The association between Helicobacter-pylori-induced inflammation and gastric adenocarcinoma is well documented and it has been suggested that the pro-mitotic and apoptotic effect of Cyclooxygenase-2 and Osteopontin on the epithelial cells of the gastric mucosa may have a role in carcinogenesis of the gastric mucosa.
The aim of this study was to determine the expression of Cyclooxygenase-2 and Osteopontin in normal gastric mucosa, mucosa with gastritis and gastric mucosa with intestinal metaplasia, in relation to Helicobacter-pylori infection and grade of inflammation. Immunohistochemistry was performed on 108 gastric biopsies in order to detect Cyclooxygenase-2 and Osteopontin expression. The intensity and percentage of staining were evaluated using the H-Score, and its association with grade of inflammation, Helicobacter pylori infection and intestinal metaplasia was determined. Expression of Cyclooxygenase-2 and Osteopontin was higher in gastric biopsies (values shown respectively) with Helicobacter-pylori infection (179.9/142.3), intestinal metaplasia (208.8/179.3) or higher grades of inflammation (190/135.7) in comparison to normal gastric mucosa (100.7/80) or mild grade of inflammation (128.4/128.4), (p<0.05).There is an overexpression of Cyclooxygenase-2 and Osteopontin in gastric mucosa with H. pylori infection, intestinal metaplasia and high grades of inflammation, suggesting a constant up-regulation of protein expression in response to the inflammatory process generated by a Helicobacter-pylori infection, leading to the development of intestinal metaplasia.
Se ha documentado una asociación entre la inflamación inducida por Helicobacter-pylori y el adenocarcinoma gástrico, con posible participación del efecto pro-mitótico y anti-apoptótico inducido por Ciclo-oxigenasa-2 y Osteopontina en las células epiteliales gástricas. El objetivo de este estudio fue determinar la expresión de estas dos proteínas en mucosa gástrica normal, con gastritis, y, con metaplasia intestinal, en relación con infección por Helicobacter-pylori y el grado de inflamación. Se evaluó la intensidad y porcentaje de células con tinción inmunohistoquímica para Ciclo-oxigenasa-2 y Osteopontina en 108 biopsias gástricas. Se analizó la tinción de cada marcador y su asociación con: grado de inflamación, Helicobacter-pylori, y metaplasia intestinal. Los niveles de expresión de Ciclooxigenasa-2 y Osteopontina respectivamente, fueron superiores en las biopsias con Helicobacter-pylori (179.9, y 142.3), metaplasia intestinal (208.8, y 179.3), o, inflamación severa (190, y 135.7), comparados con la mucosa normal (100.7, y 80), o, la inflamación leve (128.4, y 128.4), (p <0.05). En conclusión, hay sobre-expresión de Ciclooxigenasa-2 y Osteopontina en la mucosa gástrica con Helicobacter-pylori, metaplasia intestinal, e inflamación severa. Esto sugiere sobre-regulación constante de la expresión de estas proteínas en la mucosa gástrica en respuesta al proceso inflamatorio generado por la infección por Helicobacter-pylori, llevando al desarrollo de metaplasia intestinal.
Gastric cancer (GC) is the fifth most common malignancy and the third leading cause of cancer deaths in both sexes worldwide.1 It is estimated that there will be 27,510 new cases of GC in the United States of America in 2019, causing 11,140 deaths.2 Genetic, environmental and infectious factors have been associated with the development of GC3,4 with chronic infection by Helicobacter pylori (H. pylori) proposed as the most important identified risk factor for this neoplasia; this is supported by epidemiological evidence.5,6
The current understanding of gastric oncogenesis includes a model of H. pylori-associated lesions with well-defined sequential stages: chronic active gastritis→chronic atrophic gastritis→intestinal metaplasia→dysplasia and, finally, invasive carcinoma.7–9H. pylori infection is the main cause of chronic gastritis,10,11 which in the long term produces chronic multifocal atrophic gastritis that propitiates cellular dedifferentiation with posterior development of gastric cancer.4,12–17 However, gastric carcinogenesis cannot be explained exclusively by H. pylori infection; despite the eradication of infection, gastric cancer may occur due to the continuous progression of pre-malignant lesions or irreversible changes associated with inflammation in the gastric mucosa.3,14,18,19 In a small percentage of patients these changes may initiate chronic atrophic inflammatory changes accompanied by increased gastrin expression and release, enhancement of cyclooxygenase-2 (COX-2) expression and prostaglandin generation, as well as numerous morphological and biochemical changes leading to the transformation of mucosal cells into malignant cells.20
Cyclooxygenase-2 is an enzyme involved in prostaglandin synthesis, metabolizing arachidonic acid to prostaglandins and thromboxanes, COX-2 mRNA and protein is undetectable in normal tissues but frequently found in activated macrophages and other cells at the site of inflammation, as well as in human malignancies and animal models of carcinogenesis.20,21 It is expressed constitutively in several types of cancer, predominantly by stromal cells, promoting tumor growth and metastasis, and can be induced in many cell types by the action of mitogens, growing factors and tumor promoters.21 The direct role of COX-2 in gastric re-epithelialization and its overexpression in chronic inflammation processes has been described. It has also been suggested that selective COX-2 inhibitors reduce the inflammation and thus could block the progression of premalignant lesions in the gastrointestinal tract, by partially suppressing cell proliferation and inducing apoptosis.22,23
Osteopontin (OPN) is a glycophosphoprotein that mediates cell adhesion and stimulates the production of metalloproteinases and it is believed to play an important role in the survival of tumor cells and metastatic processes.24–26
COX-2 and OPN are involved in processes of inflammation, cellular proliferation and angiogenesis, in response to cytokines and growth factors, and have been described as possible participants in gastric carcinogenesis.22,27–29 Overexpression of both proteins has been found in gastric lesions associated with alterations of cell cycle, increased cell proliferation and angiogenesis, inhibition of apoptosis, weakening of intercellular joints and underlying connective tissue, all of which have been related to the progression of premalignant lesions to cancer.24,25 However, their relation to the development and progression of gastric precancerous lesions and their association with H. pylori infection and grade of inflammation have not as yet been established. The purpose of this study was to determine the expression level of COX-2 and OPN in normal gastric mucosa, mucosa with gastritis and gastric mucosa with intestinal metaplasia in relation to H. pylori infection, and grade of inflammation.
