) |
| Triple negative | 9/18 (69.2%) |
| Axillary response according to breast response (complete or partial) | |
| Luminal A | 0/0 (0.0%) |
| Luminal B Her2− | 3/5 (60.0%) |
| Luminal B Her2+ | 10/11 (90.9%) |
| Her2+ | 11/12 (91.7%) |
| Triple negative | 6/7 (85.7%) |
SN, sentinel node; pCR, partial complete response.
Identification and concordance of axillary marking.
| Patients n = 81 | |
|---|---|
| Identification of clip and wire placement | 81 (100%) |
| Excision of wire-marked lymph node | 80 (98.8%) |
| Sentinel gland biopsy | 72 (88.9%) |
| Concordance between clip-marked node and sentinel node | 56/71 (78.9%) |
| Ability of the marked lymph node to detect axillary involvement | 32/36 (88.9% |
Axillary treatment. Of the patients included in the study, 85.2% (69) had metastatic involvement of only 1 node; 4 women (4.9%) had 2 nodes suspicious for metastatic involvement, and 8 patients (9.9%) had 3 nodes. The axillary marking clip was detected by ultrasound in all the patients in the study group, which allowed wire placement in all patients (Table 4). The clip/wire-marked lymph node was removed in 80 of 81 patients (98.8%), while isotope/dye-marking identified the SN in 72 patients (88.9%). This SN was the same as the clip-marked node in 56 patients (78.9%).
Axillary treatment according to lymph node involvement.
| Without axillary treatment | Axillary lymph node dissection | Axillary lymph node dissection | Axillary lymph node dissection + radiotherapy | |
|---|---|---|---|---|
| ypN0 | 7 (8.6%) | 0 (0%) | 37 (45.7%) | 1 (1.2%) |
| ypN1mi | 0 (0%) | 0 (0%) | 12 (14.8%) | 2 (2.5%) |
| ypN1 | 0 (0%) | 0 (0%) | 6 (7.4%) | 16 (19.8%) |
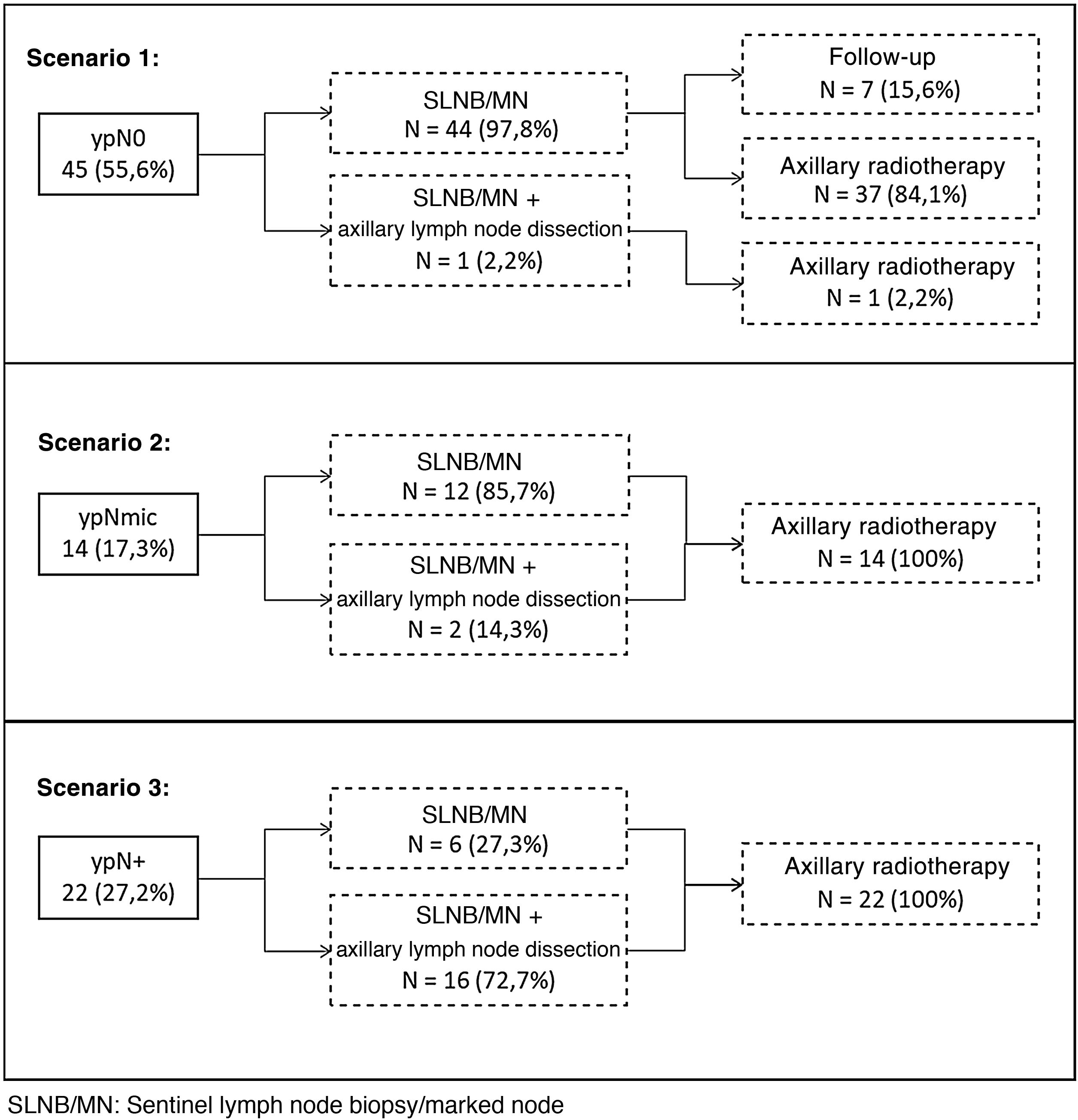
A total of 45 patients (55.6%) had a complete response in the axilla after PST. With these pathological findings the breast committee recommended AL in 18 patients (23.5%) (Fig. 1):
- -
ypN0. An AL was not performed in 97.8% of the patients with axillary response (44 out of 45 women). One of the 45 patients without involvement of the marked lymph node underwent axillary AL, as no SN was detected, and the axilla appeared macroscopically affected. Seven of these patients in whom AL was avoided (8.6%) did not receive axillary radiotherapy either (Fig. 1).
