COVID-19 vaccinations are intended to help produce neutralizing antibodies which target surface spike protein to combat the SARS-Cov-2 virus. Similarly, COVID-19 recovered patients exhibit high levels of SARS-CoV-2 neutralizing antibodies, which predominantly target the surface spike protein and are associated with the occurrence of health consequences in survivors.
ObjectiveThe aim of the study is to explore the long-term health consequences of the COVID-19 vaccines.
MethodologyA prospective, exploratory observational study conducted both online and offline using various questionnaires with all immunized individuals who had been inoculated for at least a month following their last COVID-19 vaccine either AZD1222® or BBV152® vaccines.
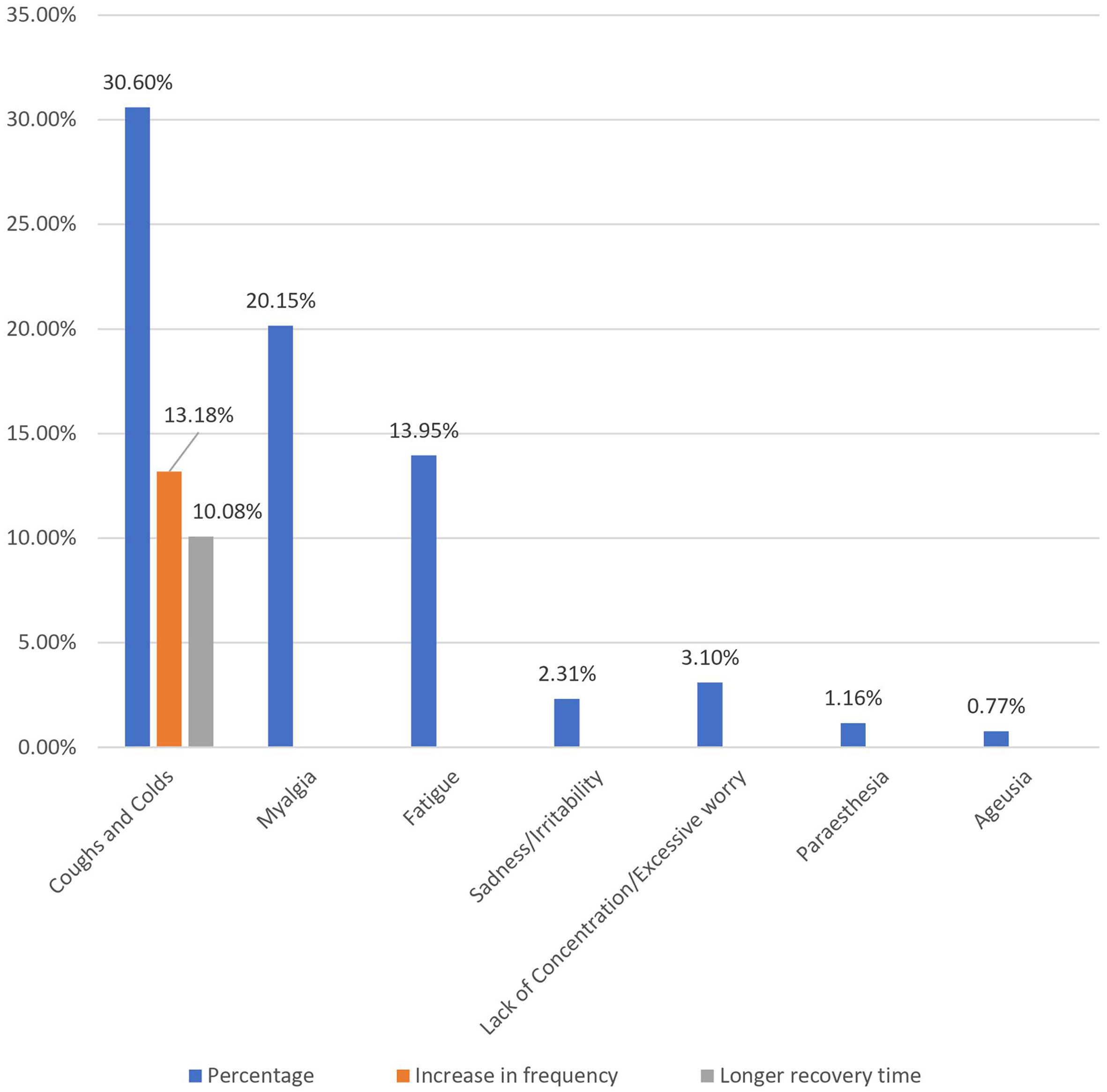
ResultsWe evaluated 258 individuals who had taken the COVID vaccine and found that females made up the majority (54.3%) and that the mean age was 24 years. Post-vaccination long-term health issues were reported by 36.05% (93) of the participants, with 37.86% (53) of females and 33.9% (40) of males (p = 0.292). Myalgia was reported by 20.15% (52), fatigue was 13.95% (36), paresthesia was 1.16% (3), ageusia was 0.77% (2), sadness/irritability was 2.31% (6), and lack of concentration/excessive worry was 3.1% (8).
ConclusionsMyalgia, fatigue, paresthesia, ageusia, coughs and colds, dyspnea, sadness/irritability, and lack of concentration/excessive worry are health consequences related to the COVID-19 vaccination which follow a similar pattern of post-COVID syndrome.
El objetivo de las vacunas frente a la COVID-19 es el de ayudar a producir anticuerpos neutralizantes dirigidos a la proteína de la espícula para combatir el virus SARS-Cov-2. De igual modo, los pacientes que se recuperan de la COVID-19 exhiben grandes niveles de anticuerpos neutralizantes de SARS-CoV-2, que se dirigen predominantemente a la superficie de la proteína de la espícula, y están asociados a la incidencia de consecuencias para la salud de los sobrevivientes.
ObjetivoEl objetivo del estudio es explorar las consecuencias para la salud a largo plazo de las vacunas frente a la COVID-19.
MetodologíaEstudio prospectivo, exploratorio y observacional realizado tanto online como offline, utilizando diversos cuestionarios con todos los individuos vacunados a quienes se había inoculado en el plazo de al menos un mes, tras la última vacuna frente a la COVID-19, bien fueran las vacunas AZD1222® o BBV152®.
ResultadosEvaluamos a 258 individuos que habían recibido la vacuna frente a la COVID, y encontramos que la mayoría eran mujeres (54,3%) con una edad media de 24 años. Las cuestiones de salud a largo plazo, tras la vacuna, fueron reportadas por un 36,05% (93) de los participantes, con un 37,86% (53) de mujeres y un 33.9% (40) de varones (p = 0,292). Se reportó mialgia en el 20,15% (52) de los casos, fatiga en el 13,95% (36), parestesia en el 1,16% (3), ageusia en el 0,77% (2), tristeza/irritabilidad en el 2,31% (6), y falta de concentración/preocupación excesiva en el 3,1% (8).
ConclusionesMialgia, fatiga, parestesia, ageusia, tos y resfriado, disnea, tristeza/irritabilidad, y falta de concentración/preocupación excesiva son las consecuencias de salud relacionadas con la vacuna frente a la COVID-19, y siguen un patrón similar al del síndrome post-COVID.
