Editado por: Dra. Núria Torner CIBER Epidemiologia y Salud Publica CIBERESP Unitat de Medicina Preventiva i Salut Pública Departament de Medicina, Universitat de Barcelona
Más datosThis study aimed to see how measles, mumps, and rubella vaccination affected the increase in SARS-CoV-2 immunoglobulin levels in individuals who had received the second dose of the SARS-CoV-2 vaccine.
Material and methodThis research is a quasi-experimental type with a pre-post test design. The population studied were adults who had received the second dose of the SARS-CoV-2 vaccine, consisting of 30 people.
ResultsThe results of this study were that most (60%) research subjects experienced an increase in IgM and some subjects (46.6%) experienced an increase in anti-SARS-CoV-2 IgG levels. The administration of the MR vaccination had no effect on increasing anti-SARS-CoV-2 IgG and IgM levels. This happened because the increase in IgM and IgG levels in the pre and post-tests in most research subjects was relatively low.
ConclusionThe administration of the MR vaccine to adults who had received a second dose of the SARS-CoV-2 vaccine elicited a response with low levels of IgG and IgM SARS-CoV-2.
Este estudio tuvo como objetivo ver cómo la vacunación contra el sarampión, las paperas y la rubéola afectó el aumento de los niveles de inmunoglobulina contra el SARS-CoV-2 en personas que habían recibido la segunda dosis de la vacuna contra el SARS-CoV-2.
Material y MétodoEsta investigación es de tipo cuasi-experimental con un diseño pre-post test. La población estudiada fueron adultos que habían recibido la segunda dosis de la vacuna contra el SARS-CoV-2, conformada por 30 personas.
ResultadosLos resultados de este estudio fueron que la mayoría de los sujetos de investigación (60%) experimentaron un aumento de IgM y algunos sujetos (46,6%) experimentaron un aumento en los niveles de IgG anti-SARS-CoV-2. Según los resultados de la prueba T pareada, el valor de p fue superior a 0,05, lo que indica que no hubo cambios significativos en los niveles de IgG e IgM antes y después de la vacunación con MR. Esto sucedió porque el aumento en los niveles de IgM e IgG en las pruebas previas y posteriores en la mayoría de los sujetos de investigación fue relativamente bajo.
ConclusiónLa administración de la vacuna MR a adultos que habían recibido una segunda dosis de la vacuna SARS-CoV-2 provocó una respuesta con niveles bajos de IgG e IgM SARS-CoV-2.
The outbreak of respiratory disease caused by the coronavirus is the biggest health problem in the world. This is a new type of coronavirus, namely the 2019-nCoV, or SARS-CoV-2 virus (severe acute respiratory syndrome coronavirus 2).1 This disease was first reported in the Chinese city of Wuhan in December 2019, hence it was called COVID-19, which stands for Coronavirus disease 2019. COVID-19 was originally zoonotic, but it has evolved and now can be transmitted from human to human.2
The incidence of COVID-19 is increasing at the international and national levels. The number of cases of COVID-19 in the world in September 2020 was 28.2 million cases, with a death rate of 910 thousand people. Incidents in Indonesia in September 2020 were 211,000 cases, of these case there were 8544 people who died. East Java Province is an area with a high incidence rate of 48.161 cases.1
Efforts to handle COVID-19 should break the chain between the host, agent, and environmental factors increasing host resistance is a strategy to break the chain. Humans can transfer the COVID-19 virus from one person to another as hosts. Human immune system antibodies will decide whether or not exposure to the virus can result in infection. Several variables influence body immunity, including food intake, stress management, age, and immunization.3
Vaccination is an effective and efficient form of disease prevention to avoid the spread of infectious diseases. Vaccination aims to increase the degree of immunity and induce a memory response to pathogens. Prevention of infectious diseases through vaccination is a step further in immunoprophylaxis efforts for both children and adults.4 Using an online antibody prediction engine, researchers discovered 30 amino acid similarities between the SARS-CoV-2 glycoprotein, the measles virus F1 glycoprotein, and the rubella virus glycoprotein E1. This similarity acts as an epitope involved in antibody production.5 Thus, this study aimed to assess the levels of anti-SARS-CoV-2 IgM before and after MR (Measles and Rubella) immunization in individuals who had received the second dose of the SARS-CoV-2 vaccine.
