Editado por: Dra. Núria Torner CIBER Epidemiologia y Salud Publica CIBERESP Unitat de Medicina Preventiva i Salut Pública Departament de Medicina, Universitat de Barcelona
Más datosCOVID-19 is a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The characteristics of this infectious disease vary from a country to another and from one peak to the next. The aim of the present study was to describe the COVID-19 patients hospitalized in Kermanshah, a city in the west of Iran, in the third peak of the disease and to identify in-hospital mortality determinants in this disease.
MethodsIn this retrospective study, the clinical and demographic characteristics, laboratory findings, prescribed treatments and outcome of all COVID-19 patients (definitive, suspected, and probable) were collected from the medical records department of Farabi Hospital affiliated with Kermanshah University of Medical Sciences, Kermanshah, Iran from 22 October to 20 November 2020.
ResultsIn total, 665 COVID-19 patients (265 females and 400 males, mean age: 58.7 years) were enrolled, including 479 confirmed (72%), 156 probable (23.5%), and 30 suspected cases (4.5%). About 84% of the patients presented with low oxygen saturation levels. The most common comorbidities were hypertension (15%), diabetes (10%), and cardiovascular disease (3%). The median (IQR) length of hospital stay was 6 (4–8) and 7 (2–14) day in discharged and deceased patients, respectively. Eighty-two out of 655 patients admitted to the hospital and 39 of the 60 patients admitted to the ICU died. In total, in-hospital mortality rate was 12.33%. Regarding lab variables, in the adjusted model, no significant difference was observed between discharged and deceased patients.The results of multivariable logistic regression showed that each one-unit increase in oxygen saturation (SPO2) increased the odds of survival by 0.88 times (95% CI 0.78–0.99, p = 0.043). Moreover, each one-day increase in the length of ICU stay reduced the odds of mortality by 0.49 times (95% CI 0.26–0.95, p = 0.035).
ConclusionHospitalized COVID-19 patients were generally more ill during the third peak so that about 85% of the patients had SPO2 < 93%. The in-hospital mortality rate was also high. Demographic and paraclinical variables (except SPO2 level) were not suitable predictors of mortality.
COVID-19 es una enfermedad causada por el síndrome respiratorio agudo severo coronavirus 2 (SARS CoV 2). Las características de esta enfermedad infecciosa varían de un país a otro y de un pico a otro. El objetivo del presente estudio fue describir a los pacientes con COVID-19 hospitalizados en Kermanshah, una ciudad al oeste de Irán, durante la tercera ola pandémica e identificar los determinantes de mortalidad hospitalaria de esta enfermedad.
MétodosEn este estudio retrospectivo, las características clínicas y demográficas, los hallazgos de laboratorio, los tratamientos prescritos y el resultado de todos los pacientes ingresados por COVID-19 (definitivo, sospechoso y probable) se recopilaron a partir de los registros médicos del Hospital Farabi afiliado a la Universidad de Medicina de Kermanshah. Sciences, Kermanshah, Irán, del 22 de octubre al 20 de noviembre de 2020.
ResultadosEn total, se registraron 665 pacientes ingresados por COVID-19 (265 mujeres y 400 hombres), de los cuales 479 casosfueron confirmados (72%), 156 probables (23.5%) y 30 sospechosos (4.5%). La edad promedio del total de casos fue de 58,7 años. Alrededor del 84% de los pacientes presentaron niveles bajos de saturación de oxígeno. Las comorbilidades más comunes fueron hipertensión (15%), diabetes (10%) y enfermedad cardiovascular (3%). La mediana (RIC) de estancia hospitalaria fue de 6 (4–8) y 7 (2–14) días en pacientes dados de alta y fallecidos, respectivamente. El 82 de los 655 pacientes ingresados en el hospital y el 39 de los 60 pacientes ingresados en UCI fallecieron. En total, la tasa de mortalidad hospitalaria fue del 12,33%. En cuanto a las variables de laboratorio, en el modelo ajustado no se observaron diferencias significativas entre los pacientes dados de alta y los fallecidos. Los resultados de la regresión logística multivariable mostraron que cada aumento de una unidad en la saturación de oxígeno (SPO2) aumentó las probabilidades de supervivencia en 0,88 veces (IC del 95% 0,78-0,99, p = 0,043). Además, cada aumento de un día en la duración de la estancia en la UCI redujo las probabilidades de mortalidad en 0,49 veces (IC del 95%: 0,26-0,95, p = 0,035).
ConclusiónLos pacientes hospitalizados con COVID-19 generalmente estaban más enfermos durante el tercer pico, de modo que aproximadamente el 85% de los pacientes tenían SPO2 < 93%. La tasa de mortalidad hospitalaria también fue alta. Las variables demográficas y paraclínicas (excepto el nivel de SPO2) no fueron predictores adecuados de mortalidad.
In December 2019, the first cases of COVID-19 infection, an emerging respiratory infection caused by SARS-CoV-2, were reported from Wuhan, China.1–3 Many people across the world were soon infected in a short time due to the high transmission rate of this infection. In Iran, 7 million people have been confirmed infected with a death toll of about 140,000 since the onset of COVID-19 infection about two years ago. Despite great efforts to manage and control this infection, Iran has experienced six peaks so far, each taller and more severe than the previous one.4 The first peak occurred in late February 2020 with the death of the first patient in Qom, which continued through May 2020. The second peak started immediately after it in May 2020 with early opening of public places and continued until August 2020. The third peak started in late September 2020 and continued through early January 2021, and the fourth peak started in March and continued until late May 2021. Immediately after a decrease in the number of patients in May 2021, an increasing trend started due to the Delta variant representing the fifth peak. Finally, the sixth peak started in late January 2022 and continued through late March 2022.
