To assess dual time point 2-deoxy-2-[18F]fluoro-d-glucose 18FFDG PET-CT accuracy in nodal staging and in detection of extra-axillary involvement.
Material and methodsDual time point [18F] FDG PET/CT scan was performed in 75 patients. Visual and semiquantitative assessment of lymph nodes was performed. Semiquantitative measurement of SUV and ROC-analysis were carried out to calculate SUVmax cut-off value with the best diagnostic performance. Axillary and extra-axillary lymph node chains were evaluated.
ResultsSensitivity and specificity of visual assessment was 87.3% and 75%, respectively. SUVmax values with the best sensitivity were 0.90 and 0.95 for early and delayed PET, respectively. SUVmax values with the best specificity were 1.95 and 2.75, respectively. Extra-axillary lymph node involvement was detected in 26.7%.
ConclusionFDG PET/CT detected extra-axillary lymph node involvement in one-fourth of the patients. Semiquantitative lymph node analysis did not show any advantage over the visual evaluation.
Valorar la precision diagnóstica de la PET-CT con 2-deoxi-2-[18F]fluor-d-glucosa [18F] FDG en doble fase en la estadificación ganglionar y en la detección de afectación extra-axilar.
Material y métodosSe realizó una [18F] FDG PET-TC en doble fase a 75 pacientes. Se valoraron los ganglios linfáticos de forma visual y semicuantitativa. Se realizaron medidas del SUV y análisis ROC para calcular el valor de SUV max con la mejor precisión diagnóstica. Se evaluaron los niveles axilares y extra-axilares.
ResultadosLa sensibilidad y especificidad del análisis visual fue del 87.3% y 75% respectivamente. Los valores de SUVmax con la mejor sensibilidad fueron de 0.90 y 0.95 para el PET en fase precoz y tardía respectivamente. Los valores de SUV max con la mejor especificidad fueron de 1.95 y 2.75 respectivamente. Se detectó afectación ganglionar extra-axilar en el 26.7%.
ConclusiónLa PET-TC con FDG detectó afectación ganglionar extra-axilar en una cuarta parte de las pacientes. El análisis semicuantitativo no pareció aportar ninguna ventaja sobre la valoración visual.
The presence of axillary lymph node metastases is the most important prognostic factor in breast cancer. No imaging technique currently available allows for an accurate evaluation of axillary lymph node involvement, and even though positron emission tomography (PET) with 2-deoxy-2-[18F]fluoro-d-glucose ([18F]FDG) is less sensitive than sentinel node biopsy for the detection of lymph node metastases, its specificity is high, ranging from 85% to 100%.1
For [18F]FDG PET, a revision reported a sensitivity and specificity that ranged from 57 to 100% and 66 to 100%, respectively.2 However the sensitivity depends on the high a priory likelihood of voluminous lymph node metastases and intrinsic tumour characteristics like grade and type.3,4
Locally advanced breast tumors have a higher incidence of axillary, extra-axillary involvement and distant metastases.5
Several studies have demonstrated that [18F]FDG PET is superior to conventional diagnostic techniques in the detection of extra-axillary nodal metastases, particularly to the internal mammary lymph nodes.6 The rate of detection is particularly high in patients in advanced stages (III or IV) of breast cancer.
In addition it has been proposed that dual-time-point [18F]FDG PET may improve the sensitivity and accuracy of [18F]FDG PET in assessing patients with primary breast cancer.7 On the contrary this procedure seems not to improve the overall performance of standard PET in detecting axillary lymph node metastasis in breast cancer patients.8
The aim of this study was to evaluate the accuracy of dual time point [18F]FDG-PET/computed tomography (CT), in nodal staging and in the detection of extra-axillary involvement and determine whether the use of a semiquantitative lymph node analysis has any advantage over the visual evaluation for the correct classification of lymph nodes in patients with locally advanced breast cancer.