MethodEthical approvalThis research was a quasi-experimental research with a pre-post test research design conducted for clinical trials of the MR vaccine to determine the immune response of SARS-COV-2 research subjects. This study has been ethically approved by number 159/KEPK-POLKESMA/2021.
Study populationThe population in this study was a group of young adults aged 18–30 years who lived in the Mojoroto and Pesantren sub-districts, Kediri city, East Java, Indonesia. Participant inclusion criteria were men or women aged 18–30 years, had received the SARS-CoV-2 vaccine, and good nutritional status with BMI indicators ≥18.5–22.9. Also fulfills the requirements (indications/contra-indications) for MMR vaccination, i.e. have never received MMR vaccination, did not suffer from other diseases during the study, and had no physical contact with people infected with COVID-19 in the last week during this study. For women, they did not currently pregnant or planning a pregnancy during the study, no history of allergies, thrombocytopenia, ITP (idiopathic, thrombocytopenic, purpura). In addition, they are willing to become research subjects by signing an informed consent. While the exclusion criteria were sufferers of other diseases during the study, physical contact with other people who were infected with COVID-19 and those who withdrew as research subjects. The characteristics of each participant could be seen at Table 1.
Characteristics of each participant.
| Participants | Age(Years old) | Sex | Vaccination | Interval between each vaccine(month) | |||
|---|---|---|---|---|---|---|---|
| COVID-19 | The Interval between COVID-19 vaccination to blood sampling (month) | MR | The Interval between MR vaccination to blood sampling (day) | ||||
| Participant 1 | 21 | female | Sinovac | 3 | M-M-R-II Merck &Co (USA) | 14 | 3 |
| Participant 2 | 20 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 3 | 22 | female | Sinovac | 1 | M-M-R-II Merck &Co (USA) | 14 | 1 |
| Participant 4 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 5 | 20 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 6 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 7 | 20 | female | Sinovac | 4 | M-M-R-II Merck &Co (USA) | 14 | 4 |
| Participant 8 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 9 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 10 | 20 | female | Sinovac | 4 | M-M-R-II Merck &Co (USA) | 14 | 4 |
| Participant 11 | 20 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 12 | 20 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 13 | 20 | female | Sinovac | 4 | M-M-R-II Merck &Co (USA) | 14 | 4 |
| Participant 14 | 20 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 15 | 20 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 16 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 17 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 18 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 19 | 20 | female | Sinovac | 3 | M-M-R-II Merck &Co (USA) | 14 | 3 |
| Participant 20 | 20 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 21 | 20 | female | Sinovac | 3 | M-M-R-II Merck &Co (USA) | 14 | 3 |
| Participant 22 | 20 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 23 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 24 | 21 | female | Sinovac | 3 | M-M-R-II Merck &Co (USA) | 14 | 3 |
| Participant 25 | 20 | female | Sinovac | 3 | M-M-R-II Merck &Co (USA) | 14 | 3 |
| Participant 26 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 27 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 28 | 20 | female | Sinovac | 3 | M-M-R-II Merck &Co (USA) | 14 | 3 |
| Participant 29 | 21 | female | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
| Participant 30 | 21 | male | Sinovac | 2 | M-M-R-II Merck &Co (USA) | 14 | 2 |
The selection of research participants was made by simple random sampling. The selected subjects will had their blood samples taken intravenously in a predetermined laboratory. IgG anti-SARS-CoV-2 and IgM anti-SARS-CoV-2 were examined using microfluidic immunoassays with the reagent from Friend Nanoentek. ECLIA test (Electro Chemilunescense Immunoassay test) was used for anti-SARS-CoV-2 IgG examination.
Statistical analysisA paired two-sample T-test was used for data analysis. The p-value used was 0.05 for indicating whether there was any significant change in IgG and IgM levels before and after MR vaccination. IBMSPSS version 24 was used to analyzed the data.