Rapid emergence of new SARS-CoV-2 variants due to viral mutations increases its ability to escape the immune system and infect more patients, renders immunization ineffective, and affects the effectiveness of public health measures.5 In September 2020, Alpha (lineage B.1.1.413) became the dominant variant in Iran,6 which was associated with double the risk of hospitalization during 14 days of a positive molecular test in the UK and Scotland compared to previous variants.7 However, this variant predominated in Iran before starting vaccination, which made it one of the worst COVID-19 peaks so that the daily death toll exceeded 700.
Considering COVID-19 treatment management during the third wave, use of remdesivir and corticosteroids, hydroxychloroquine, lopinavir, and increased.8,9 However, the effect of the Alpha variant on the mortality of COVID-19 patients is not yet clear in Iran. Knowledge of the demographic and clinical characteristics and outcomes of COVID-19 improves our understanding of the virus behavior and disease management and helps to allocate more resources to patients with a higher risk of mortality. The aim of the present study was to provide a description of the demographic and clinical characteristics, administered drugs, and outcomes of COVID-19 during the third wave in one of the largest hospitals dedicated to COVID-19 in Kermanshah Province, west of Iran.
MethodsSetting and study designThis retrospective study was conducted in Farabi Hospital affiliated with Kermanshah University of Medical Sciences, Kermanshah, Iran. This hospital was one of the two large hospitals dedicated to the management of COVID-19 patients in Kermanshah. During the pandemic, almost all ward and ICU beds were exclusively dedicated to COVID-19 patients.
All adult patients admitted to the hospital with a confirmed, suspected, or probable diagnosis of COVID-19 from 22 October to 20 November 2020 were eligible for inclusion. The criteria for hospitalization followed the guidelines published by the Iranian Ministry of Health and Medical Education (MOHME), including moderate to severe COVID-19 infection (SPO2 ≤ 93 on room air and/or an absolute lymphocyte count <1100/μl, blood pressure <90 mmHg or decreased level of consciousness). The patients without a positive RT-PCR test, positive CT findings, or clinical signs and symptoms consistent with COVID-19 were excluded from the study. In addition, patients with re-hospitalization, duplicate data, or missing data were also excluded.
Study definitionsAccording to the MOHME guidelines, COVID-19 patients are categorized as confirmed, suspected, or probable cases. Suspected COVID-19 cases are individuals with symptoms such as cough, fever, or acute respiratory disease. Probable cases are defined as suspected cases that have a chest CT scan indicating COVID-19 findings. Confirmed cases are patients with a positive nasopharyngeal swab RT-PCR test for SARS-CoV-2 with or without radiological findings consistent with a diagnosis of COVID-19 infection.
Data sources and collectionThe medical records of the patients infected with SARS-CoV-2 were evaluated and the data were extracted and recorded in a checklist. The collected data included demographic characteristics (age, gender, comorbidity), clinical manifestations (signs and symptoms upon admission), SPO2 level, chest CT scan report and RT-PCR result, drugs administered during admission (anti-viral, anti-bacterial, anti-coagulant, immunomodulators), lab data on admission, length of stay in hospital, ICU admission, and outcome (death or survival).
Statistical analysisData was analyzed using the STATA software version 14.1. Data distribution normality was evaluated using the Shapiro–Wilk test. Data with normal distribution are presented as mean and standard deviation (SD) and data with a non-normal distribution are described as median and interquartile range (IQR). Moreover, qualitative data are expressed as number and percentage.
To compare patients in different groups, t-test, chi-square, and Fisher's exact tests were applied to variables with a normal distribution and Mann–Whitney U test and rank sum test were used for variables with a non-normal distribution. The level of significance was set at 0.05. Logistic regression analysis with odds ratio (OR) and confidence interval was used to evaluate the predictors of mortality in hospitalized patients. In the regression logistic model, the outcome variable was the occurrence of death and other variables were considered as independent variables. First, the variables were entered into a univariate model separately. In case of p < 0.2, they were included in the multivariable model. The multivariable model was done using the “forward” approach. In the multivariable model, adjusted OR was estimated after adjusting for the effect of other variables in the model.
ResultsDuring the study, 715 patients with a diagnosis of COVID-19 were admitted to Farabi Hospital, of whom 10 were excluded due to duplicate data or re-hospitalization and 40 were excluded due to incomplete data or a final diagnosis other than COVID-19 infection.
In total, 665 patients with a mean age of 58.7 ± 18.3 years including 265 (39.8%) females and 400 males (60.2%) were enrolled including 479 confirmed (72%), 156 probable (23.5%), and 30 suspected cases (4.5%). Of 655 admitted patients with COVID-19, 82 (12%) were deceased and 583 (88%) were discharged. The mean age of the deceased and discharged patients was 63.0 ± 17.4 and 58.1 ± 18.3 years respectively, indicating a significant difference (P = 0.02). The majority of the patients were male (60.2%, 400/665); however, gender did not have any significant effects on the outcome (P = 0.57). The most common presentations in the time of admission were dyspnea (92.5%), cough (13.5%), muscle pain (7.7%) and headache (3.9%), which cough and headache were significantly more frequent in patients that survived compared to the deceased (p = 0.03 and p = 0.04, respectively).