The combined action of antibodies in the biological fluids, which neutralize viral particles, and the killer activity of lymphocytes, which track down and kill virus-infected cells, is responsible for the majority of patients recovering from a viral illness.1 The status of COVID-19 is unknown; however, recovered patients often have high levels of SARS-CoV-2 neutralizing antibodies that primarily target the surface spike protein, which is the focus of vaccine design.2,3 Both artificial antibodies and natural neutralizing antibodies from the blood of recovered COVID-19 patients were successful in preventing viral invasion, as per Schmidt et al., although viruses that had mutated in a way that allowed them to evade the antibodies remained alive in the situation.4 As a possible consequence, vaccines designed against the old Wuhan-Hu-1 reference virus may be ineffective against the recently identified and significantly mutated virus.5,6 However, immunization against COVID-19 is the most effective way to prevent the infection, as it lowers morbidity and mortality while also being less expensive than treatment.7
The prevalence of lingering effects among COVID-19 survivors has been observed in multiple studies around the world, including fatigue and dyspnoea, anosmia or dysgeusia, impaired pulmonary function, chest image abnormalities, hearing loss, sleep difficulties, and anxiety or depression.8–13 The persistence of COVID-19 symptoms is associated with elevated SARS-CoV-2 IgG antibody titters, increased IL-6 levels, and co-morbidities.14 COVID-19 vaccinations are intended to help produce neutralizing antibodies and combat the SARS-Cov-2 virus. None of the immunizations are without side effects or risks of adverse consequences.15 Anaphylaxis, cerebrovascular accident, thrombosis with thrombocytopenia, Guillain-Barre Syndrome (GBS), myocarditis or pericarditis, vasovagal reaction, epilepsy, pneumonia, multisystem inflammatory syndrome, and even mortality have all been recorded as adverse events following immunization.6,16 In this study, we aim to explore the long-term health consequences of the COVID-19 vaccine.
Methods and methodologyStudy design and sample sizeA prospective, exploratory, observational study was conducted in the premises of Adichunchanagiri University (ACU) which is situated in the rural part of Mandya, India, between September and November 2021 after obtaining approval from the Institutional Ethical Committee of Adichunchinagiri Hospital and Research Center (AH & RC), approval number (IEC/AH & RC/AC/022/2021). The study was registered in the Clinical Trials Registry of India (CTRI) trials registry with the identifier CTRI/2021/08/035660.
We considered the total number of students, lecturers, healthcare professionals, and other workers such as housekeepers, public relations officers, and hospital administrators from different institutions (Medical, Pharmacy, Nursing, Engineering, Science, and Education) and hospitals (AH&RC) that are affiliated with the ACU as the population size. The estimated sample size for the study was 258 with a margin of error of 5%, a confidence level of 90%, and a response distribution of 50% using the Roasoft® sample size calculator.
ParticipantsThe study included individuals over the age of 18 who satisfied the Ministry of Health and Family Welfare's vaccination eligibility criteria (https://www.mohfw.gov.in/covid_vaccination/vaccination/index.html) and had been immunized with the Oxford/AstraZeneca COVID-19 vaccine (AZD1222®) or Bharat Biotech COVID-19 vaccine (BBV152®) for at least a month after their last jab of vaccine before participating in the study. Those who had received any other COVID-19 vaccines, or who had been inoculated with study vaccines but did not complete the full dose or a month after the second dose within the study's duration, were excluded from the study. Participants with chronic diseases such as diabetes, arthritis, asthma, or COPD and those who had recovered from COVID-19 infection were also excluded from the study.
Study procedure and data collectionWe approached and communicated the details of the study to the Dean & Principal or Medical Superintendent of all seven institutes affiliated with the ACU for the conductance of the study. All seven institutes agreed and gave their approval for the survey to be conducted, with the suggestion that it be conducted online to increase participation.
The validated data collection form was then translated into a Google form that comprised informed consent, epidemiological data (age, gender, personal habits, co-morbidities, vaccination date, and name of the vaccine), recent health issues, and various questionnaires to obtain evidence on possible long-term effects of online participation. Participants in the study have the option of choosing either a face-to-face or an online mode to participate in the study. Those who were interested in face-to-face participation were requested to visit the Department of Pharmacy Practice located in AH&RC.
The screening questionnaire was circulated by the respective institute management to all students, lecturers, healthcare professionals, and other employees. At the screening, those who met the criteria for inclusion were invited to participate in the study. After obtaining informed consent from participants, we collected epidemiological data (name, age, gender, habits, occupation, name of the vaccine inoculated with, date of vaccination, any side effects of the vaccine, medication taken to relieve the side effects, leaves taken due to vaccine reactions, recent coughs and colds), recent health issues faced by participants, and administered the EQ-5D-5L17 (https://euroqol.org/) questionnaire and modified British Medical Research Council (mMRC) dyspnoea scale18 to obtain evidence on possible long-term effects. Any health risk that emerges in an individual one month after vaccines and cannot be explained by another ailment is taken into consideration as long-term effects. As per the date of the last jab of vaccination, participants were categorized into 30–89 days, 90–179 days, and more than 180-day groups.
AZD1222®The ChAdOx1 nCoV-19 vaccine (AZD1222®) contains the replication-deficient simian (Chimpanzee) adenovirus vector ChAdOx1, which contains the full-length structural surface glycoprotein of SARS-CoV-2 as well as a tissue plasminogen activator leader sequence. The coding sequences for the spike protein codon are optimized by ChAdOx1 nCoV-19 expression.5,19
BBV152®BBV152® was created using the virus strain (NIV-2020-770) with the Asp614Gly mutation, which was isolated and sequenced from a Covid-19 patient. It's a toll-like receptor (TLR) 7/8 agonist molecule adsorbed to alum, which is a complete virion propiolactone-inactivated SARS-CoV-2 vaccine (Algel-IMDG).5,20
EQ-5D-5L instrumentThe EuroQol group created the EQ-5D-5L scale in 2011, which is often used to assess health-related quality of life (HRQoL).21 Mobility, self-care, usual activities, pain/discomfort, and anxiety/depression are among the five health dimensions of the EQ-5D-5L. Each health dimension offers five categories of response possibilities to define all relevant health states (no problems, slight problems, moderate problems, severe problems, and unable to/extreme problems). Using a scoring algorithm based on public preferences, EQ-5D-5L health statuses are transformed into a single index utility score.22 Each participant's utility score can be calculated by deducting the sum of the coefficients of each health dimension from 1.23
mMRC dyspnoea scaleThe mMRC scale is a self-rating instrument that uses a range of 0–4 to rate the severity of breathlessness. “Where Grade 0 indicates no shortness of breath except during strenuous exercise, Grade 1 indicates shortness of breath when hurrying on the level or walking up a slight hill, Grade 2 indicates walks slower on the level than people of similar age due to breathlessness or having to stop to catch a breath when walking at their own pace on the level, Grade 3 indicates stops for breath after walking 100 m or after a few minutes on the level, and Grade 4 indicates too breathless to leave the house or breathless when dressing or undressing.”24
Data analysisStatistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 26 software. Categorical variables including epidemiological details, vaccines used, side effects of vaccines, medication taken to relieve side effects, leave taken post-vaccination, post-vaccination long-term health issues, coughs and colds, mMRC assessments, and EQ5D5L assessments were assessed for frequency and percentage, while numerical variables including age and utility score were assessed using mean, standard deviation (S.D) and interquartile range (IQR). An independent sample t-test was used to compare the utility scores between vaccines and Chi-Square was used to determine the relationship between various variables and vaccines, considering 0.05 as a significant level.