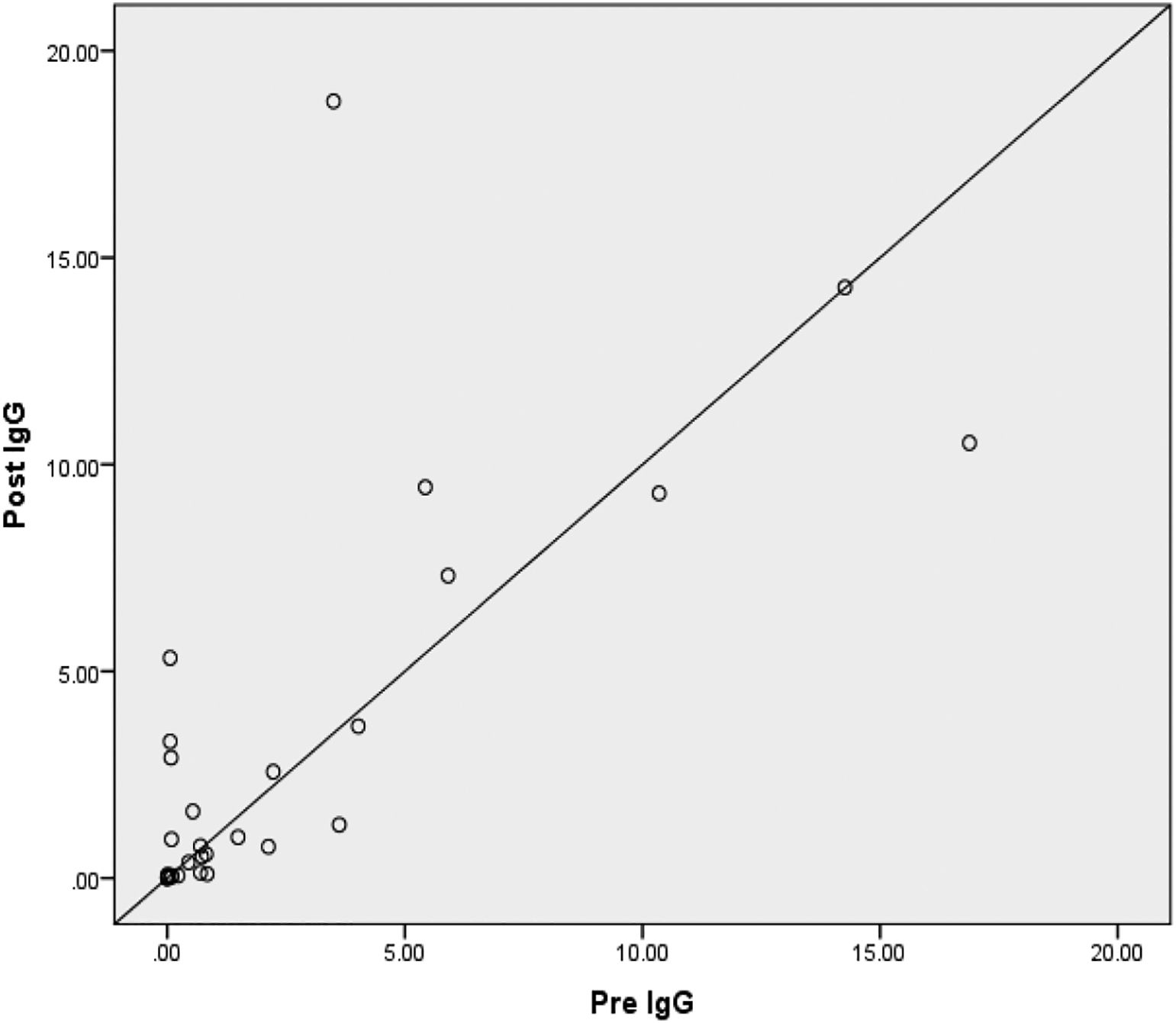
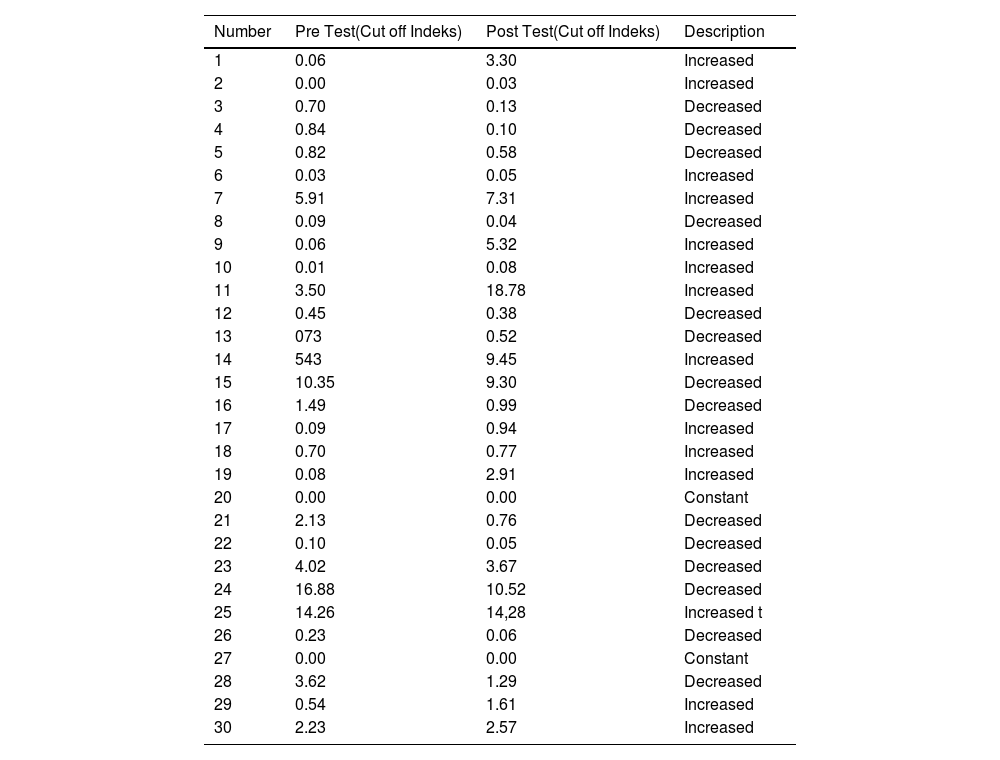
ResultIgG anti-SARS-CoV-2 test results in adultsTable 1 showed that the results of the IgG anti-SARS-CoV-2 test in adults before and after the MR vaccine were 2 subjects (6.6%) did not change, 14 subjects (46.6%) decreased, and 14 subjects (46.6%) experienced an increase. Table 2 shows the complete results of the anti-SARS-CoV-2 IgG test.
Results of Anti-SARS-CoV-2 I IgG Examination.
| Number | Pre Test(Cut off Indeks) | Post Test(Cut off Indeks) | Description |
|---|---|---|---|
| 1 | 0.06 | 3.30 | Increased |
| 2 | 0.00 | 0.03 | Increased |
| 3 | 0.70 | 0.13 | Decreased |
| 4 | 0.84 | 0.10 | Decreased |
| 5 | 0.82 | 0.58 | Decreased |
| 6 | 0.03 | 0.05 | Increased |
| 7 | 5.91 | 7.31 | Increased |
| 8 | 0.09 | 0.04 | Decreased |
| 9 | 0.06 | 5.32 | Increased |
| 10 | 0.01 | 0.08 | Increased |
| 11 | 3.50 | 18.78 | Increased |
| 12 | 0.45 | 0.38 | Decreased |
| 13 | 073 | 0.52 | Decreased |
| 14 | 543 | 9.45 | Increased |
| 15 | 10.35 | 9.30 | Decreased |
| 16 | 1.49 | 0.99 | Decreased |
| 17 | 0.09 | 0.94 | Increased |