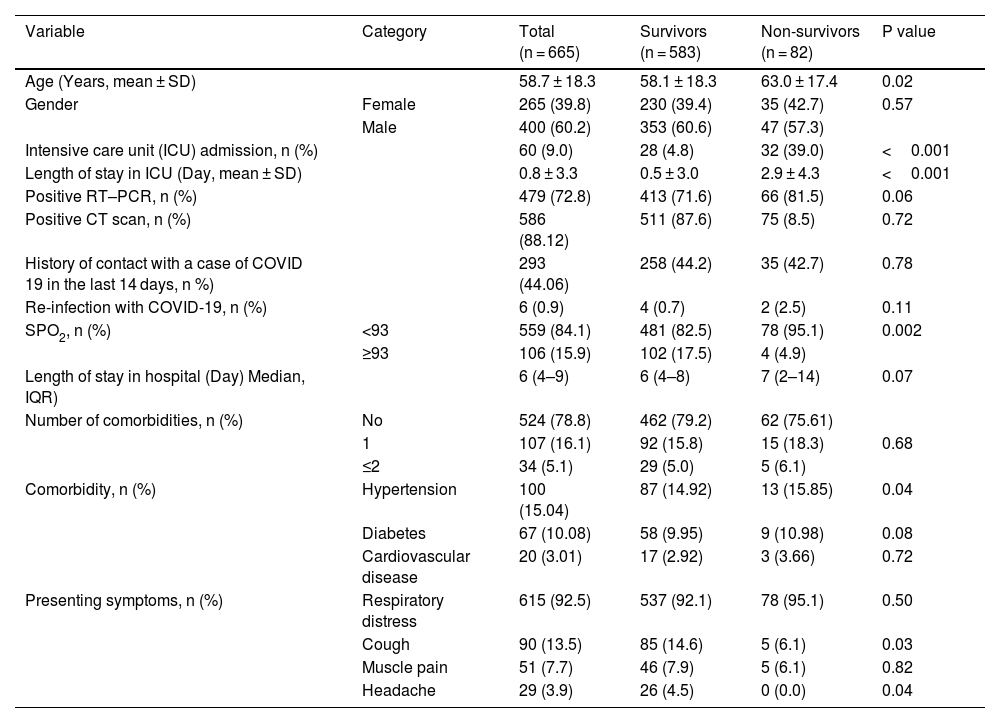
Hypertension (15%), diabetes (10%), and cardiovascular disease (3%) were the most frequent comorbidities. The frequency of hypertension was significantly higher in the deceased compared to survived patients (p = 0.04). In total, the SPO2 level was below 93% in 559 (84%) and above 93% in 106 hospitalized patients (16%). The SPO2 level was below 93% in 82.5% of the survived and 95.1% of the deceased patients, indicating a significant difference (p = 0.002). Sixty patients (9%) were admitted to the ICU of whom 39% (n = 32) died. The median length of stay was 6 (IQR: 4–9) days, no significant difference was observed in the median length of stay between the two groups (p = 0.07). Table 1 presents the demographic characteristics, admission-related variables, and disease outcome between the discharged and deceased patients.
Clinical and demographic characteristics for 665 cases involved in this study.
| Variable | Category | Total (n = 665) | Survivors (n = 583) | Non-survivors (n = 82) | P value |
|---|---|---|---|---|---|
| Age (Years, mean ± SD) | 58.7 ± 18.3 | 58.1 ± 18.3 | 63.0 ± 17.4 | 0.02 | |
| Gender | Female | 265 (39.8) | 230 (39.4) | 35 (42.7) | 0.57 |
| Male | 400 (60.2) | 353 (60.6) | 47 (57.3) | ||
| Intensive care unit (ICU) admission, n (%) | 60 (9.0) | 28 (4.8) | 32 (39.0) | <0.001 | |
| Length of stay in ICU (Day, mean ± SD) | 0.8 ± 3.3 | 0.5 ± 3.0 | 2.9 ± 4.3 | <0.001 | |
| Positive RT–PCR, n (%) | 479 (72.8) | 413 (71.6) | 66 (81.5) | 0.06 | |
| Positive CT scan, n (%) | 586 (88.12) | 511 (87.6) | 75 (8.5) | 0.72 | |
| History of contact with a case of COVID 19 in the last 14 days, n %) | 293 (44.06) | 258 (44.2) | 35 (42.7) | 0.78 | |
| Re-infection with COVID-19, n (%) | 6 (0.9) | 4 (0.7) | 2 (2.5) | 0.11 | |
| SPO2, n (%) | <93 | 559 (84.1) | 481 (82.5) | 78 (95.1) | 0.002 |
| ≥93 | 106 (15.9) | 102 (17.5) | 4 (4.9) | ||
| Length of stay in hospital (Day) Median, IQR) | 6 (4–9) | 6 (4–8) | 7 (2–14) | 0.07 | |
| Number of comorbidities, n (%) | No | 524 (78.8) | 462 (79.2) | 62 (75.61) | |
| 1 | 107 (16.1) | 92 (15.8) | 15 (18.3) | 0.68 | |
| ≤2 | 34 (5.1) | 29 (5.0) | 5 (6.1) | ||
| Comorbidity, n (%) | Hypertension | 100 (15.04) | 87 (14.92) | 13 (15.85) | 0.04 |
| Diabetes | 67 (10.08) | 58 (9.95) | 9 (10.98) | 0.08 | |