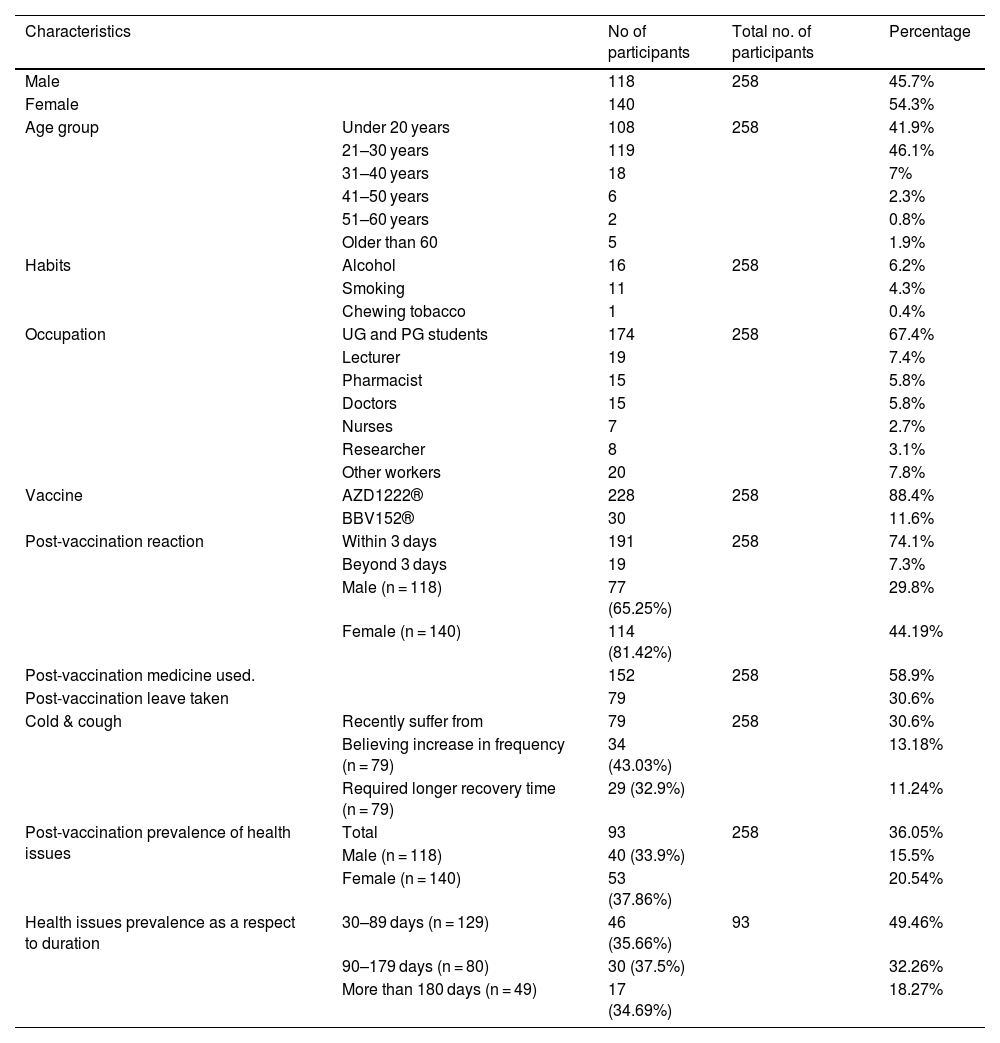
ResultsOf the 498 responses to the screening questionnaire, 197 did not meet the inclusion criteria, and 43 did not agree to participate in the study. 74 people volunteered in person and 194 people volunteered digitally, bringing the total number of participants to 258. We evaluated 258 individuals who had taken the COVID vaccine and observed that females made up the majority, 54.3% (140). The average age of the participants was 24.13 years (SD 8.62). With a maximum age of 66 and a minimum age of 18 (IQR, 6; Q3 = 25 and Q1 = 19), this group had a wide range of ages. The age group of 21–30 years old had the most participants, 46.1% (119). In total, 6.2% (16) had the habit of drinking alcohol, 4.3% (11) had the habit of smoking cigarettes, and 0.4% (1) had the habit of chewing tobacco. A total of 67.4% (174) were undergraduate and postgraduate students, 7.7% (20) were other workers, 7.4% (19) were lecturers, 5.8% (15) were doctors, 3.1% (8) were researchers, and 2.7% (7) were nurses (Tables 1 & 2).
Demographic details of participants and prevalence of health consequences relating to vaccination.