| 18 | 0.70 | 0.77 | Increased |
| 19 | 0.08 | 2.91 | Increased |
| 20 | 0.00 | 0.00 | Constant |
| 21 | 2.13 | 0.76 | Decreased |
| 22 | 0.10 | 0.05 | Decreased |
| 23 | 4.02 | 3.67 | Decreased |
| 24 | 16.88 | 10.52 | Decreased |
| 25 | 14.26 | 14,28 | Increased t |
| 26 | 0.23 | 0.06 | Decreased |
| 27 | 0.00 | 0.00 | Constant |
| 28 | 3.62 | 1.29 | Decreased |
| 29 | 0.54 | 1.61 | Increased |
| 30 | 2.23 | 2.57 | Increased |
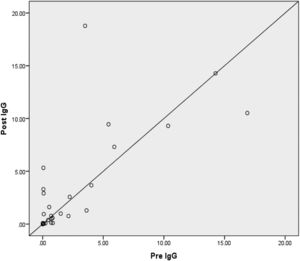
Based on Fig. 1, there is no difference in anti-SARS-CoV-2 IgG levels in respondents before and after giving the MR vaccine. This happens because IgG will increase from the 20th day after the antigen enters the body, while in this study the post test was carried out on the 14th day after administration of the vaccine. So that IgG levels have not shown an increase in the examination. A few days after immunization, serum will appear outside the germinal center (GC), namely IgM and IgG antibodies with low affinity. CD4+ T cells that have differentiated into effector cells will leave the lymph node (LN) to the inflamed tissue (vaccine inoculation site) and carry out their