| Cardiovascular disease | 20 (3.01) | 17 (2.92) | 3 (3.66) | 0.72 | |
| Presenting symptoms, n (%) | Respiratory distress | 615 (92.5) | 537 (92.1) | 78 (95.1) | 0.50 |
| Cough | 90 (13.5) | 85 (14.6) | 5 (6.1) | 0.03 | |
| Muscle pain | 51 (7.7) | 46 (7.9) | 5 (6.1) | 0.82 | |
| Headache | 29 (3.9) | 26 (4.5) | 0 (0.0) | 0.04 |
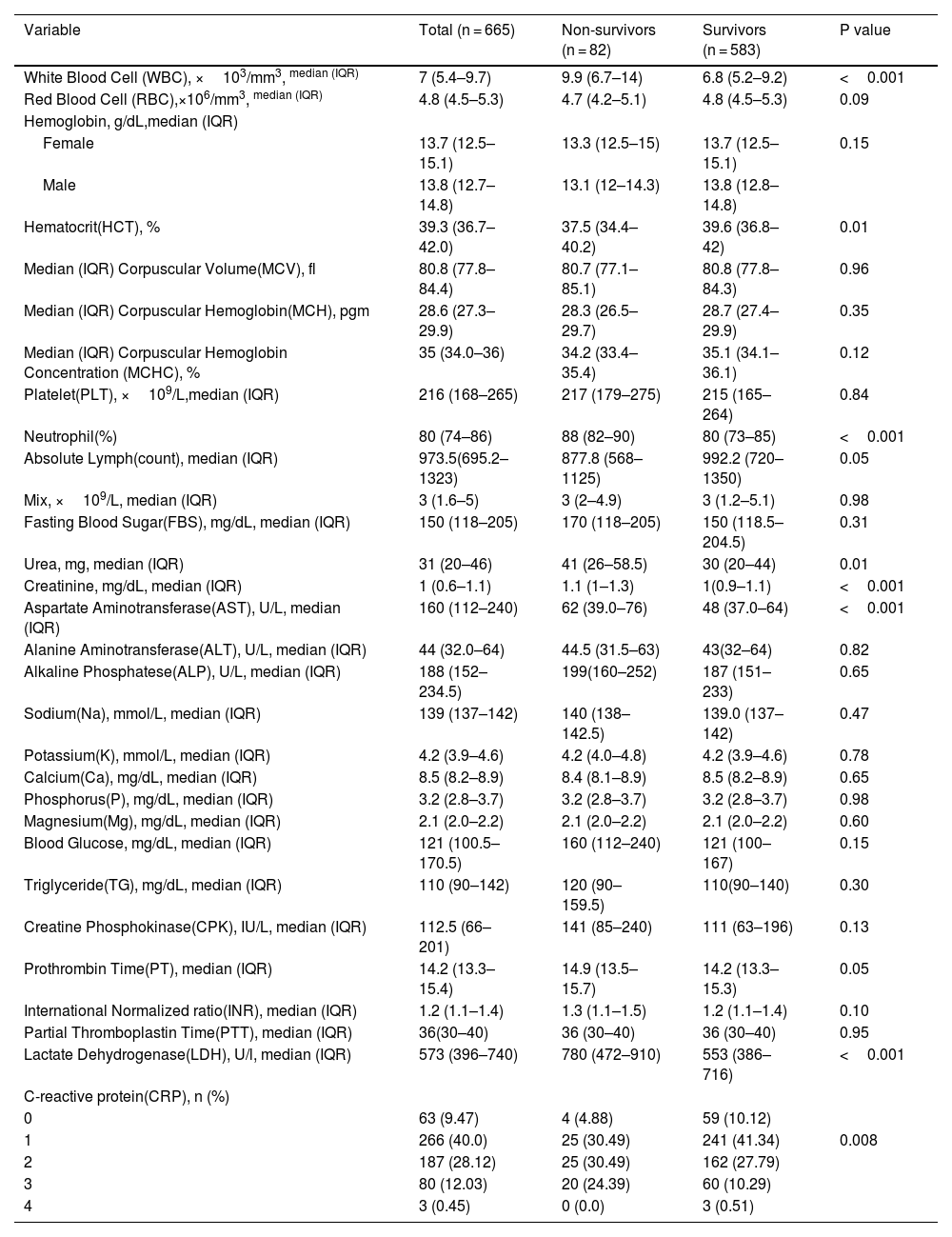
On admission, patients that eventually died had significantly higher levels of WBC, Urea, Cr, AST, PT, LDH and neutrophil percentage and lower levels of HCT and absolute lymphocyte count compared to those that survived (p < 0.05). Moreover, a significant difference was observed in CRP between the discharged and deceased patients (p = 0.008). There were no significant differences in other variables between the two groups (Table 2).
Laboratory findings of hospitalized COVID-19 patients (n = 665).
| Variable | Total (n = 665) | Non-survivors (n = 82) | Survivors (n = 583) | P value |
|---|---|---|---|---|
| White Blood Cell (WBC), ×103/mm3, median (IQR) | 7 (5.4–9.7) | 9.9 (6.7–14) | 6.8 (5.2–9.2) | <0.001 |
| Red Blood Cell (RBC),×106/mm3, median (IQR) | 4.8 (4.5–5.3) | 4.7 (4.2–5.1) | 4.8 (4.5–5.3) | 0.09 |
| Hemoglobin, g/dL,median (IQR) | ||||
| Female | 13.7 (12.5–15.1) | 13.3 (12.5–15) | 13.7 (12.5–15.1) | 0.15 |
| Male | 13.8 (12.7–14.8) | 13.1 (12–14.3) | 13.8 (12.8–14.8) | |
| Hematocrit(HCT), % | 39.3 (36.7–42.0) | 37.5 (34.4–40.2) | 39.6 (36.8–42) | 0.01 |
| Median (IQR) Corpuscular Volume(MCV), fl | 80.8 (77.8–84.4) | 80.7 (77.1–85.1) | 80.8 (77.8–84.3) | 0.96 |
| Median (IQR) Corpuscular Hemoglobin(MCH), pgm | 28.6 (27.3–29.9) | 28.3 (26.5–29.7) | 28.7 (27.4–29.9) | 0.35 |
| Median (IQR) Corpuscular Hemoglobin Concentration (MCHC), % | 35 (34.0–36) | 34.2 (33.4–35.4) | 35.1 (34.1–36.1) | 0.12 |
| Platelet(PLT), ×109/L,median (IQR) | 216 (168–265) | 217 (179–275) | 215 (165–264) | 0.84 |
| Neutrophil(%) | 80 (74–86) | 88 (82–90) | 80 (73–85) | <0.001 |
| Absolute Lymph(count), median (IQR) | 973.5(695.2–1323) | 877.8 (568–1125) | 992.2 (720–1350) | 0.05 |
| Mix, ×109/L, median (IQR) | 3 (1.6–5) | 3 (2–4.9) | 3 (1.2–5.1) | 0.98 |
| Fasting Blood Sugar(FBS), mg/dL, median (IQR) | 150 (118–205) | 170 (118–205) | 150 (118.5–204.5) | 0.31 |
| Urea, mg, median (IQR) | 31 (20–46) | 41 (26–58.5) | 30 (20–44) | 0.01 |