Material and methodsThis prospective and multicentric study was approved by the local ethics committee of our institution and Investigation Board (2009/40) and includes 7 hospitals of our region. The Institutional Review Boards in each institution approved the protocol of this study.
PatientsWritten informed consent was obtained from all patients. Seventy-five patients (all women; mean age 52.5, SD±11.8) were studied.
The inclusion criteria were newly diagnosed unilateral breast cancer with indication of neoadjuvant treatment (stage II or III), with histological confirmation of lymph node state by a previous fine-needle aspiration (FNA) or sentinel node biopsy (SNB), performed after the PET/CT study. The exclusion criteria were presence of distant metastasis confirmed by other methods previous to the request of a PET/CT.
All the patients were imaged using digital mammography, ultrasonography, as conventional diagnostic imaging (CDI), and [18F]FDG PET/CT performed in a unique reference centre.
FDG-PET/CT imagingPatients fasted for at least 4h before the PET/CT examination and had blood glucose levels lower than 160mg/dl at the time of injection. PET/CT was performed with a dedicated whole-body PET/CT machine following a standardized protocol in three-dimensional (3D) mode, 3min/bed position.9 Transmission scans were performed for all patients to provide attenuation correction with CT. The PET section thickness was 3.8mm. Iterative reconstruction and scatter correction of image was done.
All the patients underwent dual time point imaging with an average interval time of approximately 120min between the two phases. The first examination was performed as whole-body image from head to thigh 60min after the intravenous administration of approximately 370MBq of [18F]FDG (PET-1). The second examination imaged the chest only, with acquisition of one or two bed positions (PET-2).
Image interpretationFor visual interpretation images were displayed in three orthogonal projections and as whole-body maximum-pixel-intensity projection images. Two experienced nuclear medicine physicians, who were blinded to the conventional staging investigations, interpreted in consensus the [18F] FDG PET/CT studies.
Metabolic images were considered positive if areas in the lymph node took up more FDG than the surrounding tissue in the early PET (PET-1) and kept or increased activity in delayed image (PET-2).
Homogeneously hypoechoic lymph nodes with a diameter of ≥1cm in oval or round shape were defined by CDI as positive.
A combined assessment, metabolical and clinical attending the information of CDI, was performed considering it as pathologic if any technique showed signs of lymph node involvement.
The breast areas, supraclavicular and internal mammary lymph node chains were evaluated to establish the percentage of extra-axillary involvement.
Semiquantitative measurement of SUVmax was done on the axillary or extra-axillary focus with the highest abnormal FDG uptake in PET-1 (SUV-1) and PET-2 (SUV-2). The percentage difference in the SUVmax or retention index (RI) between SUV-1 and SUV-2 was calculated.
We obtained the short axial diameter of the lymph node with the greatest FDG uptake in each case using the CT portion of the PET. The lymph node with the highest FDG uptake was classified attending to its size in group 1 (short axial diameter <1cm) or group 2 (short axial diameter ≥1cm).
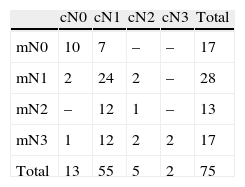
The clinical and metabolic N stages (cN and mN) were established according to the clinical exploration and the results of morphological imaging techniques and the recommendations of the American Joint Committee on Cancer (AJCC) 7th edition.10
Histopathological analysisHistological confirmation of the suspicious lymph node, according to morphological imaging, was obtained by FNAB (fine needle aspiration biopsy) previous to PET/CT scan or sentinel node biopsy (SNB) after PET/CT scan.
Statistical analysisAll statistical tests were two-sided with a significance level of p<0.05. SPSS 18.0.1 for Windows was used for all analyses.
All semiquantitative data were expressed in terms of mean±SD.
Sensitivity, specificity, positive and negative predictive values (PPV, NPV) and accuracy of [18F]FDG PET/CT imaging for lymph node staging were analyzed using standard statistical analyses. The 95% confidence intervals (CI) were calculated using the binomial distribution.