Materials and methodsStudy design and samples selectionA descriptive study was conducted at the Hospital Universitario del Caribe, and the University of Cartagena, Colombia. 120 archived formalin-fixed and paraffin-embedded (FFPE) tissues from gastric biopsies were selected from the Pathology Department in the Hospital Universitario del Caribe, Cartagena, Colombia. These samples included normal gastric mucosa (n=30), gastric mucosa with chronic gastritis (n=30), gastric mucosa with chronic gastritis and H. pylori infection, (n=30), and gastric mucosa with chronic gastritis and intestinal metaplasia (n=30). There were no cases with H. Pylori infection and intestinal metaplasia. Two pathologists (I.B., L.B.) unaware of the original diagnoses examined the hematoxylin-eosin (H&E) stained tissue sections and assigned each sample to the corresponding group according to their histologic characteristics. COX-2 and OPN expression were determined in all the samples by immunohistochemical staining, and their association to Helicobacter pylori infection and grade of inflammation was evaluated.
Histological evaluationTissue sections stained with H&E were evaluated by two pathologists to determine the presence of normal gastric mucosa, chronic gastritis and intestinal metaplasia. A diagnosis of H pylori infection was made when the bacteria were seen on histopathological examination by identification of characteristic curved or spiral bacilli. Chronic inflammation was evaluated according to the Updated Sidney system, and scored as mild, moderate or severe inflammation.30 In case of discordance the H&E slides were re-evaluated together by both to reach a consensus.
ImmunohistochemistryTwo additional sections of 4μm width were obtained from each FFPE tissue specimen, mounted on Superfrost Plus adhesion slides (Lomb Scientific, Sydney, Australia) and heated in a conventional oven at 60°C for 30min. Sections were deparaffinized in xylene and rehydrated in alcohol according to standard procedures. Subsequently they were steamed in 10mM citrate buffer (pH 6.0) to unmask the epitopes for 30min and each slide incubated with a rabbit/IgG, polyclonal, anti-cyclooxigenase-2 antibody (ThermoFisher Scientific®, Rockford, IL, USA), and a rabbit/IgG, polyclonal, Osteopontin, antibody (ThermoFisher Scientific®, Rockford, IL, USA), respectively, at 1:100 dilution for 3h at room temperature. The secondary antibody, Ultravision ONE HRP polymer® (Thermo-Fisher Scientific®, Rockford, IL, USA) was incubated for 30min at room temperature and antibody binding was detected using DAB Plus Chromogen® (3-3′-diamono-benzidine) (Ultravision ONE Detection System® Kit, Thermo Scientific, Fremont, CA, USA). Negative control slides were incubated in absence of the primary antibody. All slides were counterstained with hematoxylin, dehydrated and mounted.
Two pathologists (IB and LB), who were blinded to which group each sample belonged, evaluated immunostaining intensity at X100 and X400 final magnification, using an Eclipse 400 microscope connected to a DSFi1 camera (Nikon, Japan). Staining intensity was scored using NIS-Elements-3.0 software by the H-Score: 10 fields were chosen at random at X100 magnification and the staining intensity in epithelial cells of normal gastric mucosa, gastric mucosa with chronic gastritis, gastric mucosa with chronic gastritis and H. pylori infection, and gastric mucosa with chronic gastritis and intestinal metaplasia was scored with 0, 1, 2, or 3, corresponding to negative, weak, intermediate and strong brown staining, respectively. The total number of cells in each field and the number of cells stained at each intensity were counted. The positive average percentage was calculated and the following formula was applied: H-score=(% of cells stained at intensity category 1×1)+(% of cells stained at intensity category 2×2)+(% of cells stained at intensity category 3×3). The staining score of each marker was analyzed, as well as its association with H. pylori infection, presence of intestinal metaplasia and grade of inflammation.
Statistical analysisAssociations between H. pylori infection, grade of inflammation and presence of intestinal metaplasia were evaluated using Chi-square test. H-score values for COX-2 and Osteopontin, obtained in each tissue category (normal gastric mucosa, gastric mucosa with chronic gastritis, gastric mucosa with chronic gastritis, and H. pylori infection and gastric mucosa with chronic gastritis and intestinal metaplasia) were compared by t-test and/or ANOVA test. COX-2 and OPN expressions were compared to grade of inflammation and H. pylori infection by t-test. The GraphPad Prisma® v 5.00 software (GraphPad® Software Inc, San Diego, CA) was used and a p<0.05 was considered statistically significant.
Declaration of ethical aspectsThe study was approved by the Ethics Committee of the Universidad de Cartagena and the Ethics Review Board of the Hospital Universitario del Caribe and all data was handled anonymously.
ResultsGastric biopsy specimens from 108 patients, classified as normal gastric mucosa (n=22), chronic gastritis (n=27), chronic gastritis with H. pylori infection (n=30), and chronic gastritis with intestinal metaplasia (n=29) were included in the study. Of the 120 cases initially selected, 12 were eliminated due to insufficient tissue in the FFPE block.
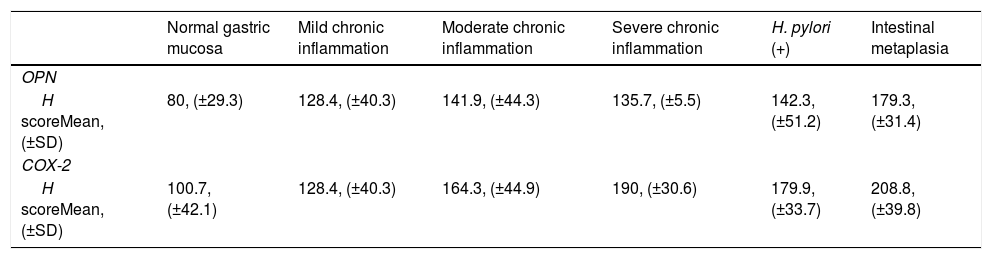
Chronic inflammation was a frequent finding, present in 86 (79.6%) biopsies, most (n=46, 53.5%) corresponding to moderate chronic inflammation, 26 (24.07%) to mild chronic inflammation, and 14 (23.8%) to severe chronic inflammation. Table I shows grades of inflammation in each group of samples.