| Creatinine, mg/dL, median (IQR) | 1 (0.6–1.1) | 1.1 (1–1.3) | 1(0.9–1.1) | <0.001 |
| Aspartate Aminotransferase(AST), U/L, median (IQR) | 160 (112–240) | 62 (39.0–76) | 48 (37.0–64) | <0.001 |
| Alanine Aminotransferase(ALT), U/L, median (IQR) | 44 (32.0–64) | 44.5 (31.5–63) | 43(32–64) | 0.82 |
| Alkaline Phosphatese(ALP), U/L, median (IQR) | 188 (152–234.5) | 199(160–252) | 187 (151–233) | 0.65 |
| Sodium(Na), mmol/L, median (IQR) | 139 (137–142) | 140 (138–142.5) | 139.0 (137–142) | 0.47 |
| Potassium(K), mmol/L, median (IQR) | 4.2 (3.9–4.6) | 4.2 (4.0–4.8) | 4.2 (3.9–4.6) | 0.78 |
| Calcium(Ca), mg/dL, median (IQR) | 8.5 (8.2–8.9) | 8.4 (8.1–8.9) | 8.5 (8.2–8.9) | 0.65 |
| Phosphorus(P), mg/dL, median (IQR) | 3.2 (2.8–3.7) | 3.2 (2.8–3.7) | 3.2 (2.8–3.7) | 0.98 |
| Magnesium(Mg), mg/dL, median (IQR) | 2.1 (2.0–2.2) | 2.1 (2.0–2.2) | 2.1 (2.0–2.2) | 0.60 |
| Blood Glucose, mg/dL, median (IQR) | 121 (100.5–170.5) | 160 (112–240) | 121 (100–167) | 0.15 |
| Triglyceride(TG), mg/dL, median (IQR) | 110 (90–142) | 120 (90–159.5) | 110(90–140) | 0.30 |
| Creatine Phosphokinase(CPK), IU/L, median (IQR) | 112.5 (66–201) | 141 (85–240) | 111 (63–196) | 0.13 |
| Prothrombin Time(PT), median (IQR) | 14.2 (13.3–15.4) | 14.9 (13.5–15.7) | 14.2 (13.3–15.3) | 0.05 |
| International Normalized ratio(INR), median (IQR) | 1.2 (1.1–1.4) | 1.3 (1.1–1.5) | 1.2 (1.1–1.4) | 0.10 |
| Partial Thromboplastin Time(PTT), median (IQR) | 36(30–40) | 36 (30–40) | 36 (30–40) | 0.95 |
| Lactate Dehydrogenase(LDH), U/l, median (IQR) | 573 (396–740) | 780 (472–910) | 553 (386–716) | <0.001 |
| C-reactive protein(CRP), n (%) | ||||
| 0 | 63 (9.47) | 4 (4.88) | 59 (10.12) | |
| 1 | 266 (40.0) | 25 (30.49) | 241 (41.34) | 0.008 |
| 2 | 187 (28.12) | 25 (30.49) | 162 (27.79) | |
| 3 | 80 (12.03) | 20 (24.39) | 60 (10.29) | |
| 4 | 3 (0.45) | 0 (0.0) | 3 (0.51) |
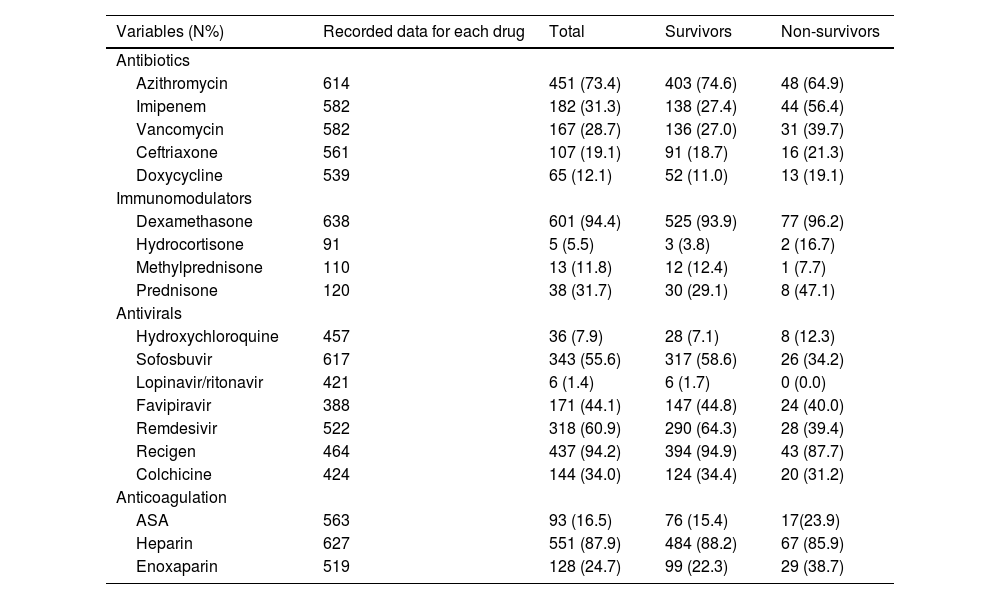
Table 2 summarizes the drugs administered during admission. According to Table 3, azithromycin (73.4%), heparin (87.9%), sovodak (sofosbuvir + daklatasvir) (55.6%), and dexamethasone (94.4%) were the most common prescribed antibiotic, anticoagulant, antiviral, and immune modulator drug, respectively. No significant difference was observed in the prescribed drugs between the two groups (p > 0.05).