| Characteristics | No of participants | Total no. of participants | Percentage | |
|---|---|---|---|---|
| Male | 118 | 258 | 45.7% | |
| Female | 140 | 54.3% | ||
| Age group | Under 20 years | 108 | 258 | 41.9% |
| 21–30 years | 119 | 46.1% | ||
| 31–40 years | 18 | 7% | ||
| 41–50 years | 6 | 2.3% | ||
| 51–60 years | 2 | 0.8% | ||
| Older than 60 | 5 | 1.9% | ||
| Habits | Alcohol | 16 | 258 | 6.2% |
| Smoking | 11 | 4.3% | ||
| Chewing tobacco | 1 | 0.4% | ||
| Occupation | UG and PG students | 174 | 258 | 67.4% |
| Lecturer | 19 | 7.4% | ||
| Pharmacist | 15 | 5.8% | ||
| Doctors | 15 | 5.8% | ||
| Nurses | 7 | 2.7% | ||
| Researcher | 8 | 3.1% | ||
| Other workers | 20 | 7.8% | ||
| Vaccine | AZD1222® | 228 | 258 | 88.4% |
| BBV152® | 30 | 11.6% | ||
| Post-vaccination reaction | Within 3 days | 191 | 258 | 74.1% |
| Beyond 3 days | 19 | 7.3% | ||
| Male (n = 118) | 77 (65.25%) | 29.8% | ||
| Female (n = 140) | 114 (81.42%) | 44.19% | ||
| Post-vaccination medicine used. | 152 | 258 | 58.9% | |
| Post-vaccination leave taken | 79 | 30.6% | ||
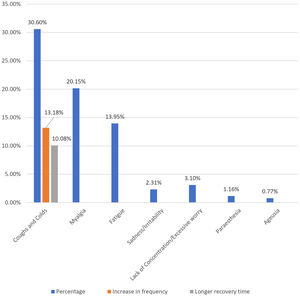
| Cold & cough | Recently suffer from | 79 | 258 | 30.6% |
| Believing increase in frequency (n = 79) | 34 (43.03%) | 13.18% | ||
| Required longer recovery time (n = 79) | 29 (32.9%) | 11.24% | ||
| Post-vaccination prevalence of health issues | Total | 93 | 258 | 36.05% |
| Male (n = 118) | 40 (33.9%) | 15.5% | ||
| Female (n = 140) | 53 (37.86%) | 20.54% | ||
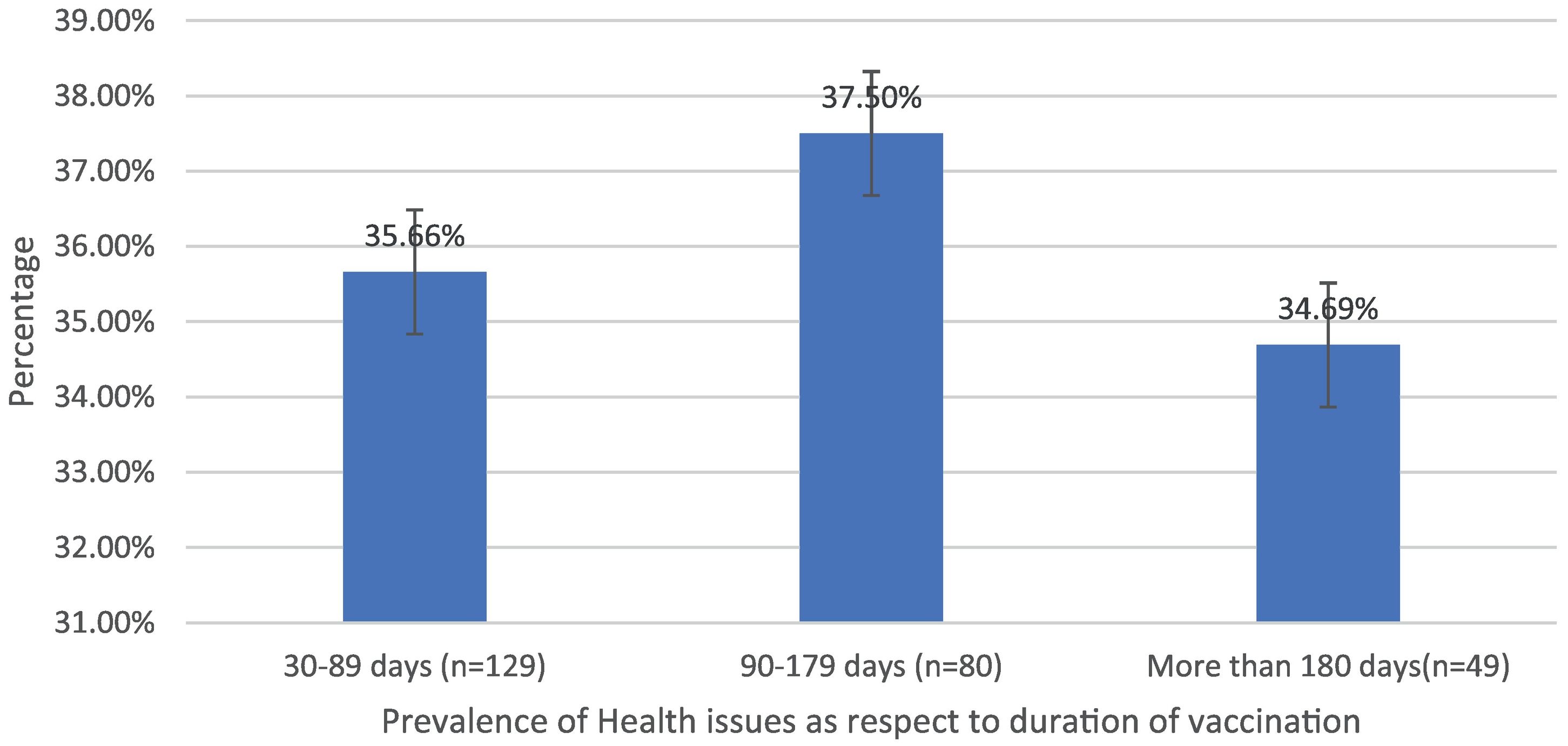
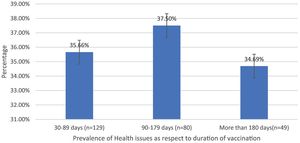
| Health issues prevalence as a respect to duration | 30–89 days (n = 129) | 46 (35.66%) | 93 | 49.46% |
| 90–179 days (n = 80) | 30 (37.5%) | 32.26% | ||
| More than 180 days (n = 49) | 17 (34.69%) | 18.27% | ||
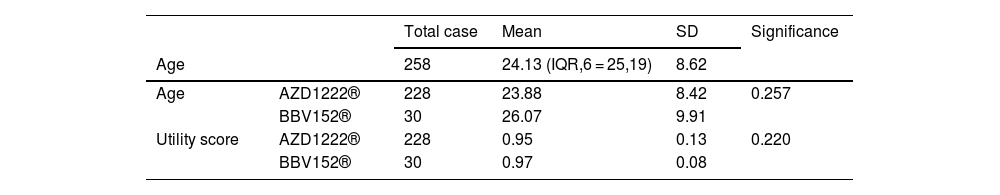
The age of the participants and utility scores were used to calculate the mean, standard deviation, and significant value.