Grade of inflammation in relation to sample groups.
| No inflammation n (%) | Mild chronic inflammation n (%) | Moderate chronic inflammation n (%) | Severe chronic inflammation n (%) | Total | |
|---|---|---|---|---|---|
| Normal Gastric Mucosa | 22 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 22 (20.37%) |
| HP (−) Chronic Gastritis | 0 (0%) | 10 (37.03%) | 13 (48.14%) | 4 (14.81%) | 27 (25%) |
| HP (+) Chronic Gastritis | 0 (0%) | 10 (33.33%) | 16 (54%) | 4 (13.33%) | 30 (27.77%) |
| Chronic Gastritis with Intestinal Metaplasia | 0 (0%) | 6 (20.68%) | 17 (58.62%) | 6 (20.68%) | 29 (26.85%) |
| Total | 22 (20.37%) | 26 (24.07%) | 46 (42.59) | 14 (12.96%) | 108 |
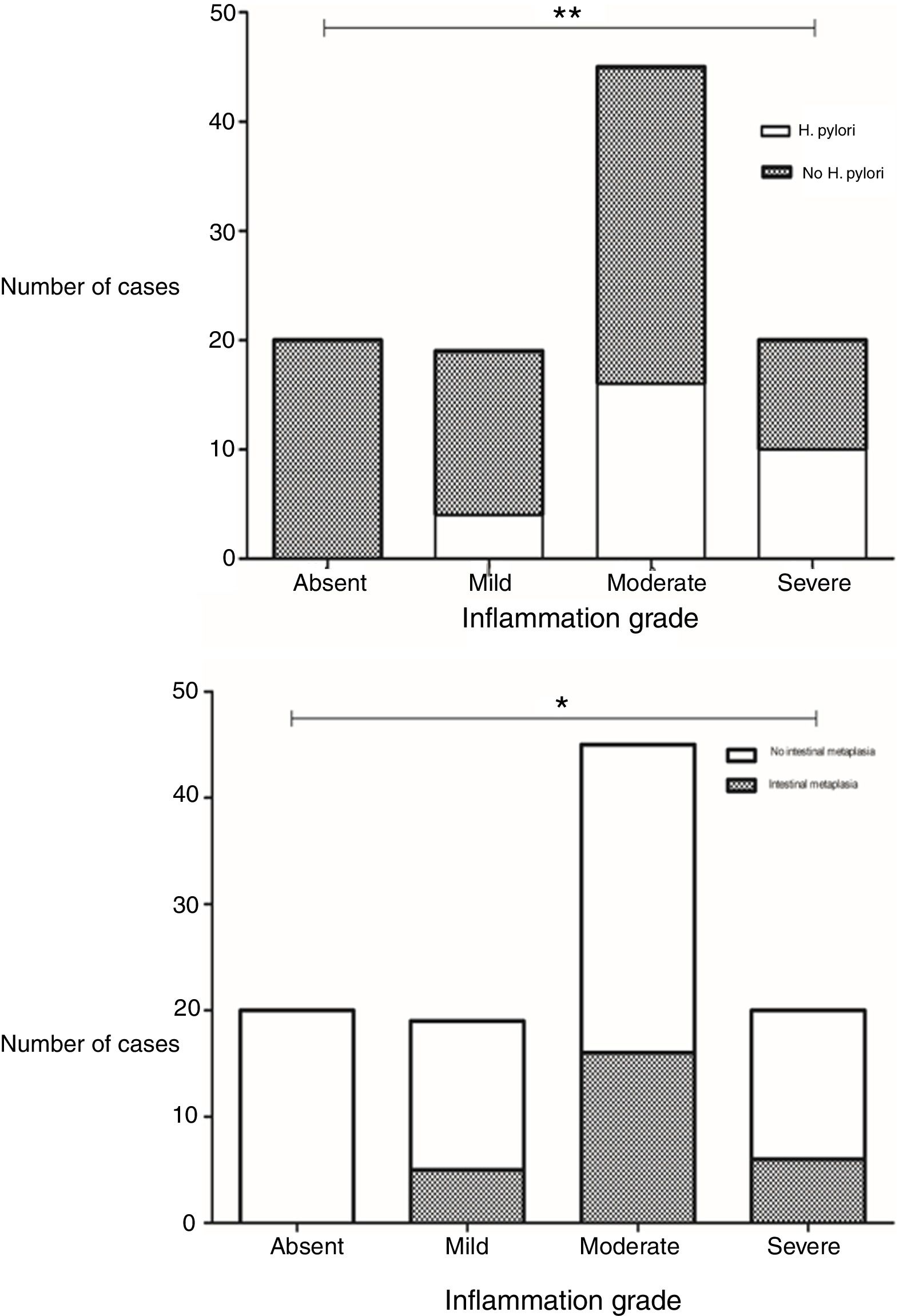
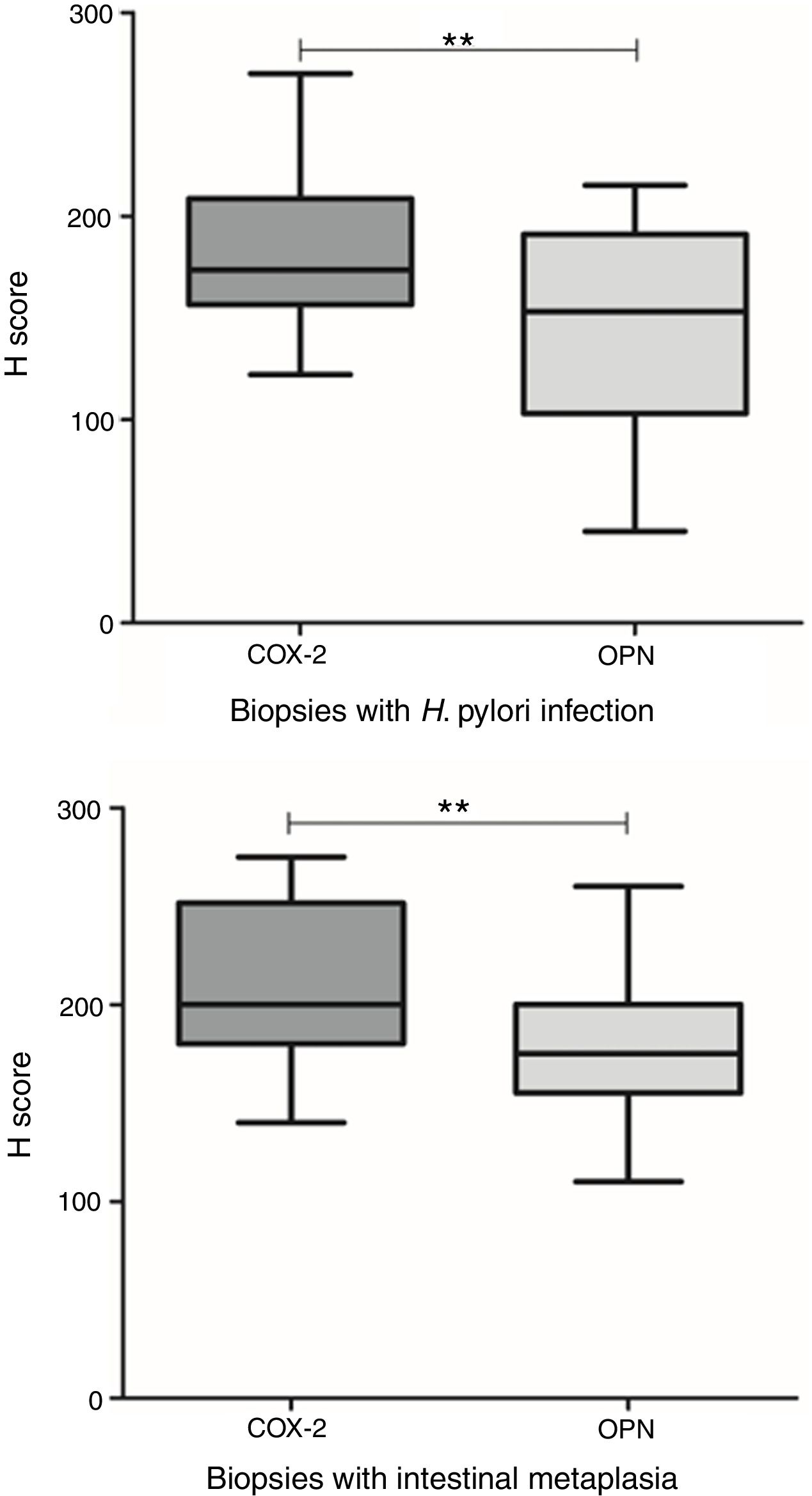
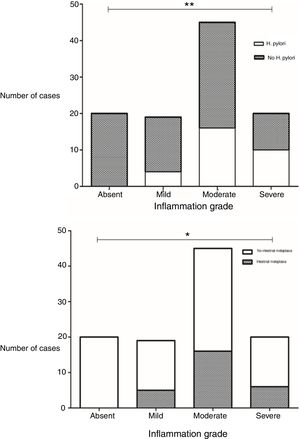
Presence of H. pylori was observed in 28.8% of the biopsies. A statistically significant difference was found in the grade of inflammation between biopsies with and without H. pylori infection (p=0.002, x2) (Fig. 1). There was also a significant difference in inflammation grade between biopsies with and without intestinal metaplasia (p=0.025, x2), with a higher number of biopsies with chronic inflammation associated to H. pylori infection and to presence of intestinal metaplasia (Fig. 1).