function as an effector function. After the GC reaction ends, the long-lived plasma cells leave the LN and migrate to the bone marrow. During the GC reaction, some of the B cells that have differentiated into memory cells will rest in the extrafollicular area of the LN, and will be active again after receiving a booster immunization. Memory T cells circulate in the T cell zone of the LN, bone marrow or in tissues. Administration of an immunization booster will induce re-activation of T and B memory cells that have been formed during the first immunization. Memory B cells will undergo maturation and differentiate into plasma cells that produce large amounts of high-affinity antibodies.10
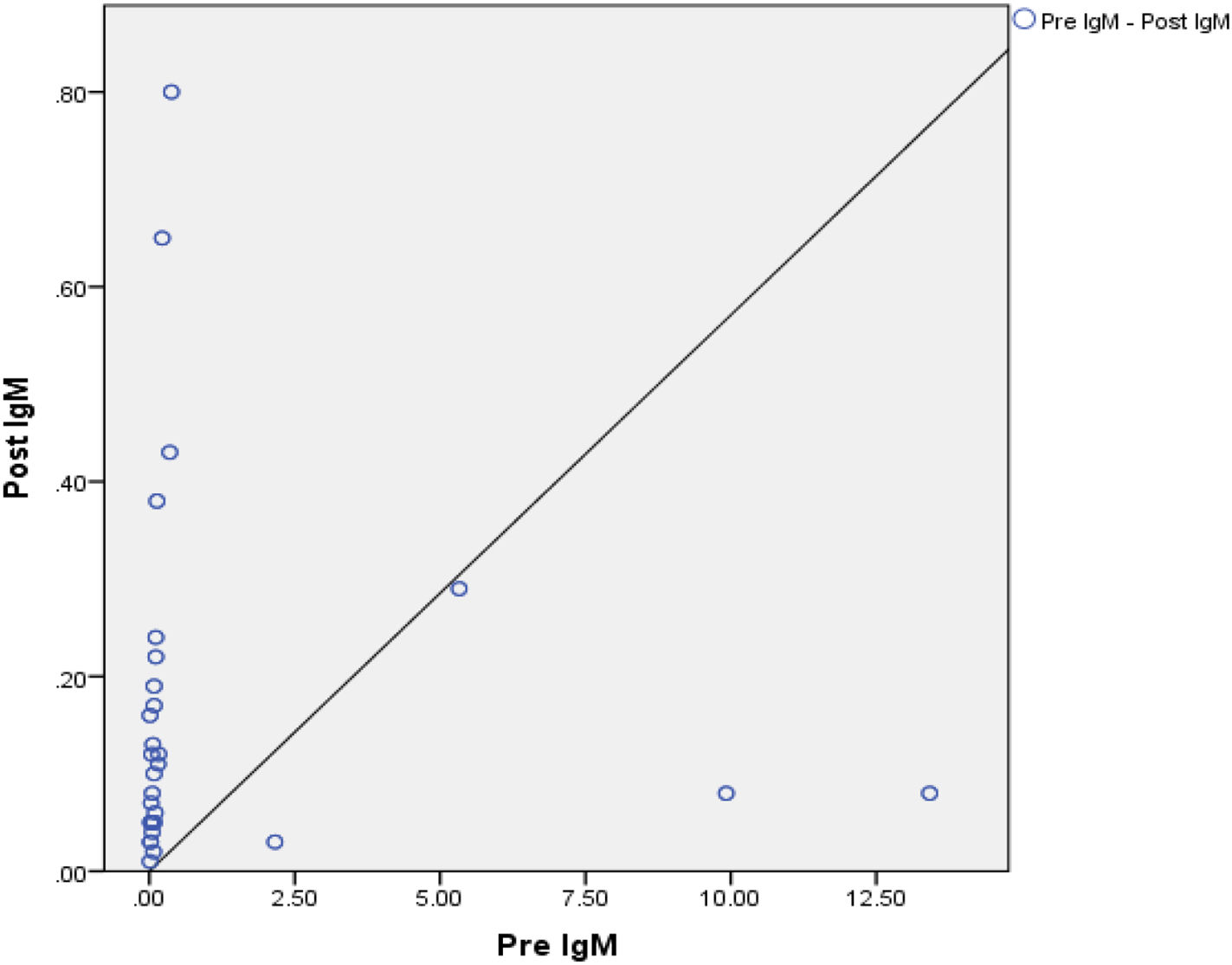
IgM anti-SARS-CoV-2 test results in adultsTable 2 shows the results of the anti-SARS-CoV-2 IgM test before and after the MR vaccine. Two research subjects (6.6%) did not experience a change, 10 research subjects showed (33.3%) decreased, and 18 research subjects (60%) showed an increase in IgM. The complete results of the anti-SARS-CoV-2 IgM test can be seen in Table 3 below.