| Total case | Mean | SD | Significance | ||
|---|---|---|---|---|---|
| Age | 258 | 24.13 (IQR,6 = 25,19) | 8.62 | ||
| Age | AZD1222® | 228 | 23.88 | 8.42 | 0.257 |
| BBV152® | 30 | 26.07 | 9.91 | ||
| Utility score | AZD1222® | 228 | 0.95 | 0.13 | 0.220 |
| BBV152® | 30 | 0.97 | 0.08 | ||
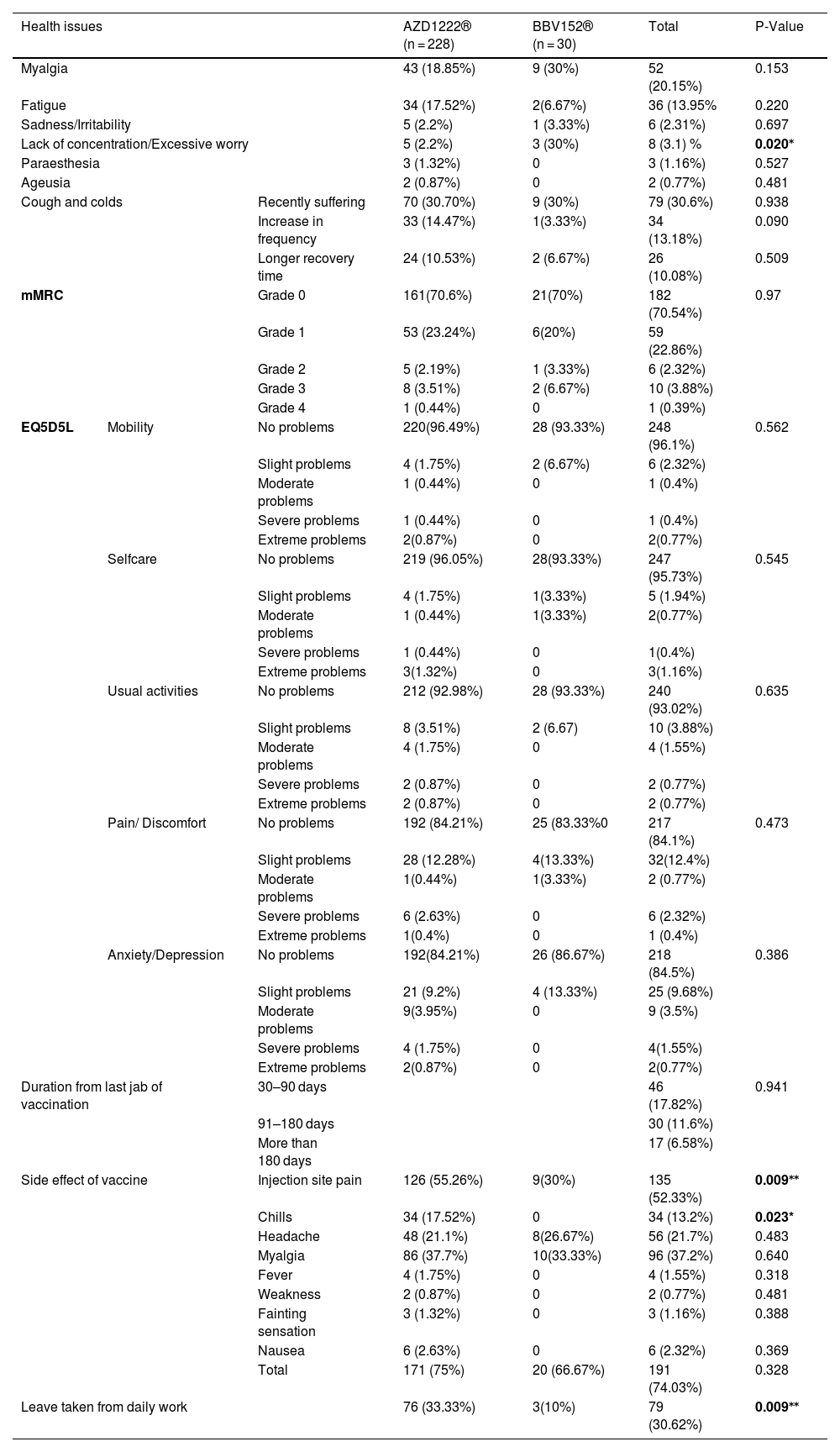
AZD1222® was used by 88.4% (228) of the participants, while BBV152® was used by 11.6% (30). 74.1% (191) of participants experienced side effects within three days of vaccination, which included injection site pain 52.3% (135), myalgia 37.2% (96), headache 21.7% (56), chills 13.2% (34), nausea 2.3% (6), fever 1.6% (4), fainting sensation 1.2% (3), and weakness 0.8% (2). There was a statistically significant difference between male and female vaccination side effects, with 65.25% vs 81.2% (P = 0.003), respectively. 75% (171) of AZD1222® and 66.7% (20) of BBV152® inoculated participants had experienced side effects. The side effect was extended beyond 3 days in 7.3% (19) of the participants. There were no significant differences in the occurrence of side effects between the two vaccines (P = 0.328). 30.6% (79) of the participants were required to take time off after vaccination (P = 0.009) (Tables 1 & 3).
Association of side effects and post-vaccination health issues with vaccines.
| Health issues | AZD1222® (n = 228) | BBV152® (n = 30) | Total | P-Value | ||
|---|---|---|---|---|---|---|
| Myalgia | 43 (18.85%) | 9 (30%) | 52 (20.15%) | 0.153 | ||
| Fatigue | 34 (17.52%) | 2(6.67%) | 36 (13.95% | 0.220 | ||
| Sadness/Irritability | 5 (2.2%) | 1 (3.33%) | 6 (2.31%) | 0.697 | ||
| Lack of concentration/Excessive worry | 5 (2.2%) | 3 (30%) | 8 (3.1) % | 0.020⁎ | ||
| Paraesthesia | 3 (1.32%) | 0 | 3 (1.16%) | 0.527 | ||
| Ageusia | 2 (0.87%) | 0 | 2 (0.77%) | 0.481 | ||
| Cough and colds | Recently suffering | 70 (30.70%) | 9 (30%) | 79 (30.6%) | 0.938 | |
| Increase in frequency | 33 (14.47%) | 1(3.33%) | 34 (13.18%) | 0.090 | ||
| Longer recovery time | 24 (10.53%) | 2 (6.67%) | 26 (10.08%) | 0.509 | ||
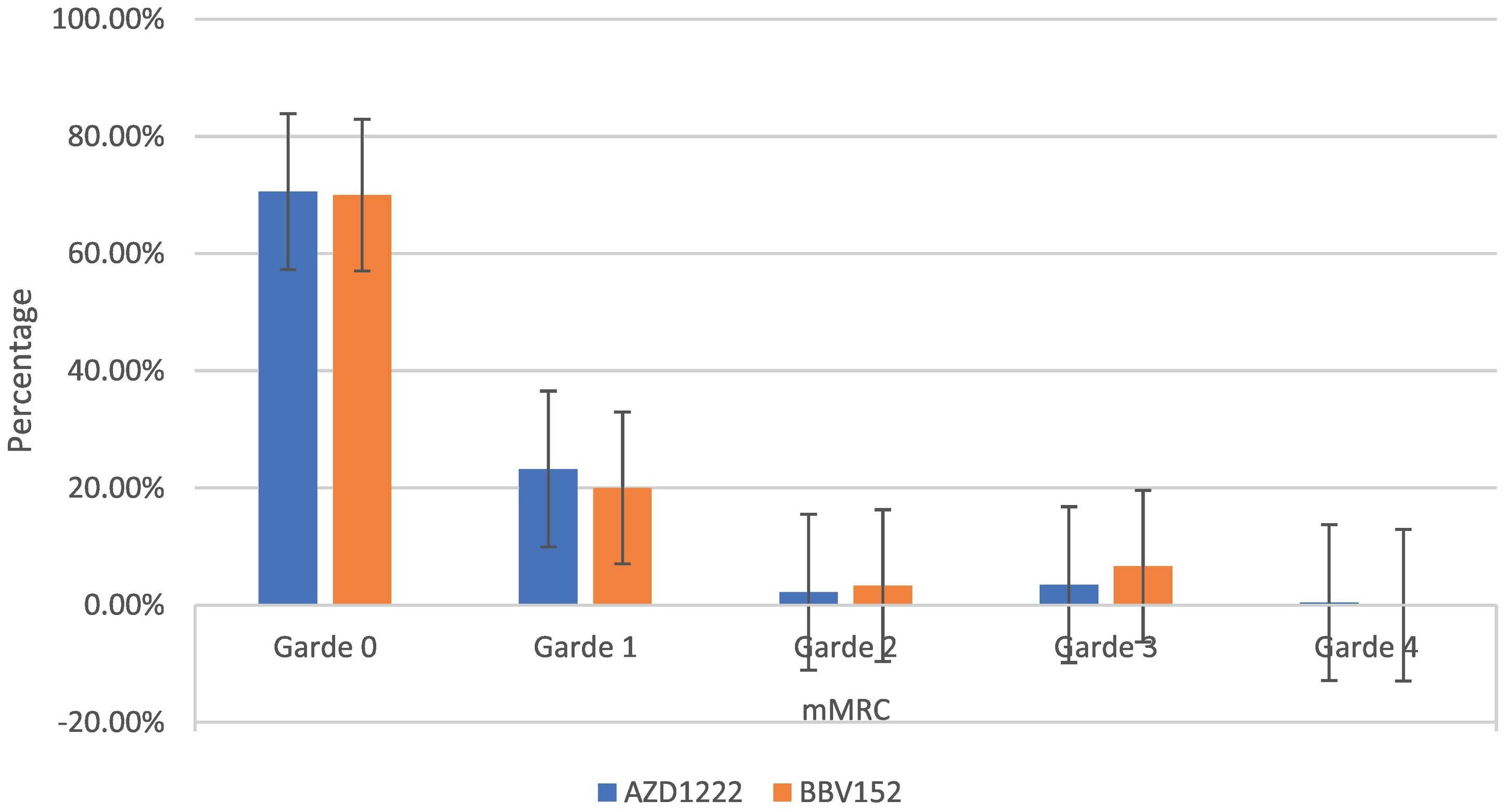
| mMRC | Grade 0 | 161(70.6%) | 21(70%) | 182 (70.54%) | 0.97 | |
| Grade 1 | 53 (23.24%) | 6(20%) | 59 (22.86%) | |||
| Grade 2 | 5 (2.19%) | 1 (3.33%) | 6 (2.32%) | |||
| Grade 3 | 8 (3.51%) | 2 (6.67%) | 10 (3.88%) | |||
| Grade 4 | 1 (0.44%) | 0 | 1 (0.39%) | |||
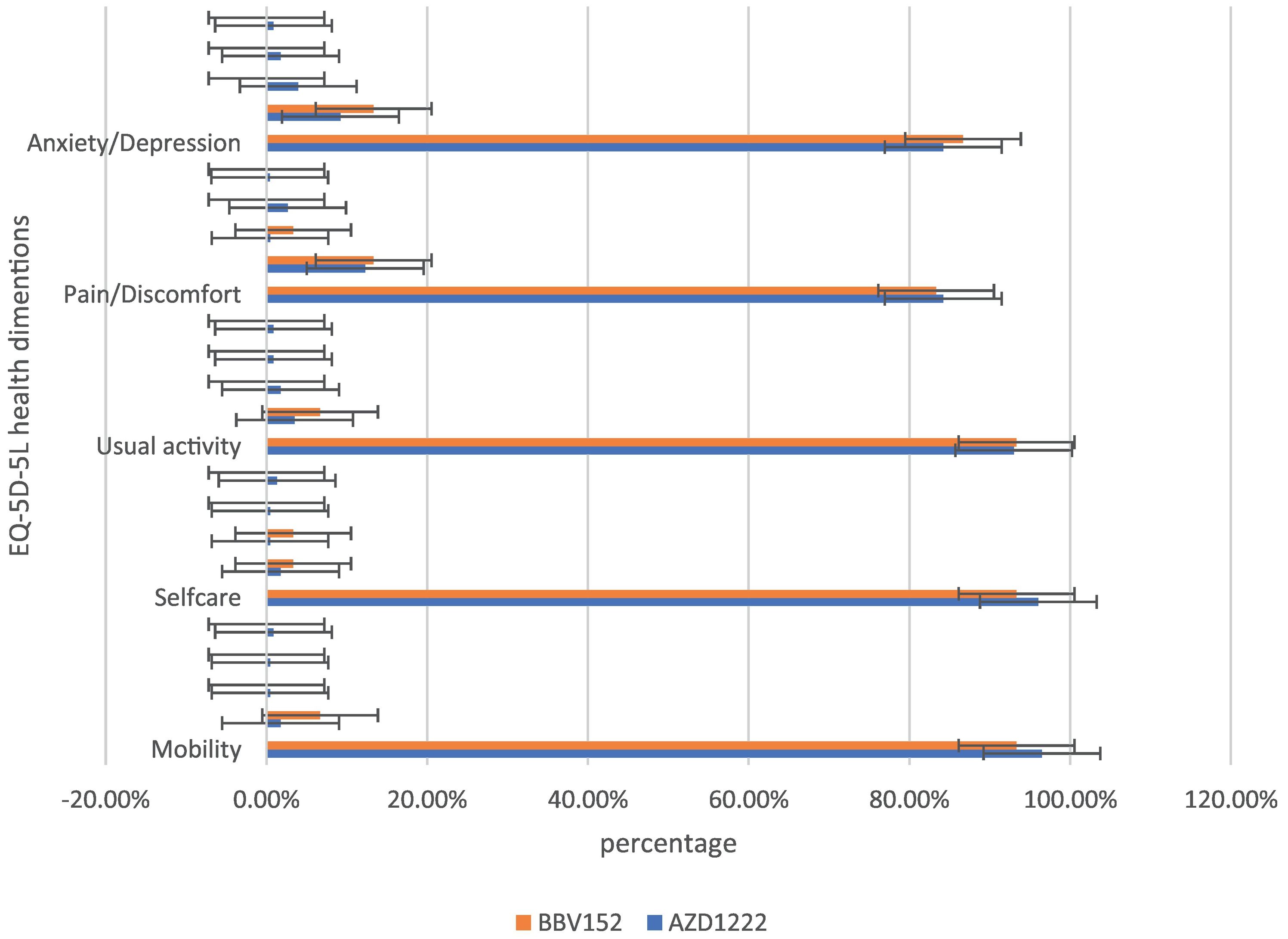
| EQ5D5L | Mobility | No problems | 220(96.49%) | 28 (93.33%) | 248 (96.1%) | 0.562 |
| Slight problems | 4 (1.75%) | 2 (6.67%) | 6 (2.32%) | |||
| Moderate problems | 1 (0.44%) | 0 | 1 (0.4%) | |||
| Severe problems | 1 (0.44%) | 0 | 1 (0.4%) | |||
| Extreme problems | 2(0.87%) | 0 | 2(0.77%) | |||
| Selfcare | No problems | 219 (96.05%) | 28(93.33%) | 247 (95.73%) | 0.545 | |
| Slight problems | 4 (1.75%) | 1(3.33%) | 5 (1.94%) | |||
| Moderate problems | 1 (0.44%) | 1(3.33%) | 2(0.77%) | |||
| Severe problems | 1 (0.44%) | 0 | 1(0.4%) | |||
| Extreme problems | 3(1.32%) | 0 | 3(1.16%) | |||
| Usual activities | No problems | 212 (92.98%) | 28 (93.33%) | 240 (93.02%) | 0.635 | |
| Slight problems | 8 (3.51%) | 2 (6.67) | 10 (3.88%) | |||