Differences in grades of inflammation in relation to H. pylori infection and presence of intestinal metaplasia. The grade of inflammation was significantly different between biopsies with and without H. pylori infection (p=0.002, x2), and between biopsies with and without intestinal metaplasia (p=0.002, x2). Top: Grade of inflammation in relation to H. pylori infection. Bottom: Grade of inflammation in relation to presence of intestinal metaplasia.
The expression of OPN and COX-2 proteins was studied in the groups of gastric tissue samples: normal gastric mucosa, gastric mucosa with chronic gastritis, gastric mucosa with chronic gastritis and H. pylori infection and gastric mucosa with chronic gastritis and intestinal metaplasia.
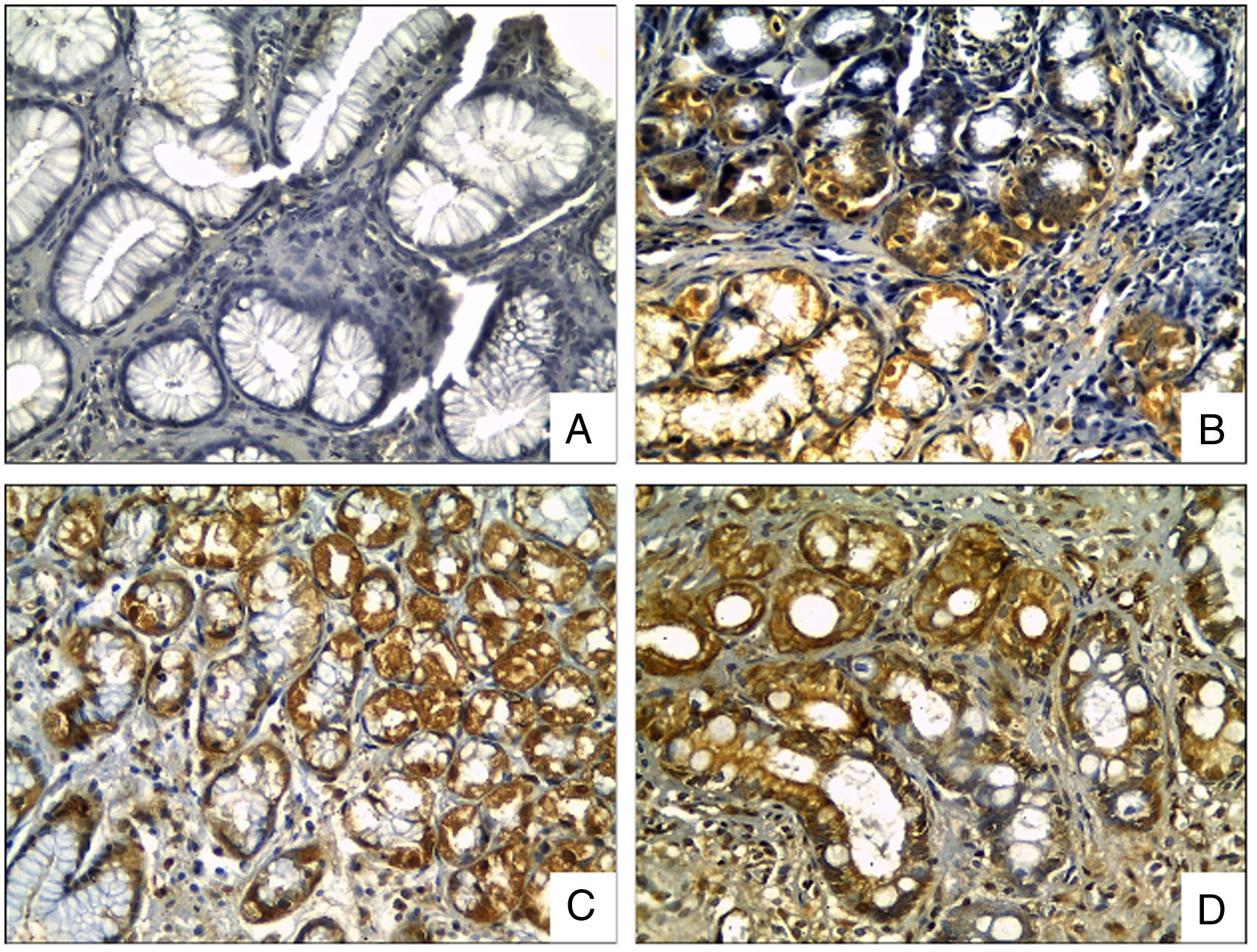
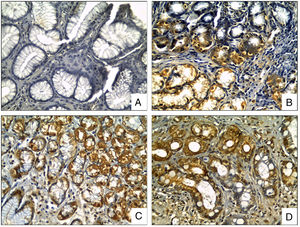
OPN staining was observed in the cytoplasm of the epithelial cells, located predominantly at the neck and the base of gastric glands, being displaced to the basal region in the mucous cells of the surface. OPN expression was significantly increased in biopsies with H. pylori compared to biopsies without it (p=0.003, t test), and, significantly higher in areas with intestinal metaplasia in comparison to biopsies without it (p<0.0001, t test), (Fig. 2).