- -
ypN1. An AL was not performed in 50% of the patients with persistent metastatic lymph node involvement. Of these patients, 66.7% (12 of 18) had micrometastatic lymph node involvement and all received axillary radiotherapy. Five of the 6 patients with macrometastases (83.3%), without axillary AL, had other sentinel nodes without disease.
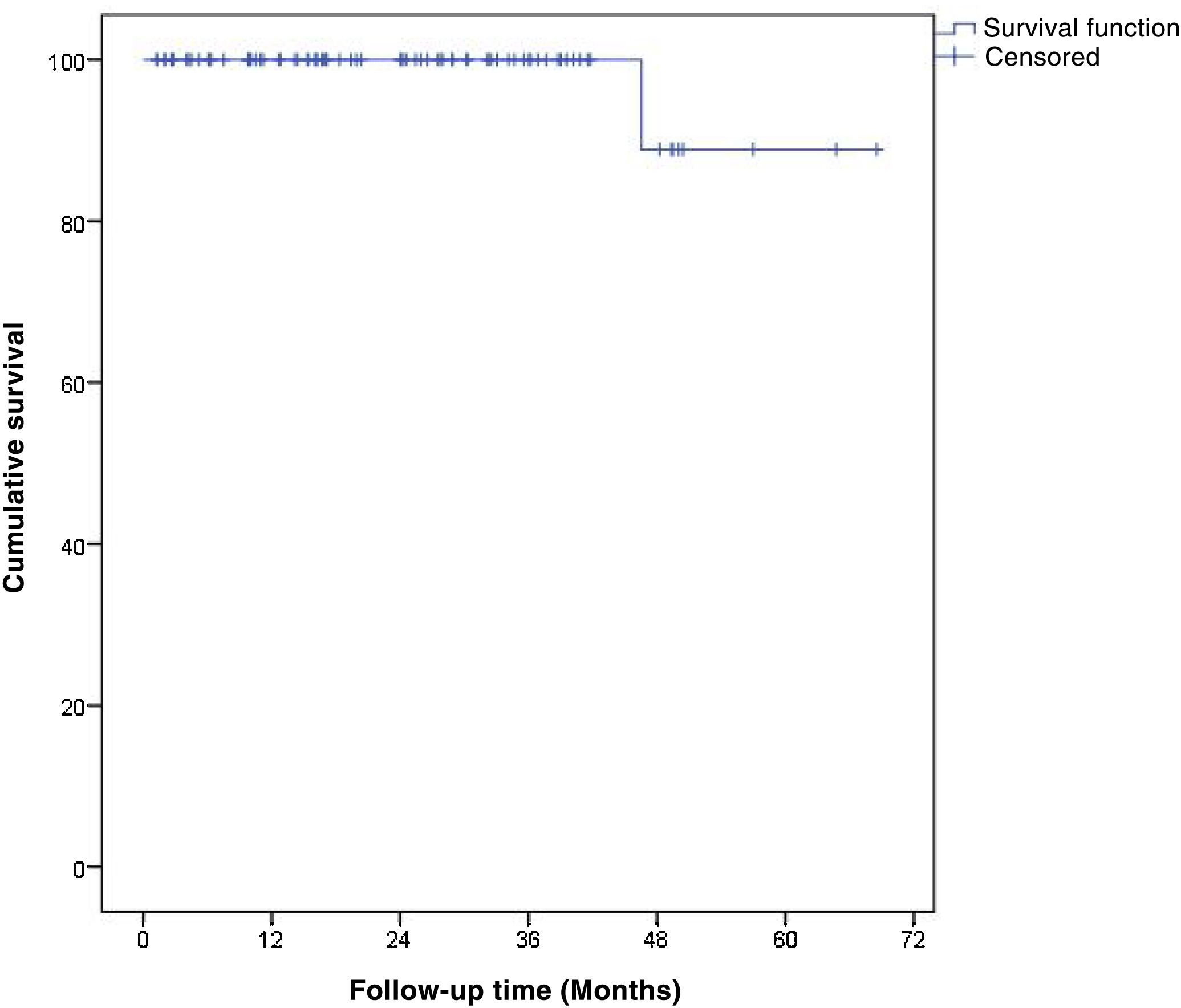
Oncological events. The mean follow-up time was 24.2 ± 16 months (range: 1.3–68.5) and no axillary relapses were detected during follow-up. One patient who underwent AL and lymph node radiotherapy died 47 months after surgery due to metastatic progression of her breast cancer, resulting in an OS at 48 months of 88.9% (95%CI 78.4%–99.4%) (Fig. 2).
DiscussionAxillary surgery in women with breast cancer has been de-escalated in recent years. However, axillary surgery after PST has not been shown to be similarly less intensively performed, due to the absence of prospective studies evaluating these women. This study analyses the efficacy of clip marking the affected lymph node after PST. This efficacy depends on the method used for marking and therefore several studies have evaluated these methods. Donker et al.12 marked the axillary node with radioactive iodine (125I) seeds (MARI procedure) with an identification rate of 97% and an FN rate of 7%. Caudle et al.7 used the TAD method to evaluate the response of metastatic axillary nodes to PST. The identification rate achieved by this group was 95%, with an FN rate of 2%. In contrast, when only the marked node or the SN was removed, the FN rate was 4.2% and 10.1%, respectively. Boughey et al.13 demonstrated in the ACOSOG Z1071 study that placing a clip on the affected node during diagnosis of the disease combined with SNB is an appropriate method to decrease the FN rate. This rate decreased from 19.9% to 6.8% in patients with SNB and selective marking of the biopsied node, respectively. The retrospective study by Simons et al.14 used radioactive iodine seeds to identify the biopsied lymph node after PST combined with SNB, obtaining an identification rate of 92.8% and an FN rate