The results (mN) were related to the previous cN established attending to the clinical exploration and morphological imaging techniques. The integration of both imaging techniques (morphologic and metabolic) was evaluated considering as positive any lymph node that was positive with any technique.
The mean SUV-1 and SUV-2 values were obtained in group 1 and group 2 lymph nodes.
A ROC-analysis (receiver-operating characteristics) was performed, and the cut-off value was calculated as to obtain the best diagnostic test parameters to establish the lymph node involvement in PET-1 and PET-2.
The concordance between cN and mN was assessed (kappa index) classifying the results as: poor (<0.20), weak (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80) and very good (0.81–1.00).
ResultsOf the 75 patients, 63 had lymph node involvement confirmed by histopathology. Sixty-seven patients underwent FNAB guided by ultrasonography in the suspicious lymph node. The rest of cases were confirmed by SNP. Thus, the prevalence of lymph node involvement was high (84%).
In 19 patients lymph nodes were smaller than 1cm attending to the CT portion of the PET (group 1). In the rest of cases the mean size was of 17.5mm±6.3.
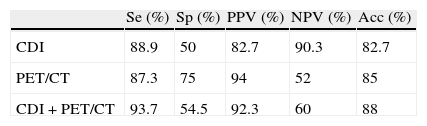
The sensitivity, specificity, PPV, NPP and accuracy of [18F]FDG-PET/CT for the diagnosis of lymph node metastasis were 87.3%, 75.0%, 94.8%, 52.9% and 85%, respectively.
In this work, none of the 8 FN cases in the early PET showed significant FDG uptake in the delayed PET.
The combined interpretation (clinical and metabolic) did not improve the diagnostic results. Table 1 shows the distribution of the results.
Statistical diagnostic parameters in lymph node status of conventional diagnostic imaging, metabolic imaging and combined.
| Se (%) | Sp (%) | PPV (%) | NPV (%) | Acc (%) | |
| CDI | 88.9 | 50 | 82.7 | 90.3 | 82.7 |
| PET/CT | 87.3 | 75 | 94 | 52 | 85 |
| CDI+PET/CT | 93.7 | 54.5 | 92.3 | 60 | 88 |
CDI: conventional diagnostic imaging; Se: sensitivity; Sp: specificity; PPV: positive predictive value; NPV: negative predictive value; Acc: accuracy.
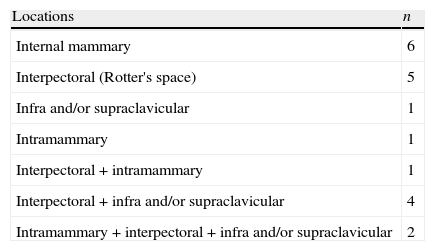
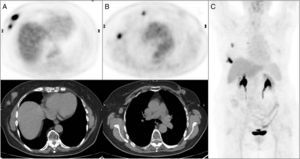
Extra-axillary lymph node involvement was detected by [18F]FDG PET/CT in 20/75 (26.7%) of patients (Fig. 1). Table 2 shows the distribution of the results.