OPN Immunohistochemical staining in gastric biopsies with mucosal lesions. OPN staining was significantly higher in biopsies with H. pylori infection and areas with intestinal metaplasia. (A) normal gastric mucosa; (B) chronic gastritis; (C) chronic gastritis with H. pylori infection; (D) chronic gastritis with intestinal metaplasia (Osteopontin IHC, original magnification ×400).
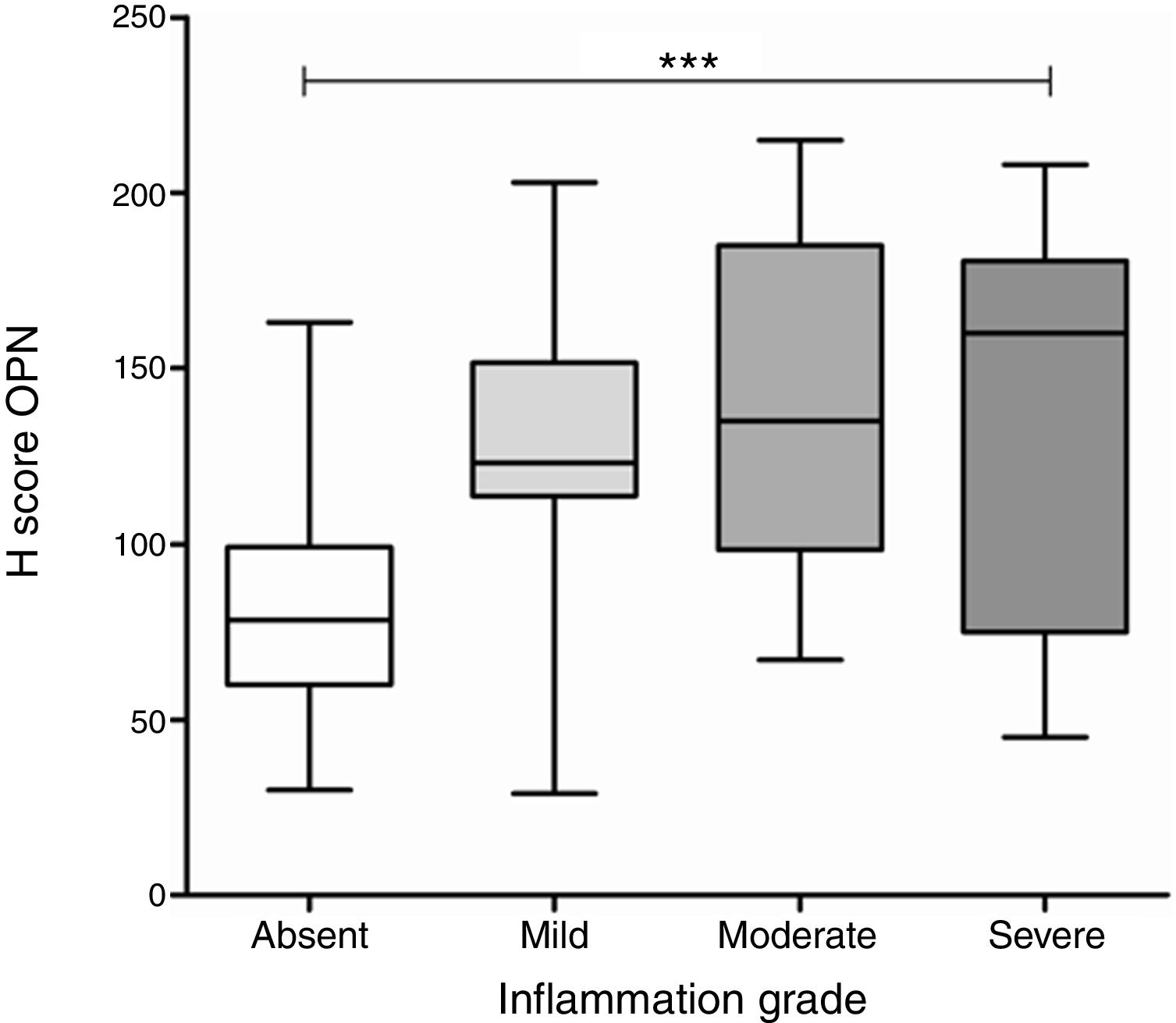
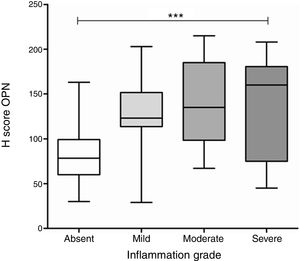
A significant difference was found in OPN expression between gastric biopsies with different grades of inflammation (p<0.0001, ANOVA test), with differences in the average levels of expression specific to each grade of inflammation, compared to the level of expression found in biopsies without inflammation (Fig. 3). In the group of biopsies with H. pylori infection, there was not a significant difference in the expression of OPN between the biopsies with different grades of inflammation.