Frequency of prescribed treatments and outcomes in hospitalized patients with COVID19 during the third wave.
| Variables (N%) | Recorded data for each drug | Total | Survivors | Non-survivors |
|---|---|---|---|---|
| Antibiotics | ||||
| Azithromycin | 614 | 451 (73.4) | 403 (74.6) | 48 (64.9) |
| Imipenem | 582 | 182 (31.3) | 138 (27.4) | 44 (56.4) |
| Vancomycin | 582 | 167 (28.7) | 136 (27.0) | 31 (39.7) |
| Ceftriaxone | 561 | 107 (19.1) | 91 (18.7) | 16 (21.3) |
| Doxycycline | 539 | 65 (12.1) | 52 (11.0) | 13 (19.1) |
| Immunomodulators | ||||
| Dexamethasone | 638 | 601 (94.4) | 525 (93.9) | 77 (96.2) |
| Hydrocortisone | 91 | 5 (5.5) | 3 (3.8) | 2 (16.7) |
| Methylprednisone | 110 | 13 (11.8) | 12 (12.4) | 1 (7.7) |
| Prednisone | 120 | 38 (31.7) | 30 (29.1) | 8 (47.1) |
| Antivirals | ||||
| Hydroxychloroquine | 457 | 36 (7.9) | 28 (7.1) | 8 (12.3) |
| Sofosbuvir | 617 | 343 (55.6) | 317 (58.6) | 26 (34.2) |
| Lopinavir/ritonavir | 421 | 6 (1.4) | 6 (1.7) | 0 (0.0) |
| Favipiravir | 388 | 171 (44.1) | 147 (44.8) | 24 (40.0) |
| Remdesivir | 522 | 318 (60.9) | 290 (64.3) | 28 (39.4) |
| Recigen | 464 | 437 (94.2) | 394 (94.9) | 43 (87.7) |
| Colchicine | 424 | 144 (34.0) | 124 (34.4) | 20 (31.2) |
| Anticoagulation | ||||
| ASA | 563 | 93 (16.5) | 76 (15.4) | 17(23.9) |
| Heparin | 627 | 551 (87.9) | 484 (88.2) | 67 (85.9) |
| Enoxaparin | 519 | 128 (24.7) | 99 (22.3) | 29 (38.7) |
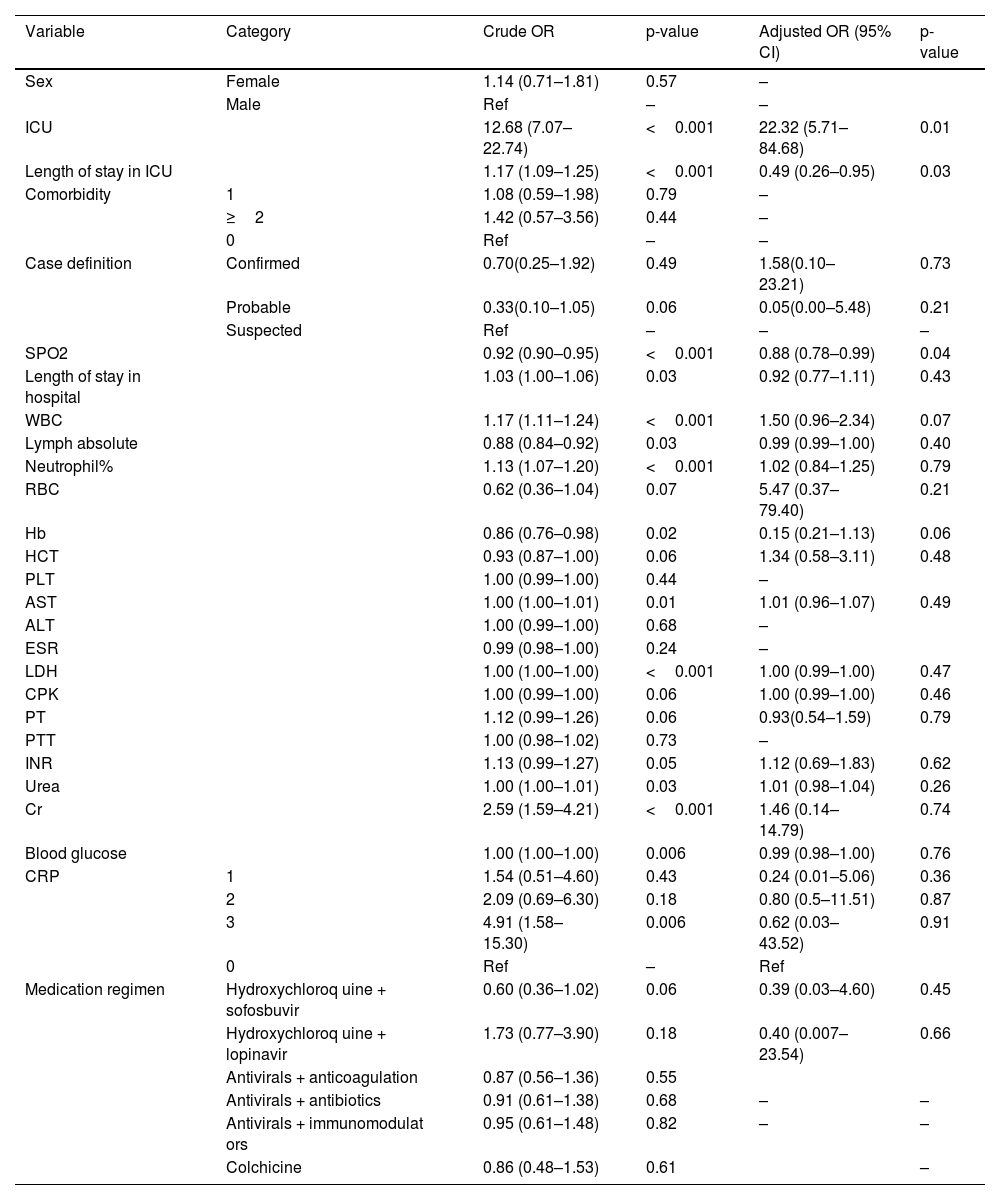
According to univariate logistic regression analysis, the variables that had a significant association with the odds of mortality in COVID-19 included ICU admission (cOR 12.68, 95% confidence interval [CI] 7.07–22.74, p < 0.001), length of ICU (cOR 1.17, 95% CI 1.09–1.25, p < 0.001) and hospital stay (cOR 1.03, 95% CI 1.00–1.06, p = 0.03), absolute lymphocyte count (cOR 0.88, 95% CI 0.84–0.92, p = 0.03), neutrophil percentage (cOR 1.13, 95% CI 1.07–1.20, p < 0.001), and blood glucose level (cOR 1.00, 95% CI 1.00–1.00, p = 0.006). The results of multivariable logistic regression showed that each one-unit increase in SPO2 increased the odds of survival by 0.88 times (95% CI 0.78–0.99, p = 0.04). Moreover, each one day increase in the length of ICU stay reduced the odds of mortality by 0.49 times (95% CI 0.26–0.95, p = 0.03) (Table 4).