Anti-SARS-CoV-2. IgM Examination Results.
| Number | Pre Test(Cut off Indeks) | Post Test(Cut off Indeks) | Description |
|---|---|---|---|
| 1 | 9.92 | 0.08 | Decreased |
| 2 | 0.11 | 0.22 | Increased |
| 3 | 0.11 | 0.24 | Increased |
| 4 | 0.38 | 0.80 | Increased |
| 5 | 0.06 | 0.13 | Increased |
| 6 | 0.02 | 0.03 | Increased |
| 7 | 0.05 | 0.08 | Increased |
| 8 | 0.04 | 0.12 | Increased |
| 9 | 13.42 | 0.08 | Decreased |
| 10 | 0.05 | 0.04 | Decreased |
| 11 | 0.22 | 0.65 | Increased |
| 12 | 0.08 | 0.19 | Increased |
| 13 | 0.10 | 0.06 | Decreased |
| 14 | 0.03 | 0.05 | Increased |
| 15 | 0.03 | 0.07 | Increased |
| 16 | 0.16 | 0.12 | Decreased |
| 17 | 2.16 | 0.03 | Decreased |
| 18 | 0.08 | 0.10 | Increased |
| 19 | 5.33 | 0.29 | Decreased |
| 20 | 0.35 | 0.43 | Increased |
| 21 | 0.02 | 0.05 | Increased |
| 22 | 0.05 | 0.05 | Constant |
| 23 | 0.13 | 0.38 | Increased t |
| 24 | 0.15 | 0.11 | Decreased |
| 25 | 0.01 | 0.01 | Constant |
| 26 | 0.08 | 0.05 | Decreased |
| 27 | 0.07 | 0.02 | Decreased |
| 28 | 0.02 | 0.03 | Increased |
| 29 | 0.01 | 0.16 | Increased |
| 30 | 0.08 | 0.17 | Increased |
Source: Laboratorium result.