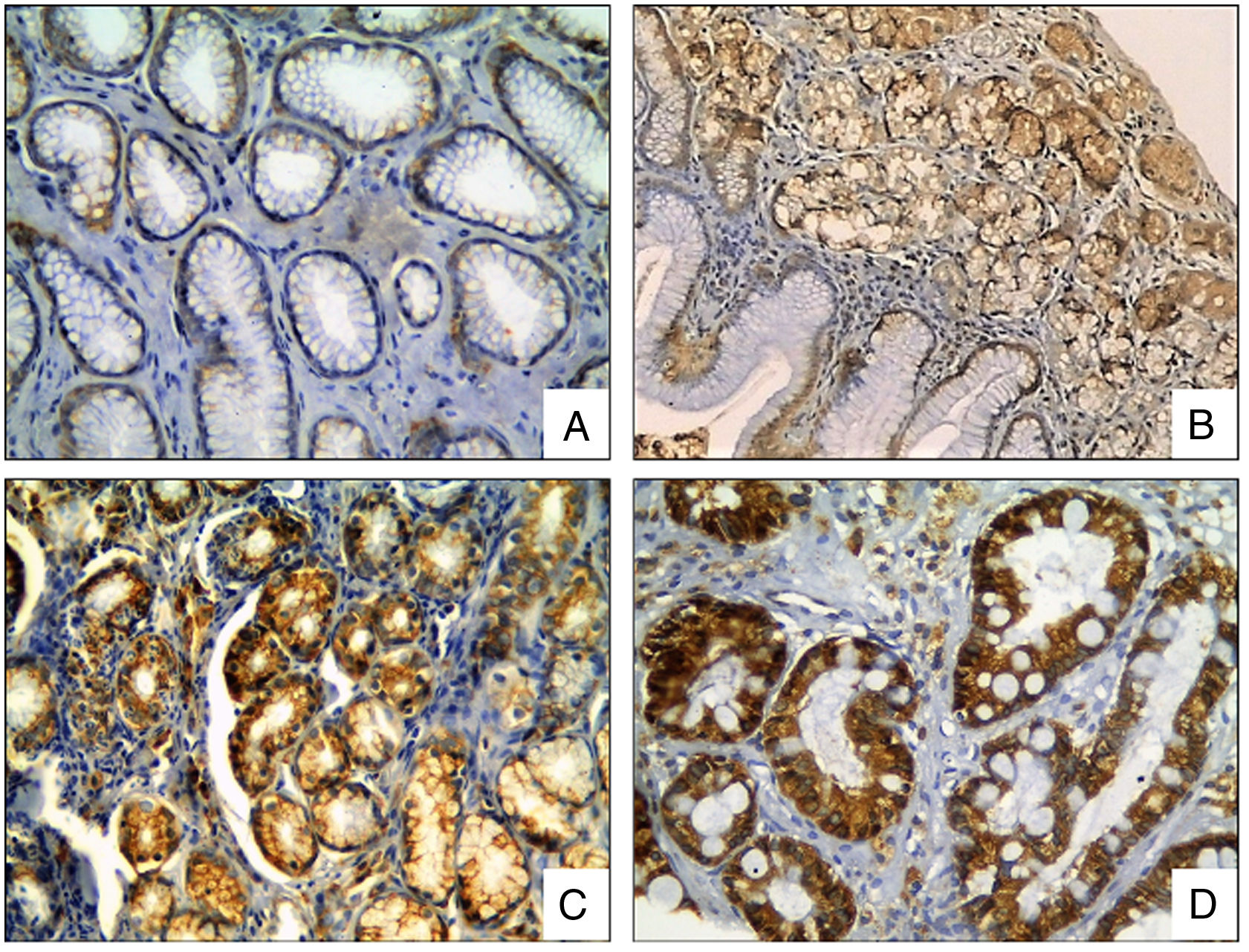
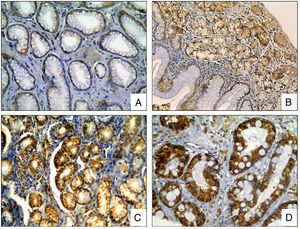
The COX-2 staining was observed in the cytoplasm of the epithelial cells. The COX-2 expression was significantly different between biopsies with and without H. pylori infection (p<0.0001, t test), and between areas with and without intestinal metaplasia (p<0.0001, t test), (Fig. 4).
COX-2 Immunohistochemical staining in gastric biopsies with mucosal lesions. COX-2 staining was significantly higher in biopsies with H. pylori infection and areas with intestinal metaplasia. (A) normal gastric mucosa; (B) chronic gastritis; (C) chronic gastritis with H. pylori infection; (D) chronic gastritis with intestinal metaplasia (COX-2 IHC, original magnification ×400).
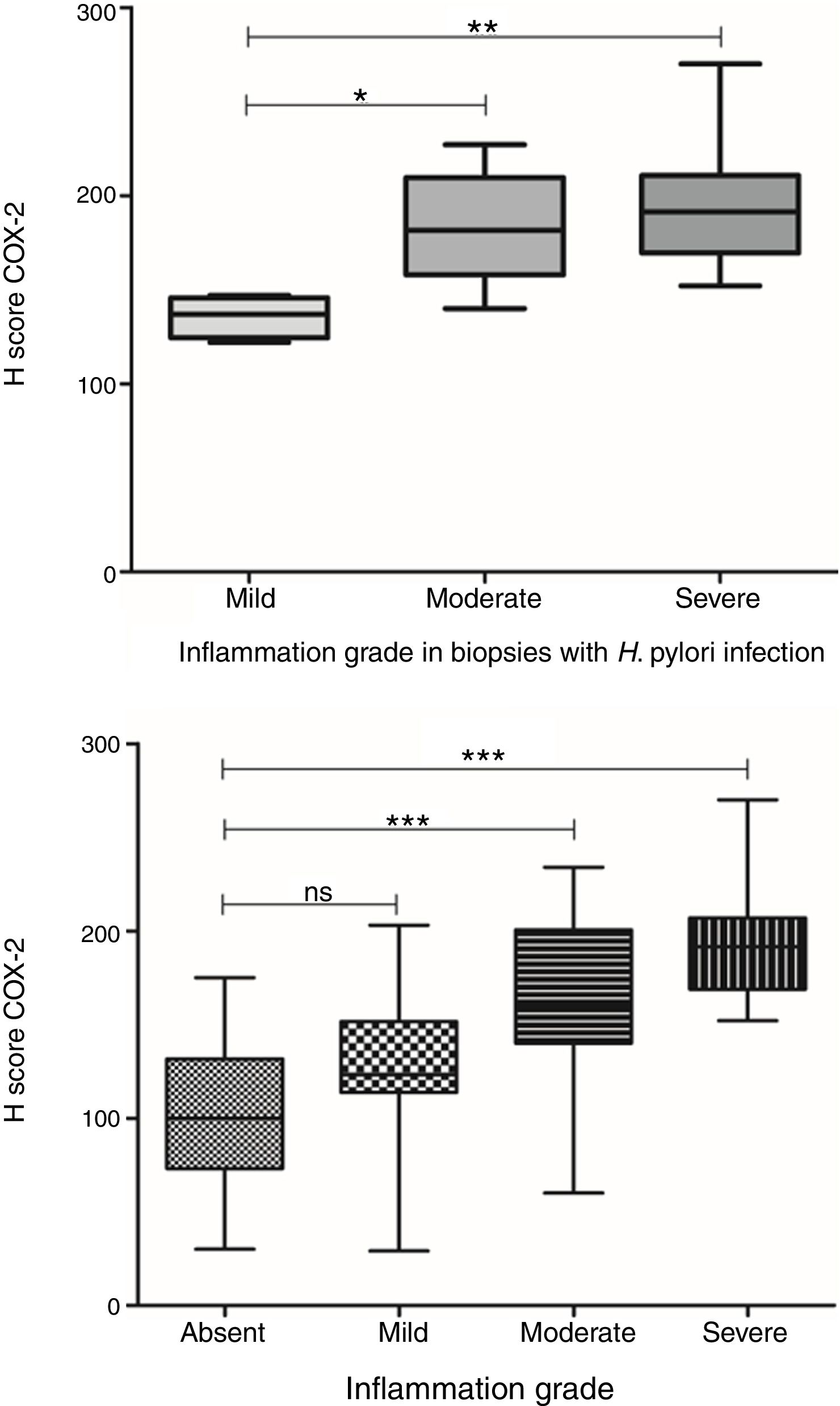
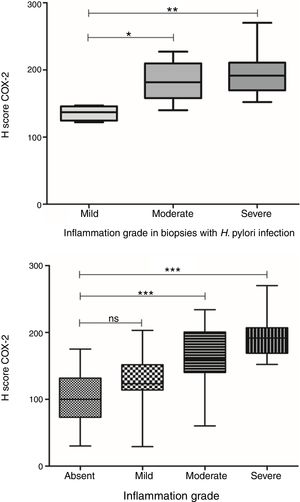
COX-2 expression was also significantly different between gastric biopsies with different grades of inflammation (p<0.0001, ANOVA test). Although there was no significant difference in the average expression levels between biopsies with mild chronic inflammation and biopsies without inflammation, a significant difference was observed in COX-2 expression levels in biopsies with moderate and severe chronic inflammation in comparison with biopsies without inflammation (Fig. 5). Furthermore, in biopsies with H. pylori infection, a significant difference was observed in COX-2 expression in relation to the grade of inflammation (p=0.009, ANOVA test). Here there was a difference in COX-2 expression in biopsies with mild chronic inflammation in comparison to biopsies with both severe and moderate chronic inflammation. This difference was not seen between biopsies with moderate and severe chronic inflammation (Fig. 5).
COX-2 expression in biopsies with H. pylori infection in relation to grade of inflammation. Top: COX-2 expression was significantly different between gastric biopsies with different grades of inflammation (p<0.0001, ANOVA test). There was not a significant difference in the COX-2 expression between biopsies with mild chronic inflammation and biopsies without inflammation; Bottom: In biopsies with H. pylori infection, there was a significant difference in COX-2 expression in relation to the grade of inflammation (p=0.009, ANOVA test).