Related factors associated with death in hospitalized COVID-19 patients.
| Variable | Category | Crude OR | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|---|
| Sex | Female | 1.14 (0.71–1.81) | 0.57 | – | |
| Male | Ref | – | – | ||
| ICU | 12.68 (7.07–22.74) | <0.001 | 22.32 (5.71–84.68) | 0.01 | |
| Length of stay in ICU | 1.17 (1.09–1.25) | <0.001 | 0.49 (0.26–0.95) | 0.03 | |
| Comorbidity | 1 | 1.08 (0.59–1.98) | 0.79 | – | |
| ≥2 | 1.42 (0.57–3.56) | 0.44 | – | ||
| 0 | Ref | – | – | ||
| Case definition | Confirmed | 0.70(0.25–1.92) | 0.49 | 1.58(0.10–23.21) | 0.73 |
| Probable | 0.33(0.10–1.05) | 0.06 | 0.05(0.00–5.48) | 0.21 | |
| Suspected | Ref | – | – | – | |
| SPO2 | 0.92 (0.90–0.95) | <0.001 | 0.88 (0.78–0.99) | 0.04 | |
| Length of stay in hospital | 1.03 (1.00–1.06) | 0.03 | 0.92 (0.77–1.11) | 0.43 | |
| WBC | 1.17 (1.11–1.24) | <0.001 | 1.50 (0.96–2.34) | 0.07 | |
| Lymph absolute | 0.88 (0.84–0.92) | 0.03 | 0.99 (0.99–1.00) | 0.40 | |
| Neutrophil% | 1.13 (1.07–1.20) | <0.001 | 1.02 (0.84–1.25) | 0.79 | |
| RBC | 0.62 (0.36–1.04) | 0.07 | 5.47 (0.37–79.40) | 0.21 | |
| Hb | 0.86 (0.76–0.98) | 0.02 | 0.15 (0.21–1.13) | 0.06 | |
| HCT | 0.93 (0.87–1.00) | 0.06 | 1.34 (0.58–3.11) | 0.48 | |
| PLT | 1.00 (0.99–1.00) | 0.44 | – | ||
| AST | 1.00 (1.00–1.01) | 0.01 | 1.01 (0.96–1.07) | 0.49 | |
| ALT | 1.00 (0.99–1.00) | 0.68 | – | ||
| ESR | 0.99 (0.98–1.00) | 0.24 | – | ||
| LDH | 1.00 (1.00–1.00) | <0.001 | 1.00 (0.99–1.00) | 0.47 | |
| CPK | 1.00 (0.99–1.00) | 0.06 | 1.00 (0.99–1.00) | 0.46 | |
| PT | 1.12 (0.99–1.26) | 0.06 | 0.93(0.54–1.59) | 0.79 | |
| PTT | 1.00 (0.98–1.02) | 0.73 | – | ||
| INR | 1.13 (0.99–1.27) | 0.05 | 1.12 (0.69–1.83) | 0.62 | |
| Urea | 1.00 (1.00–1.01) | 0.03 | 1.01 (0.98–1.04) | 0.26 | |
| Cr | 2.59 (1.59–4.21) | <0.001 | 1.46 (0.14–14.79) | 0.74 | |
| Blood glucose | 1.00 (1.00–1.00) | 0.006 | 0.99 (0.98–1.00) | 0.76 | |
| CRP | 1 | 1.54 (0.51–4.60) | 0.43 | 0.24 (0.01–5.06) | 0.36 |
| 2 | 2.09 (0.69–6.30) | 0.18 | 0.80 (0.5–11.51) | 0.87 | |
| 3 | 4.91 (1.58–15.30) | 0.006 | 0.62 (0.03–43.52) | 0.91 | |
| 0 | Ref | – | Ref | ||
| Medication regimen | Hydroxychloroq uine + sofosbuvir | 0.60 (0.36–1.02) | 0.06 | 0.39 (0.03–4.60) | 0.45 |
| Hydroxychloroq uine + lopinavir | 1.73 (0.77–3.90) | 0.18 | 0.40 (0.007–23.54) | 0.66 | |
| Antivirals + anticoagulation | 0.87 (0.56–1.36) | 0.55 | |||
| Antivirals + antibiotics | 0.91 (0.61–1.38) | 0.68 | – | – | |
| Antivirals + immunomodulat ors | 0.95 (0.61–1.48) | 0.82 | – | – | |
| Colchicine | 0.86 (0.48–1.53) | 0.61 | – |
While the second peak of COVID-19 started after a slight fall relative to the first peak in Iran, the second and third peaks followed each other closely so that the third peak started in late September 2020 and continued through January 2021. In late August, concurrent with the opening of schools, in addition to a reduction in observing health protocols and changes in the social behavior of people, some asymptomatic carriers embarked on travels. Moreover, despite formulating strict protocols for holding Muharram ceremonies (commemoration rituals of Shia Muslims), some people did not observe them and became infected. With an increase in the daily death toll, very strict measures were applied for COVID-19 management starting from late November, including night and out-of-state travel restrictions, minimum staff presence at workplace, and closure of many guilds. The present study was conducted to describe the clinical characteristics and outcomes of COVID-19 patients admitted to a referral hospital in the west of Iran during the third wave of the epidemic. It should be noted that mass vaccination did not start in Iran until after the third wave. According to the results of the present study, ICU admission were associated with an increase in the odds of mortality while a higher SPO2 and increased length of ICU was associated with a significantly lower risk of in-hospital mortality.