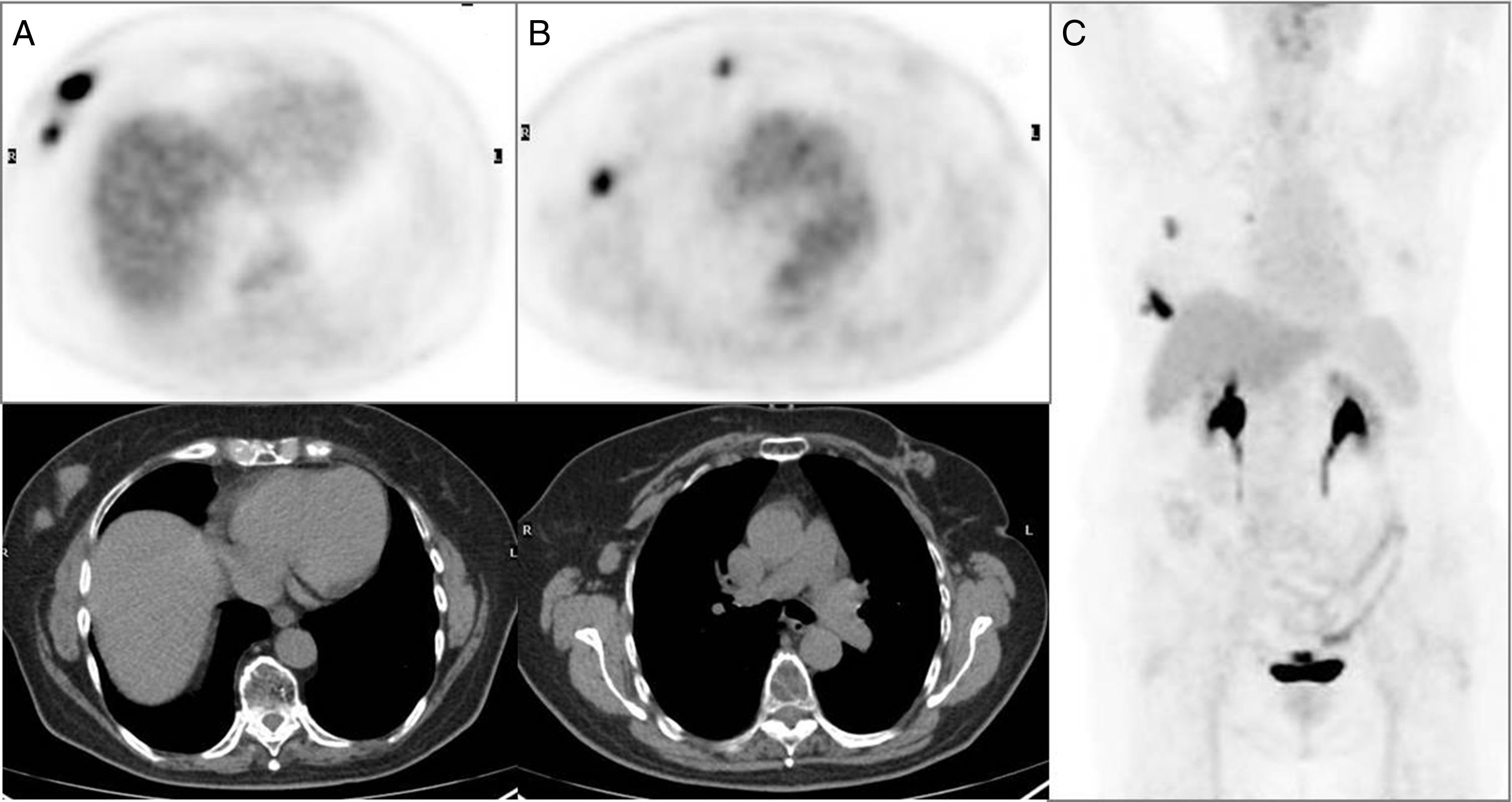
Multifocal right breast cancer in PET/CT axial images (A) with a clinically pathologic right axillary lymph node and a pathologic lymph node in right mammary territory showed in axial images of the PET/CT (B) unsuspected in conventional imaging techniques. Summary of the results on the maximum intensity projection PET imaging (C).
Metabolic lymph node involvement: distribution of extra-axillary locations.
| Locations | n |
| Internal mammary | 6 |
| Interpectoral (Rotter's space) | 5 |
| Infra and/or supraclavicular | 1 |
| Intramammary | 1 |
| Interpectoral+intramammary | 1 |
| Interpectoral+infra and/or supraclavicular | 4 |
| Intramammary+interpectoral+infra and/or supraclavicular | 2 |
n: number.
[18F]FDG PET/CT upgraded the cN stage in 29/75 patients. This assessment was correct in 28/75 patients (37.3%). Furthermore, PET/CT detected distant metastases in 8 patients.