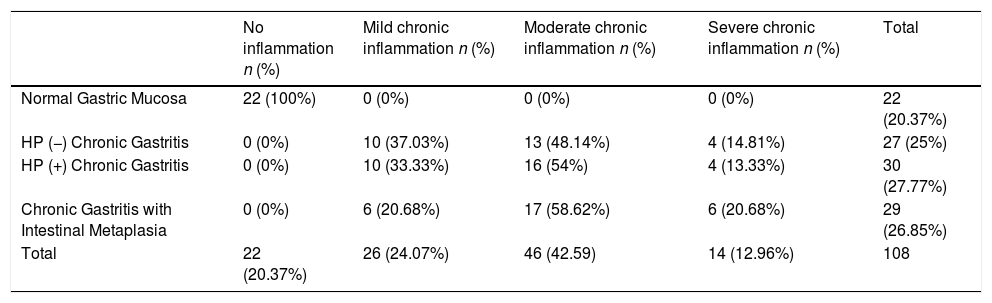
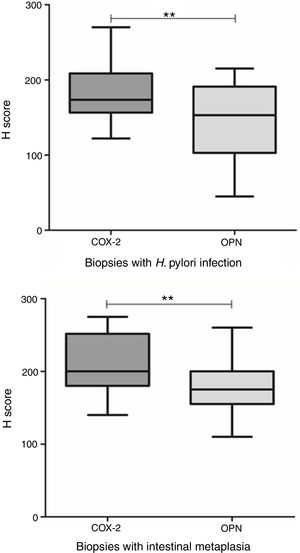
The expression of OPN and COX-2 in biopsies with H. pylori infection were significantly different (p=0.001, t test). Also, in areas with intestinal metaplasia the expression of both proteins was different (p=0.004, t test) (Fig. 6). Table II summarizes the mean H Score value for each biomarker in relation with grade of inflammation, H. pylori infection and presence of intestinal metaplasia.
OPN and COX-2 expressions in relation to H. pylori infection and intestinal metaplasia. Top: OPN and COX-2 were significantly different in biopsies with H. pylori infection (p=0.001, t test). Bottom: OPN and COX-2 were significantly different in areas with intestinal metaplasia (p=0.004, t test).
OPN and COX-2 expression according to the grade of inflammation, H. pylori infection, and presence of intestinal metaplasia.
| Normal gastric mucosa | Mild chronic inflammation | Moderate chronic inflammation | Severe chronic inflammation | H. pylori (+) | Intestinal metaplasia | |
|---|---|---|---|---|---|---|
| OPN | ||||||
| H scoreMean, (±SD) | 80, (±29.3) | 128.4, (±40.3) | 141.9, (±44.3) | 135.7, (±5.5) | 142.3, (±51.2) | 179.3, (±31.4) |
| COX-2 | ||||||
| H scoreMean, (±SD) | 100.7, (±42.1) | 128.4, (±40.3) | 164.3, (±44.9) | 190, (±30.6) | 179.9, (±33.7) | 208.8, (±39.8) |
In this study we evaluated the immunohistochemical expression of COX2 and OPN according to a previously reported association between gastric chronic inflammatory processes, H. pylori infection, and intestinal metaplasia, with gastric carcinogenesis. Differences in their expression pattern in gastric mucosa with chronic inflammation, H pylori infection and intestinal metaplasia, compared to normal gastric mucosa were also considered.