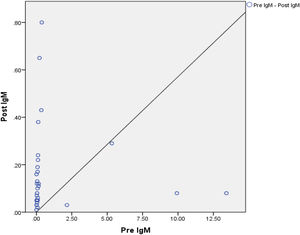
Based on Fig. 2, there is no difference in anti-SARS-CoV-2 IgM levels in respondents before and after being given the MR vaccine. The results of this analysis illustrate that although there was an increase in IgM and IgG levels before and after administration of the MR vaccine, the increase in anti-SARS-CoV-2 IgM levels was quite low. The low anti-SARS-CoV-2 IgM level during the pre-test occurs because the detection of IgM in the serum indicates a person has just been exposed to the virus. In this study, most of the research subjects received exposure to the second dose of vaccine 2 months before the pre-test, so the trend of anti-SARS-CoV-2 IgM test results showed low levels. The IgM examination results during the post test also showed quite low levels. This situation occurs due to the transition of the body's response to IgM production to IgG 14 days after exposure to the MR vaccine. A person's anti-SARS-CoV-2 IgM will be maximally produced for virus-specificity at 9 days after the onset of the disease, which then transitions to IgG which will occur in the second week. Examination of IgM and IgG is specific for SARS-CoV-2, it is important to consider that IgM values tend to disappear within 2 weeks of the start of infection.6
Effect of MR vaccination on SARS-CoV-2 immunoglobulin levels in adultsThe effect of MR vaccination on anti-SARS-Cov-2 immunoglobulin levels was then analyzed using the paired t-test. Based on the results of the paired t-test on IgM levels, a p-value of 0.118 > 0.05 was obtained. There is no significant difference in IgG and IgM values before and after MR vaccination. Analysis of the effect of MR vaccination on SARS-CoV-2 immunoglobulin levels in adults with good nutritional status was carried out using the t-test. The results of this analysis illustrate that although there was an increase in IgM and IgG levels before and after administration of the MR vaccine, the increase in anti-SARS-CoV-2 IgM levels was quite low.
DiscussionThe results from Table 2 indicate that 60% of the research subjects experienced an increase in anti-SARS-CoV-2 IgM levels after the MR vaccination. This shows that the body responds to MR vaccination as a newly recognized SARS-CoV-2 antigen, thereby stimulating the immune system to develop increased levels of anti-SARS-CoV-2 IgM.
Based on the post-test IgM anti-SARS-CoV-2 in Table 2, the research subjects with the MR vaccine showed that none (0%) of the research subjects were reactive to anti-SARS-CoV-2. This situation occurs because of the transition of the body's response to the production of IgM to IgG. A person's anti-SARS-CoV-2 IgM is maximally virus-specific nine days after disease onset, with subsequent transition to IgG occurring within the second week. As IgM and IgG are specific for SARS-CoV-2, it is important to consider that IgM values tend to disappear within 2 weeks of infection onset. Low levels of body antibodies will result in the appearance of symptoms of infection within 14 days of the decrease in antibody levels. As a result, determining when a patient was infected with the virus might be challenging in some situations. As a result, if the immunoglobulin value is not high enough, the test result is a false negative. SARS-CoV-2 infection can potentially spread between asymptomatic individuals with high viral levels. It is therefore difficult to control the virus's transmission.6
The low IgM levels in the second week were caused by the inability of most monoclonal antibodies from the original SARS-CoV to bind to SARS-CoV-2. Limited data from China showed that IgM peaked on day 9, and IgG appeared on the second week. Cross-reactions can be obtained between patients with SARS-CoV-2 and SARS-CoV but not with other coronavirus types.6
IgM molecules are bound by a J chain (Joining chain). Most B cells express IgM on their surface as antigen receptors. When compared to IgG, IgM is generated early during the initial immune response. IgM is generated quickly and gradually replaced by IgG.7
Based on the anti-SARS-CoV-2 IgM examination findings in Table 2, during the pre-test, four subjects (13.3%) tested reactive for anti-SARS-CoV-2. These results indicate the presence of new SARS-C0V-2 infection in the study subjects. However, based on the subjective data collected, none of them reported that they had been in contact with someone who was diagnosed for COVID-19. This situation can occur if a person is in contact with someone who is asymptomatic (OTG). A person who is classified as OTG must self-isolate; however, many of them still gather and socialize as usual. As a result, the risk of possible spread is increasingly unpredictable and unavoidable.8
Anti-SARS-CoV-2 IgG examination in Table 1, there were 30 research subjects carried out using the ECLIA-test method. Research subjects who have received the 2nd SARS-CoV-2 vaccination dose were then given MR vaccination in the next 1 to 4 months. Based on the examination results, 43.3% of the IgG anti-SARS-CoV-2 research subjects showed reactivity to the MR vaccine given during the post-test. Examination of anti-SARS-CoV-2 IgG during the post-test was carried out approximately 14 days after administering the MR vaccine. These results illustrate that 43.3% of the antibodies of the research subjects recognized the MR vaccine as the given SARS-CoV-2 antigen.