On the other hand, the cN was higher than mN in 9 patients. In 7/9 patients, conventional diagnostic techniques diagnosed lymph node involvement whereas metabolic imaging did not show pathologic lymph node uptake: 3 were correct and 4 corresponded to false positive.
Distribution of cN and mN stages is shown in Table 3.
The mean SUV-1, SUV-2 and RI of the PET N positive axillary lymph nodes was 1.43±0.40 in group 1 and 7.98±4.83 in group 2 (p<0.05 in both of them); 1.79±0.63 in group 1 and 8.82±5.53 in group 2 (p<0.05 in both of them) and 29.40±27.67 in group 1 and 14.89±21.35 in group 2 (p<0.05 in group 2).
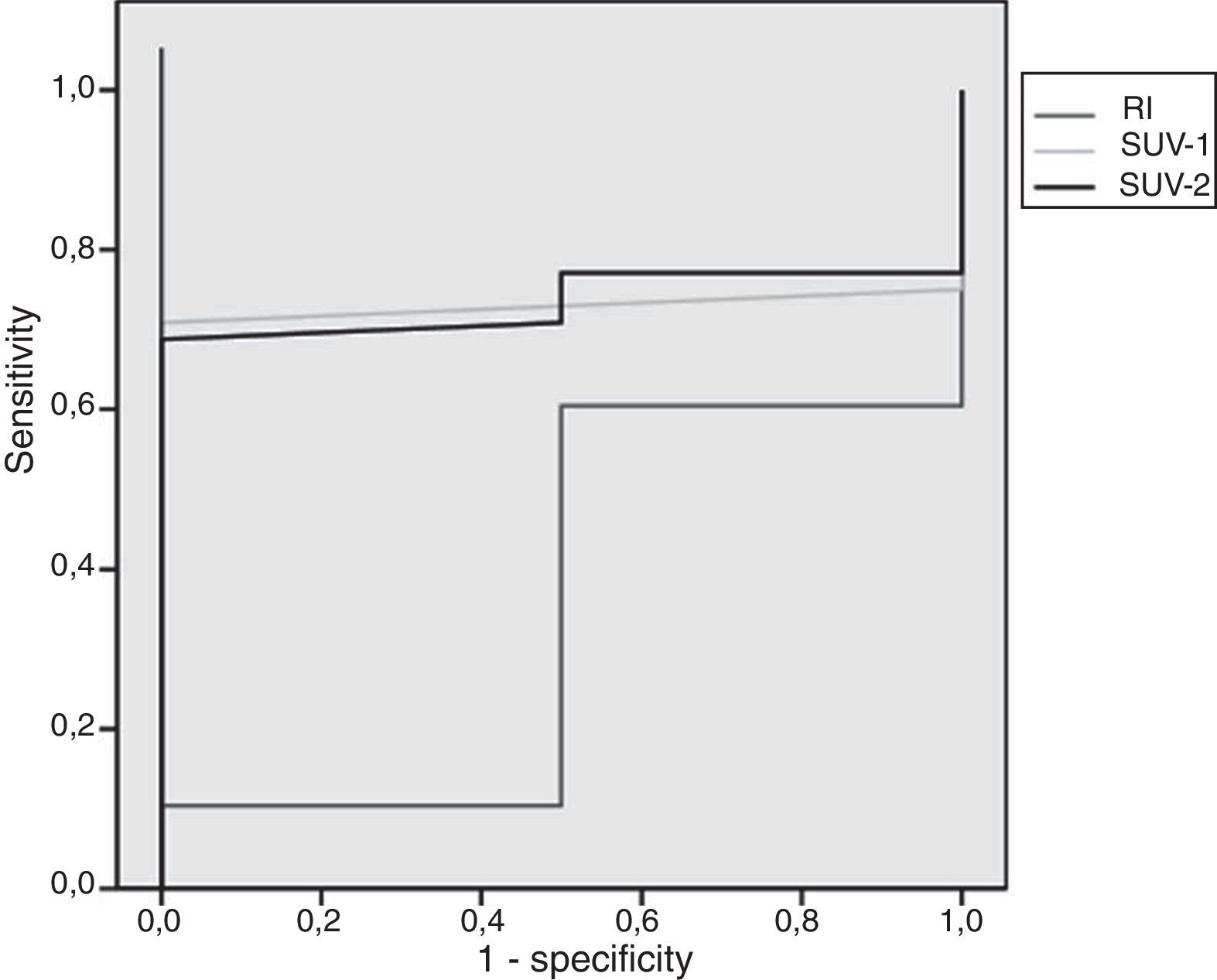
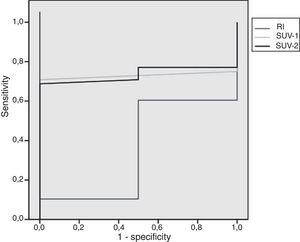
After ROC analysis, the SUVmax values of 0.90 and 0.95 for PET-1 and PET-2 showed to be the best cut-off point, with a sensitivity of 98%. On the contrary, the values of SUVmax with the best specificity (100%) were 1.95 and 2.75 for early and delayed PET/CT, respectively. The area under the ROC curve for SUV-1 was 0.73 (p>0.05), 0.73 for SUV-2 (p>0.05) and 0.35 for RI (p>0.05). The ROC analysis is presented in Fig. 2.
Attending to these results, semiquantitative lymph node analysis did not appear to have any advantage over the visual evaluation for the correct classification of lymph nodes.
The agreement between PET/CT and CDI in the establishment of lymph node pathology was very good (Kappa=0.86).
On the contrary, the concordance between cN and mN was weak (Kappa index=0.39).
DiscussionThe sensitivity of [18F]FDG PET for detecting axillary lymph node (ALN) metastases in breast cancer is reported to be low. However it has been demonstrated that PET/CT may detect extra-axillary lymph node involvement more accurately than CDI.11,12
Axillary and extra-axillary lymph node status is an important predictor regarding recurrence and survival of patients having primary breast cancer among others prognostic factors.13
In our work, PET-CT and CDI showed similar performance in the establishment of lymph node involvement, probably due to the high mean size of the biggest lymph nodes (17.7mm) and the high prevalence of lymph node disease (84%).