| Moderate problems | 4 (1.75%) | 0 | 4 (1.55%) | |||
| Severe problems | 2 (0.87%) | 0 | 2 (0.77%) | |||
| Extreme problems | 2 (0.87%) | 0 | 2 (0.77%) | |||
| Pain/ Discomfort | No problems | 192 (84.21%) | 25 (83.33%0 | 217 (84.1%) | 0.473 | |
| Slight problems | 28 (12.28%) | 4(13.33%) | 32(12.4%) | |||
| Moderate problems | 1(0.44%) | 1(3.33%) | 2 (0.77%) | |||
| Severe problems | 6 (2.63%) | 0 | 6 (2.32%) | |||
| Extreme problems | 1(0.4%) | 0 | 1 (0.4%) | |||
| Anxiety/Depression | No problems | 192(84.21%) | 26 (86.67%) | 218 (84.5%) | 0.386 | |
| Slight problems | 21 (9.2%) | 4 (13.33%) | 25 (9.68%) | |||
| Moderate problems | 9(3.95%) | 0 | 9 (3.5%) | |||
| Severe problems | 4 (1.75%) | 0 | 4(1.55%) | |||
| Extreme problems | 2(0.87%) | 0 | 2(0.77%) | |||
| Duration from last jab of vaccination | 30–90 days | 46 (17.82%) | 0.941 | |||
| 91–180 days | 30 (11.6%) | |||||
| More than 180 days | 17 (6.58%) | |||||
| Side effect of vaccine | Injection site pain | 126 (55.26%) | 9(30%) | 135 (52.33%) | 0.009⁎⁎ | |
| Chills | 34 (17.52%) | 0 | 34 (13.2%) | 0.023* | ||
| Headache | 48 (21.1%) | 8(26.67%) | 56 (21.7%) | 0.483 | ||
| Myalgia | 86 (37.7%) | 10(33.33%) | 96 (37.2%) | 0.640 | ||
| Fever | 4 (1.75%) | 0 | 4 (1.55%) | 0.318 | ||
| Weakness | 2 (0.87%) | 0 | 2 (0.77%) | 0.481 | ||
| Fainting sensation | 3 (1.32%) | 0 | 3 (1.16%) | 0.388 | ||
| Nausea | 6 (2.63%) | 0 | 6 (2.32%) | 0.369 | ||
| Total | 171 (75%) | 20 (66.67%) | 191 (74.03%) | 0.328 | ||
| Leave taken from daily work | 76 (33.33%) | 3(10%) | 79 (30.62%) | 0.009⁎⁎ | ||
Significant levels 0.05 are shown in bold.
Post-immunization long-term health concerns were observed in 36.05% (93) of participants, with 57% (53) being females and 43% (40) being males (P = 0.509) (Table 1). Fatigue was reported by 13.95% (36) participants, including 14.9% (34) of AZD1222® and 6.67% (2) of BBV152® (P = 0.220). Myalgia was reported by 20.15% (52) participants, including 18.85% (43) of AZD1222® and 30% (9) of BBV152® (P = 0.153). Lack of concentration or excessive worry was reported by 3.1% (8), including 2.24% (5) of AZD1222® and 30% (3) of BBV152® (P = 0.020). 2.31% (6) of participants, including 2.2% (5) of AZD1222® and 3.33% (1) of BBV152® (P = 0.697), reported irritability or sadness. 0.87% (2) of AZD1222® inoculated participants complained of a change in taste (P = 0.481). The AZD1222® inoculated participants reported paraesthesia in 1.32% (3) of cases (P = 0.527) (Tables 1 & 3 and Fig. 1). At the time of the survey, 30.6% (79) of the participants had coughs and colds. Among them, 43.03% (34) of participants believed immunization caused more coughs and colds, and 32.9% (26) believed that it took longer to recover. 34.69% (17) of participants who received the vaccine before 181 days, 37.5% (30) of participants who received the vaccine between 91 and 180 days, and 35.66% (46) of participants who received the vaccine between 30 and 90 days reported health concerns (P = 0.941) (Tables 1 & 3 and Fig. 1 & 4).
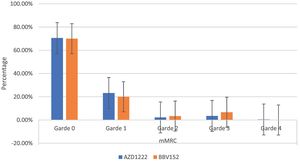