In SARS-CoV-2, the antibody response appears more rapidly, although some patients will not have long-lasting antibodies to this virus. The corresponding protection mechanism in SARS-CoV-2 has not been fully established, and the antibody repertoire is not fully understood. The S protein's receptor-binding domain (RBD) was the key target for neutralizing antibodies in the previous SARS-CoV. Most monoclonal antibodies from the old SARS-CoV cannot bind to SARS-CoV-2. Limited data from China showed that IgM peaked on day nine, and IgG appeared on the second week. Cross-reactions can be obtained between patients with SARS-CoV-2 and SARS-CoV but not with other coronaviruses.6
IgG was the most common antibody found in blood and other physiological fluids. When an antigen, such as a bacterium, virus, or particular chemical, enters the body, white blood cells “remember” the antigen and produce IgE antibodies to attack it. As a result, when the antigen re-enters the body, the immune system will quickly detect it and respond because antibodies have already been created. In antigen annihilation, IgG responds and complements act as opsonins. Phagocytic cells, monocytes, and macrophages have receptors for the Fc component of IgG. They can improve the interaction between phagocytes and target cells.7
The results of the anti-SARS-CoV-2 IgG examination at the time of the pre-test showed that 11 research subjects (36.6%) showed reactive results. This Fig. 1 shows the body's response to the previous two doses of vaccination given to research subjects. Based on subjective data, it was obtained that most of the research subjects received the second dose of Sinovac vaccination two months before the pre-test. Anti-SARS-CoV-2 IgG detects chronic SARS-CoV-2 viral infection. In this study, the Sinovac immunization was given to all research volunteers (100%). The inactivated SARS-Cov-2 virus is used in this vaccination. So that after exposure to the SARS-CoV-2 vaccine, IgG can be detected as early as eight days, and seroconversion occurs in the second week after antigen exposure.9
ConclusionMost (60%) research subjects experienced an increase in anti-SARS-CoV-2 IgM levels, 33.3% experienced a decrease in anti-SARS-CoV-2 IgM levels, and 6.66% did not experience any changes in anti-SARS-CoV-2 IgM after treatment, given the MR vaccine. Based on the cut-off IgM index, four research participants (13.3%) were reactive in the pre-test, and 0% of the research subjects were reactive in the post-test.
SuggestionFurther research is needed on changes in IgM in adults by expanding the type of SARS-CoV-2 vaccine used. The research subjects should be monitored further to see trends in IgM and IgG changes that occur later, and the characteristics of respondents need to be expanded to determine changes in IgM and IgG. IgG to the body's response according to the characteristics of research subjects. Furthermore, risk factors for disease occurrence in individuals can be influenced by intrinsic factors which will determine the level of individual susceptibility to disease occurrence. These factors include genetics, gender, age, certain anatomical and physiological factors, and nutrition. Therefore, further research is needed using bigger and more heterogent sample size to represent the real-world population.
LimitationThe limitation of this study are the small sample size, yet it was done by random sampling using informed consent. Beside, the used of single COVID-19 vaccine type and brand, similar age and female preferred participant are also considered as the limitation of this study. Based on the characteristics of the respondents in this study, all respondents were aged 18–30 years, with the gender of the respondents being 96.6% female. So that the research sample has homogeneous characteristics and risk factors for infection.
FundingThis research receives no external funding.
We want to thank all research subjects who have participated in this research, Sukorame Public Health Center, Mojoroto District, North City Health Center, and Kediri City Islamic Boarding School Health Center, who assisted in completing this research. We also thank the Directors of Poltekkes, Ministry of Health, Malang, East Java, Indonesia, who have provided budgetary support to complete this research.