In general, in extra-axillary locations, these nodes are not harvested or treated by routine surgical procedures.
Therefore [18F]FDG PET/CT may be useful as an additional imaging tool to assess extra-axillary lymph node metastasis, with impact on management.12 All these findings may have implications for treatment and survival: more extensive radiotherapy fields, possible indication for chemotherapy with more intensive schedules and potential extended long-term endocrine treatments if tumours are endocrine responsive. All this may benefit the patients since the prognosis of N3 disease has improved over the years.14
The sites of extra-axillary nodes include the internal mammary chain (IMC), the infraclavicular region, the supraclavicular fossa, the breast itself and the interpectoral (Rotter's) space.
About the rate of extra-axillary lymph node detection for [18F]FDG PET/CT, a incidence of 25–28% in stages III and IV breast cancer patients has been reported.12,15 Our results are in concordance with the rate obtained in advanced stages of the disease.
With respect to the CDI, several studies suggest that [18F]FDG PET performs better than CDI in depicting the involvement in high-level axillary (Berg level III) as well as in supraclavicular and internal mammary lymph nodes.11,12 In our series of patient, PET/CT changed the previous cN, upgrading the stage in 38.6% of cases. This percentage was higher than the 17% reported by Aukema et al.12
Axillary FDG uptake added incremental diagnostic confidence to CDI in the same way that other authors have described, although the results were not statistically significant.16 About the global diagnostic parameters, diagnostic accuracy of the combined assessment of CDI and PET/CT was 88%, similar to the 85% reported by Ueda et al.15 The differences were higher for sensitivity and specificity with a very good specificity (94%) and a moderate sensitivity (64%) compared with our results (93.7% and 54.5%, respectively) probably due to the former included patients with operable breast cancer and therefore a lower incidence of lymph node involvement (32%).