In a previous study published from Kermanshah Province during the first year of COVID-19 epidemic (peaks one to three), 9.71% of the patients died while 12% of the patients died in the present study.10 These results also are in contrast to studies conducted during the second peak of COVID-19 in Denmark, and Netherlands (concurrent with the third peak in Iran),11,12 which showed a significant decrease in the mortality rate, possibly due to the concurrence of Alpha variant with mass vaccination in some countries. Another possible reason for the increased mortality rate during the third COVID-19 peak in Iran may be an increase in the number of patients with severe infection. Eighty-five percent of the patients had SPO2 < 93% (versus 62% in the first year of the pandemic in Kermanshah Province), possibly due to different factors such as a new variant with more invasive characteristics or simply because the patients presented to the hospital later after the onset of symptoms.
In the present study, only 21.2% of the admitted patients and 24.39% of the deceased had comorbidities which were less than those reported in large cohorts studies carried out in other countries such as Austria,13 Denmark11 and Korea.14 Also, we found no differences in age nor in frequency of comorbidity between the discharged and deceased patients. An reasonable explanation could be that our insight on this epidemic became deeper over time and thus high-risk populations were identified and offered more protection. Therefore, all individuals, regardless of age, gender and occupation were susceptible to infection or death due to SARS CoV-2.
The third wave of the COVID-19 pandemic had a large impact on ICU occupancy so that in an attempt to maintain pre-epidemic standards of care, new ICU beds were located in wards other than ICUs and more staff from other wards were recruited. However, during the third wave of COVID -19, our hospital was not able to increase the ICU capacity as needed. In the current study, the overall in-hospital mortality and ICU mortality were 12.33% and 39%, respectively. The high in-hospital mortality rate may be related to the increased severity of illness in ICU admission, i.e., patients who received non-invasive respiratory support were likely to stay out of the ICU due to low ICU capacity, and more patients requiring intubation were admitted, and this has led to an increase in both in-ICU and out-ICU mortality, as well as an increase in overall in-hospital mortality.15
Considering the results of the Recovery trial that showed lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone, corticosteroids were extensively used during the third wave so that 94.4% of the patients received dexamethasone during hospitalization in the present study.16 Moreover, a number of clinical trials have shown that remdesivir administration is associated with shorter recovery time, less need for mechanical ventilation, and lower 28-day mortality in hospitalized patients when compared to placebo17,18. Therefore, since 60% of the patients received remdesivir in the present study, this could explain the mortality rate. Another breakthrough in the management of the third wave of COVID-19 pandemic was the use of anticoagulants and proning position.19 High consumption of azithromycin, doxycycline, hydroxychloroquine, and other antibiotics was observed in the present study. Although antibiotic therapy is not recommended in COVID-19, more than 90% of the patients received this treatment. No significant difference was observed in the prescribed drugs between the discharged and deceased patients. The irrational use of antibiotics has always been a major concern in Iran.20 In a multicenter cohort study during the first six months of the COVID-19 pandemic in Iran, it was estimated that each patient received 1.21 DDDs of antibiotics every day.21 Our study highlighted the need for judicious use of antibiotics in the management of COVID-19 by strengthening antimicrobial surveillance programs.
Despite significant differences in some lab variables between the discharged and deceased patients, none of the differences was significant after adjustment for other variables (Table 1). However, the plasma glucose (as an index of diabetes control) and creatinine levels were higher in the deceased versus discharged patients; nonetheless, the difference was not significant after adjustment for other variables. This finding was consistent with previous reports of higher plasma glucose and creatinine levels in critically ill COVID-19 patients.22,23
This study has some limitations, including a small sample size and a retrospective design. Moreover, during the third wave, we picked a one-month period from the middle of the wave that was concurrent with the peak to enroll a sufficient number of patients. Therefore, the results may not be extrapolated to the whole wave and caution should be exercised in interpreting the results.
In conclusion, hospitalized COVID-19 patients generally presented more severe illness during the third peak so that about 85% of the patients had oxygen saturation level below 93% and the in-hospital mortality rate was higher. Moreover, the results showed high rates of antibiotic prescription. Demographic and paraclinical variables (except SPO2 level) were not suitable predictors of mortality.
Funding sourceThis study was supported by a grant from the Vice Chancellery for Research and Technology, Kermanshah University of Medical Sciences (grant number: 4000675).
Ethics approval and consent to participateThe protocol was approved by the Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.MED.REC.1400.001).
We also like to acknowledge the cooperation of Vice Chancellor for Research of Kermanshah University of Medical Sciences. This article was part of a MD dissertation (by Younes Jesmani) supported by Kermanshah University of Medical Sciences (KUMS).