There are many reports of overexpression of COX-2 in intestinal and extra intestinal tumors24 as well as in inflammatory and premalignant lesions; the overexpression increases as the inflammation and dedifferentiation grades increase in the tissue.31 It is also associated with a worse prognosis in patients with GC.27,31
Gastric epithelial cells respond to H. pylori infection by over-regulating the expression of pro-inflammatory genes, including COX-2.32 In the H. pylori-induced inflammatory response, COX-2 acts as a key regulator.33 Furthermore, a significant interaction between COX-2 expression and H. pylori infection in high risk gastric lesions, related to the grade of inflammation has been reported, as well as a significant increased risk of progression of these gastric lesions in subjects with elevated COX-2 expression.34
Previous studies have shown that the intensity of COX-2 expression in the gastric mucosa was related to the presence of intestinal metaplasia in patients infected with H. pylori.35 Erkan et al., found statistically significant differences in both intensity and frequency of COX-2 staining between intestinal metaplasia, active chronic gastritis, and normal gastric mucosa, being greater in the intestinal metaplasia group, followed by the active chronic gastritis and normal gastric mucosa groups.36 In the present study, we also showed that such relation with the intensity of COX-2 expression was associated to the grade of inflammation, as it was significantly overexpressed in gastric mucosa with moderate and severe inflammation, compared to normal gastric mucosa, but was not overexpressed in those with mild inflammation.
A recent study proposed an additional way by which COX-2 overexpression is produced in gastric mucosa with infection by H. pylori, involving epigenetic mechanisms. They concluded that H. pylori infection induces β-catenin nuclear translocation, upregulation of the histone demethylase JMJD2B, and then stimulates COX-2 transcription by cooperating with NF-κB, which eventually promotes H. pylori-induced carcinogenic process.37
Genes that responded to selective inhibition of COX-2 were tracked in an animal model of knock-out mice for the APC gen treated with last generation inhibitors of COX enzyme. The only finding of interest reported was the inhibition of the gene promoter that encodes for A4 nuclear receptor (NR2A4), responsible for the activation of the gene that encodes for OPN. Its activity is the result of its interaction with E-2 prostaglandin (PGE-2), which is in turn a metabolite of COX-2 activity over arachidonic acid.38
It has been reported that OPN induces COX-2 expression through the C/NF-kB kinase protein pathway21; thus, it is possible that the increase of OPN expression in areas with intestinal metaplasia might cooperate with COX-2 in the induction of gastric carcinogenesis. In this sense, a positive correlation has been reported between OPN and COX-2 expression in tissue with gastric cancer.39
Previous studies have shown that OPN might be involved in a variety of chronic inflammatory diseases and cancer.40 It has been found to be overexpressed in the intestinal epithelium, especially when chronic inflammation is present. Brown et al. reported a strong expression at the luminal surface level of epithelial cells of the gastrointestinal tract.41–43 Chen et al. documented a directly proportional relationship between the grades of inflammation in the gastric mucosa and OPN expression which was significantly higher in patients with atrophic chronic gastritis compared to moderate gastritis, and in patients with gastric cancer compared to patients without it.44
It has also been suggested that OPN may play an important role in gastric inflammation associated to H. pylori. According to findings by Chang et al., this infection may increase OPN gastric expression, which correlates with gastric inflammation and the presence of intestinal metaplasia.25 Park et al. compared wild mice infected with H. pylori, versus knockout mice for OPN gene infected with H. pylori, and showed that the grade of gastritis and macrophage infiltration in the gastric mucosa and the mitotic index of mucosal cells were lower in the knockout mice group in comparison with wild mice. OPN expression was elevated in response to H. pylori infection and OPN depletion decreased production of cytokines in both gastric epithelial cells and macrophages during H. pylori infection. These results suggest that OPN is an important mediator of proinflammatory cytokine production during H. pylori infection and demonstrate the involvement of OPN in the production of proinflammatory cytokines by gastric epithelial cells in the immune response against H. pylori, in addition to inflammatory cells.45 Similarly, the present study demonstrates a significant OPN overexpression in gastric epithelium with H. pylori infection and intestinal metaplasia and that such overexpression is related to the grade of gastric mucosa inflammation.
A possible mechanism by which OPN could contribute to the development of intestinal metaplasia would be the increase in cell survival, thus predisposing them to malignant transformation.40 OPN is physiologically expressed at very low levels; in contrast it is overexpressed in tissues with chronic gastritis and intestinal metaplasia, the staining intensity being inversely proportional to the histological differentiation grade, and directly proportional to the malignant potential of the lesion.22 Its overexpression is associated with a poorer prognosis.26
There were no differences in OPN expression level in the different grades of inflammation in biopsies with H. pylori; however, COX-2 expression level showed significant differences in the same group of samples with moderate and severe chronic inflammation.
There was a higher COX-2 expression compared to OPN in biopsies with H. pylori infection associated with moderate and severe chronic inflammation and in areas with intestinal metaplasia. In the inflammation associated with the infectious condition and development of intestinal metaplasia, the inflammation mediators may induce increased expression of both proteins, particularly COX-2.
During H. pylori-induced chronic gastric inflammation, there is an increased tissue turnover that predisposes an excessive rate of cell proliferation and may result in an excess of mitotic errors and increased rate of mutagenesis. This lengthy process, commonly known as the “Correa pathway”, is dependent on continued or chronic inflammation46 in which COX2 and OPN are involved, and could play an important role.
One of the limitations of this study was that a special staining for H. pylori was not performed in those cases with severe inflammation without evidence of the bacteria in H&E stained slides to confirm its absence.
ConclusionsOPN and COX-2 were overexpressed in gastric epithelium associated with H. pylori infection, intestinal metaplasia and a high grade of chronic inflammation and their expression was correlated. These findings provide additional evidence for the up-regulation of protein expression that exists in epithelial gastric cells in response to chemical signals generated by H. pylori-associated inflammation process; an expression that is maintained into advanced stages of the chronic inflammatory process. The relationship between the overexpression of these proteins and their possible intervention in gastric carcinogenesis is not yet clear, justifying the need for additional studies to establish their role in this process.
Funding sourceThis work was financially supported by the University of Cartagena in support to the Research Group of Histopathology. The authors manifest that there is no conflict of interest to declare.
Conflict of interestThere is no conflict of interest to declare.
The authors would like to thank the staff of the Pathology Department of the Hospital Universitario del Caribe, Cartagena, for their assistance in conducting this study, especially to Doctor Cesar Redondo for his collaboration.
Al Departamento de Patología del Hospital Universitario del Caribe de Cartagena, Colombia, en especial al doctor César Redondo.