It has been reported that the metabolic detection capacity of malignancy for PET/CT in lymph nodes can be related to the tumoral mass in them. Therefore the sensitivity of FDG-PET in the detection of lymph node involvement is dependent on the size of the node and although the lack of anatomical information of FDG PET alone may affect its specificity, integrated FDG PET/CT can provide both morphologic and metabolic information, by combining anatomical CT data and functional FDG PET data.17
Due to in PET/CT we can evaluate the lymph node size in the CT portion, we studied the correlation between size and semiquantitative parameters and observed that the SUV max values in early and delayed PET/CT were higher and statistically significant in bigger lymph nodes compared to the smaller ones.
Crippa et al.18 demonstrated a difference in the sensitivity of PET for detecting axillary disease according to clinical stage: 70% for clinical stage N0 and 100% for stage N2. In our study PET/CT detected all the N2 stages. About the false negatives (FN), PET/CT demonstrated 8 and CDI 7. 5/8 FN for PET/CT was false negative for CDI. The 3 FN only for PET/CT were cN1 whereas the FN only for CDI was mN1 and mN3, respectively.
In other work, it has been reported that excisional biopsy can be a cause of false positive (FP) results in axilla.15 Inflammatory reactions to surgical excision of the primary lesion may have disseminated to the regional axillary lymph node and led to the false positive findings, suggesting that diagnosis of axilla by [18F]FDG PET/CT after excisional biopsy is not recommended.19,20 In the 3 FP showed by PET/CT, no previous biopsy was demonstrated. The lymph nodes were smaller than 1cm in diameter and the SUV-1 low or moderate (1.3–1.9). 2 of them were FP in CDI that demonstrated 4 FP more.
In relation to the cut-off values, we obtained similar results than Taira et al.21 This group, in an analysis using a SUVmax cut-off value of 2.0 for the axillary lymph node, reported a sensitivity and specificity of 37.0 and 98.5%. In ROC analysis, we obtained that the best cut-off in early PET was of 0.90 for the best sensitivity and of 1.95 for the best specificity. None single value of SUV-1, SUV-2 or RI demonstrated good diagnostic statistical parameters.
There is little experience about the application of dual time point imaging in the lymph node evaluation in breast cancer. Choi et al.8 found that the performance of 1h and 3h PET/CT were identical in the detection of axillary lymph node metastasis, showing a sensitivity of 60.3% and specificity of 84.7%. They reported than RI was not useful in distinguishing between benign and malignant lymph nodes. Their results can be explained by the low rate of malignant lymph nodes confirmed by pathology (42.7%) due to the early stage of disease in most of their patients. In our work, about the patients without lymph node involvement, only 3 cases showed positive PET uptake in early PET that increased faintly in the delayed PET in 2 cases and kept in 1.
In our sample of patients, dual time-point imaging did not improve the overall performance of [18F]FDG PET/CT in detecting axillary lymph node metastasis in breast cancer patients, as other authors reported.8 In our work, none of the 8 FN cases in the early PET showed significant FDG uptake in the delayed PET.
A limitation of this study was that we were unable to secure histopathological confirmation in all extra-axillary suspected lymph node metastases detected by [18F]FDG PET/CT imaging. Nonetheless, due to the high PPV reported by other series, routinely histopathologic confirmation in all the locations is not recommended.22,23
Conclusion[18F]FDG PET/CT was able to detect extra-axillary lymph node involvement in one-fourth of the patients with stage II–III breast cancer, similar to previously reported results.
Semiquantitative lymph node analysis does not appear to have any advantage over the visual evaluation for the correct classification of lymph nodes, although, owing to the limited literature, more investigation is necessary.
Conflicts of interestThe authors have no conflicts of interest to declare.