The mMRC dyspnoea scale revealed that 70.54% (182) of participants responded to Grade 0, with 70.6% (161) of AZD1222® and 70% (21) of BBV152®, 22.86% (59) to Grade 1, with 23.24% (53) of AZD1222® and 20% (6) of BBV152®, 2.32% (6) to Grade 2, with 2.19% (5) of AZD1222® and 3.33% (1) of BBV152, 3.88% (10) to Grade 3, with 3.51% (8) of AZD1222® and 6.67% (2) of BBV152®, and 0.39% (1) to Grade 4 from AZD1222®. The overall p-value was 0.97 (Table 3 & Fig. 3).
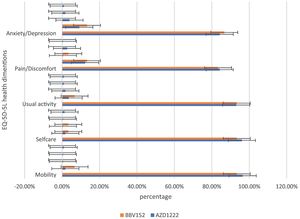
In the assessment of EQ-5D-5L, 96.1% (248) had no mobility problems (96.49% (220) of AZD1222® and 93.3% (28) of BBV152®), 2.32% (6) had slight mobility problems (1.75% (4) of AZD1222® and 6.67% (2) of BBV152®), 0.4% (1) had moderate mobility problems (0.44% (1) of AZD1222®), 0.4% had severe mobility problems (0.44% (1) of AZD1222®), and 0.8% had extreme problems in mobility (0.88% (2) of AZD1222®). The overall P-value obtained from the mobility was 0.562. 95.7% (247) had no problems with self-care (96.05% (219) of AZD1222® and 93.33% (28) of BBV152®), 1.9% (5) had minor problems with self-care (1.75% (4) of AZD1222® and 3.33% (1) of BBV152®), 0.8% (2) had moderate problems with self-care (0.44% (1) of AZD1222® and 1.33% (1) of BBV152®), 0.4% (1) had severe problems with self-care (0.44% (1) of AZD1222®), and 1.16% (3) had extreme problems with self-care (1.32% (3) of AZD1222®). The overall P-value obtained in self-care was 0.545. 93.02% (240) had no problems performing usual activities (92.98% (212) of AZD1222® and 93.33% (28) of BBV152®), 3.88% (10) had slight problems in performing usual activities (3.51% (8) of AZD1222® and 6.67% (2) of BBV152®), 1.55% (4) had moderate problems in performing usual activities (1.75% (4) of AZD1222®), 0.77% (2) had severe problems in performing usual activities (0.87% (2) of AZD1222®), and 0.77% (2) had extreme problems in performing usual activities (0.87% (2) of AZD1222®). The P-value obtained in the usual activities was 0.635. 84.1% (271) had no pain experiences (84.21% (192) of AZD1222® and 83.33% (25) of BBV152®), 12.4% (32) had slight pain experiences (12.28% (28) of AZD1222® and 13.33% (4) of BBV152®), 0.77% (2) had moderate pain experiences (0.44% (1) of AZD1222® and 3.33% (1) of BBV152®), 2.32% (6) had severe pain experiences with (2.63% (6) of AZD1222®), and 0.4% (1) had extreme pain problems (0.44% (1) of AZD1222®). The overall P-value obtained in the pain experience was 0.473. 84.5% (218) had anxiety or depression (84.21% (192) of AZD1222® and 86.67% (26) of BBV152®), 9.68% (25) had slight anxiety or depression (9.2% (21) of AZD1222® and 13.33% (4) of BBV152®), 3.5% (9) had moderate anxiety or depression (3.95% (9) of AZD1222®), 1.55% (4) had severe anxiety or depression (1.75% (4) of AZD1222®), and 0.77% (2) had extreme anxiety or depression (0.87% (2) of AZD1222®). The overall P-value obtained in the anxiety and depression tests was 0.386. The mean utility score was 0.95 (SD 0.13; 95% CI 0.94, 0.97) with values ranging from 0.063 to 1. Overall, there were no statistically significant utility scores between AZD1222® and BBV152® (mean 0.95 (SD 0.14) vs 0.97 (SD 0.08); p = 0.220) Tables 2 & 3Fig. 2).