of 2%–4% combining both procedures. Hellingman et al.15 later proposed a modification of the MARI procedure by injecting technetium-99m-labelled macroaggregated albumin into the clip-marked nodes and then removing it during surgery, achieving an identification rate of 87%. Recently the study by Martínez et al.16 evaluated the safety and efficacy of magnetic seeds as a method of axillary staging, obtaining an identification rate of 100% and a reduction in FN rate from 21.4% to 5.9% when combined with SNB and removal of the marked node. The study by Siso et al.17 used ultrasound to identify the clip-marked lymph node with an identification rate of 95.4% and an FN rate of 4.1%. Gurleyik et al.18 achieved an identification rate of 98.4% using ultrasound-guided lymph node localisation after PST. The studies by Park et al.19 and Patel et al.20 analysed the efficacy of lymph node tattooing with charcoal and black ink respectively, obtaining an FN rate of 20.0% and 13.3%. In our study, the clip was detected in 100% of patients. Wire marking identified the marked node in 80 out of 81 patients (98.8%) and isotope/dye marking identified the SN in 72 patients (88.9%). Other authors have employed a similar methodology to ours using a guidewire. Plecha et al.21 and Hartmann et al. 22 obtained mixed results. However, the study by Plecha et al.21 achieved a 97.3% success rate for the procedure, affirming that the wire-guided localisation of metastatic axillary nodes is an accurate technique and that it improves FN and surgical removal rates. In contrast, the study by Hartmann et al.22 obtained an identification rate of 70.8%, stating that this technique is not suitable for routine clinical practice. These discrepancies could be due to differences in the experience of the surgeons/radiologists participating in the study. In our opinion, this staging procedure is an efficient and easily reproducible method, as it uses the same methodology as that used for non-palpable breast lesions, thus making it a method that can be extrapolated to most breast units. In addition, it does not require investment and does not entail the radiological safety risks associated with other techniques.