DiscussionThe study was conducted with 258 participants who had received one of two vaccines, either AZD1222® or BBV152®, to determine the COVID-19 vaccine's inverse impact and its long-term health consequences. We found that 74.1% (191) of the participants had at least one of the vaccine side effects, with no statistically significant differences in the occurrence of side effects between AZD1222® and BBV152® (P = 0.328) (Tables 1 & 3). Riad et al.25 reported a higher incidence than we did, while Dziedzic et al.26 reported a lower incidence. It's possible that the difference in the occurrence of side effects between studies is attributed to the usage of drugs after immunization. In the study, 58.9% of the participants used medicine to prevent side effects (Table 1). In comparison to BBV152®, the AZD1222® vaccine is more frequently associated with injection site pain and chills (P = 0.009 and P = 0.023, respectively) (Table 3). Myalgia, headaches, nausea, fever, fainting sensation, and weakness are among the other side effects that are similar in both vaccines. Because of side effects, 30.6% of the participants had to take time-off from their regular jobs. Participants who had been vaccinated with AZD1222® appeared to take more time-off from daily work than those who had been immunized with BBV152® (P = 0.009). In comparison to male participants, females are more likely to develop the side effect (P = 0.003) (Table 3).
In the study, we observed that 36.05% (93) of the individuals had long-term health consequences following immunizations, including myalgia, fatigue, paraesthesia, ageusia, dyspnoea, coughs and colds, sadness/irritability, and lack of concentration/excessive worry. Except for a lack of concentration or excessive worry, all other post-vaccination health issues are equally evident in both vaccinations. Table 3 shows that BBV152® is more frequently associated to a lack of concentration or excessive worry than AZD1222® (30% vs 2.2%, P = 0.020). Carf et al., Huang et al., and Chopra et al. all found a similar pattern of morbidity prevalence among COVID-19 recovered people in their studies.9,10,27 The similarity in the prevalence rate and symptoms of post-vaccination health consequences with post-COVID-19 syndrome raises the question of whether the neutralizing antibody developed by the vaccine inoculation is the cause. Because there is a resemblance between vaccinated and COVID-19 recovered individuals who have a neutralizing antibody that primarily targets spike protein. COVID-19 pneumonia was also reported as an adverse event associated with the COVID-19 vaccine in India's Ministry of Health and Family Welfare's causality assessment report.6 There is no difference in the incidence of health consequences between 30–90 days, 91–180 days, and more than 180 days (P = 0.941) (Table 3). It could be because neutralizing antibodies persist after inoculation. We have no idea how long the neutralizing antibody will last once it is developed. However, according to the World Health Organization's (WHO) interim statement on booster doses, vaccine efficacy drops by only 8% after 6 months of vaccination across all age groups.28 Similarly, 80–90% of people infected with SARS-CoV-2 are protected from reinfection for up to 7 months after infection.29 It required a more detailed study with consideration of neutralizing antibodies to corroborate the results.
In the study, around half of the individuals who are suffering from coughs and colds during the study period believe that coughs and colds are becoming more frequent and that recovery time is taking longer. Coughing appears to be dry, with more episodes occurring in the morning and evening. To support the participant's experiences with coughs and colds, we used the mMRC scale to assess dyspnoea. On the mMRC scale, we observed shortness of breath from Grade 1 to Grade 4, and the incidence of coughs and colds appears to be nearly equal (29.45% vs 30.6%) (Table 3). In both immunizations, we did not observe statistically significant variations in the occurrence of coughs and colds, as well as dyspnoea. The anxiety/depression dimension of the EQ-5D-5L assessment is compared to the sum of the participant's experiences of sadness/irritability (2.31%) and lack of concentration/excessive worry (3.1%). The total number of moderate to severe problems in the EQ-5D-5L anxiety/depression dimension is nearly equal to the total number of participant's experiences of sadness/irritability and lack of concentration/excessive worry (5.82% vs 5.41%) (Table 3). Overall, the EQ-5D-5L scale assessment finds minor concerns in each of the five health dimensions, and both immunizations yield similar consequences. In both vaccinations, the utility score (mean 0.95 (SD 0.14) vs 0.97 (SD 0.08); P = 0.220), which is analogous to the participant's quality of life as measured by the EQ-5D-5L, is not statistically significant (Table 2). When compared to AZD1222®, BBV152® has a slightly higher quality of life. However, since the study shows the COVID-19 vaccination has long-term inverse health consequences, it is worthwhile to inoculate for disease prevention when there is no specific drug to cure the disease.
One of the study's limitations is that we assessed vaccination's long-term health consequences regardless of vaccine design platforms, and the number of participants for each vaccine group was not equal. Another limitation is that the study is carried out both online and offline, which may have the possibility that important details from online participants will be missed, while face-to-face or offline participation may yield better results. The study is also limited by the fact that it is a one-time observational study. It required more study with multi-time observation to confirm the findings of this study.
ConclusionIn the study, we observed that 36.05% of the participants experienced long-term health consequences post-immunization, and females are more susceptible than males. Myalgia, fatigue, paraesthesia, ageusia, coughs and colds, dyspnoea, sadness/irritability, and lack of concentration or excessive worry are health consequences related to the COVID-19 vaccination, which follow a similar pattern to the post-COVID syndrome. Both AZD1222® and BBV152® vaccines have the potential to cause an equal number of long-term health problems in vaccinated individuals. There is no difference in the incidence of long-term health consequences between 30–90 days, 91–180 days, and more than 180 days. To confirm the findings, more research with a balanced sample distribution and a large number of time observations is needed.
FundingThis study was not supported by any agency or commercial entity.
Ethical ClearanceThe study was approved from the Institutional Ethical Committee of Adichunchanagiri Hospital and Research Center (Approval No. IEC/AH&RC/AC/022/2021).
We thank Dr. B Ramesh (Dean & Principal, Sri Adichunchanagiri College of Pharmacy) and Dr. M. G. Shivaramu (Principal, Adichunchanagiri Institute of Medical Sciences) for providing a favourable environment to conduct the study.