The second objective of the study was to determine the decrease in AL according to the type of pathological response after PST in the biopsied lymph node. In our experience, the tumour profiles with the highest incidence of pCR were Her2-positive and triple-negative tumours, with responses of 91.7% and 85.7%, respectively. Comparable results were obtained in the studies of Samiei et al.23 and Gurleyik et al.,18 and in all the studies the rates of breast-conserving surgery were significantly higher in Her2-positive and triple-negative tumours. These results raise the possibility of de-escalating axillary surgery in patients with a marked lymph node pCR in a manner similar to planning in the breast after a pCR. Two factors would support this approach. The first relates to the use of breast and axillary radiotherapy in this group of women with nodal involvement at diagnosis, which guarantees oncological safety after breast and axillary node preservation. Secondly, studies such as that of Fayanju et al.24 show that the group of patients with lymph node pCR have a comparable prognosis to patients with clinically negative nodes at diagnosis.
In our study a total of 56 patients (78.9%) had concordance between the two markers, an incidence similar to that found in other studies (75.9%–77%).7,13,17 This high concordance between the marked lymph node and the SN suggests that they are the same node, which would be in line with the definition of the SN as the node most likely to metastasise.25 In these patients the disease itself will have marked their SN, which could suggest omitting SNB in these patients. In contrast, other studies have shown lower concordance rates, with values ranging from 35.7% to 65%.16,22,26 Also in this study, 21.1% of patients had no concordance between the marked node and the SN. These discrepancies may be due to lymphatic blockage and less likely, to an error in marking the biopsied node. Therefore, we believe that patient selection for lymph node staging after PST is significant, which in our study focused on women with limited involvement of the axilla (N1).
In our experience, 61 patients (76.3%) were spared AL, and the group with lymph node pCR benefitted the most. However, in 50% of patients with persistent metastatic lymph node involvement, despite the recommendation of the clinical guidelines, omission of AL was indicated after individual assessment by the tumour committee and inclusion in this prospective study. A study by Nijnatten et al.27 compares DFS and OS among patients with lymph node-positive breast cancer at diagnosis based on their response to neoadjuvant chemotherapy. The results of this study conclude that there is no significant difference between patients with axillary pCR (ypN0) and patients with isolated tumour cells (ypN0itc) or micrometastases (ypN1mi). In contrast, residual axillary macrometastases (ypN1) were associated with a less favourable prognosis. The multicentre study by Cabioglu et al.28 showed similar results, stating that AL could be avoided in patients with breast and/or lymph node pCR after neoadjuvant chemotherapy and in the case of cT1-2 or low tumour load (micrometastases or isolated tumour cells) lymph node residue in luminal subtypes, provided that axillary radiotherapy is given. In contrast, studies by Wong et al.29 and Fisher et al.30 argue that low-volume residual disease after neoadjuvant chemotherapy is associated with lower DFS, and OS rates compared to those with negative nodes. These discrepancies call for prospective studies to assess the impact of AL and axillary radiotherapy on the mid- and long-term outcomes of patients with macrometastases after PST. In our study, no axillary relapses were recorded during follow-up in patients who did not undergo AL and were treated with axillary radiotherapy, but further follow-up is needed to assess the oncological safety of this surgical de-escalation, especially in women with macrometastases after PST.
Our study has several limitations. The absence of AL in a significant group of patients prevents us from knowing the FN rate of the series. A second limitation is the short follow-up time (mean follow-up of 24 months), which prevents us from making a correct assessment of oncological safety and the impact on overall survival of the procedure in the medium term, especially in the patients in whom AL was avoided.
In conclusion, our study shows that combined axillary marking (clip in the biopsied lymph node and SNB) has a high efficacy rate for nodal staging (98.8%). Of these marked lymph nodes, 78.9% were consistent with the SN. Patients with Her2 and triple negative tumours have a high rate of pCR (91.7% and 85.7% respectively) and therefore constitute a group for whom this procedure is specially indicated. In our study, AL was avoided in 76.25% of the women, and therefore we consider that the effort involved in marking and removing the biopsied lymph node is justified.
FinancingNone.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Acea-Figueira E, García-Novoa A, Díaz Carballada C, Bouzón Alejandro A, Conde C, Santiago Freijanes P, et al. Estadificación ganglionar tras terapia sistémica primaria en mujeres con cáncer de mama y afectación ganglionar al diagnóstico. Cir Esp. 2022. https://doi.org/10.1016/j.ciresp.2022.05.004