Primary immune thrombocytopenia (pITP) is a hematological disorder characterized by the destruction of platelets by an immunological phenomenon. The main therapeutic objective is the rapid and sustained increase in platelet count to reduce the risk of bleeding. There are discrepancies between the prognostic factors in children and adult patients.
ObjectiveTo determine the prognostic factors (clinical, laboratory, sociodemographic) for the development of chronic and persistent pITP in adults in two institutions of the Department of Antioquia 2012–2018.
Materials and methodsObservational, analytical study, longitudinal design based on information obtained from a cohort of patients. Bivariate and multivariate analysis techniques (logistic regression) are used to study predictors and model development.
ResultsChronic persistent pITP corresponds to 65% of patients, predominantly women (82%). There is an association with chronicity between the variables of symptom times greater than 15 days (OR: 5.45; 95% CI: 2.3–12.5), dexamethasone treatment (OR: 0.074; 95% CI: 0.023–0.23), prednisolone treatment (OR: 0.35; 95% CI: 0.125–1.034). A predictive model was developed, including variables: time of symptoms and treatment (sensitivity of 87.5%, specificity of 52.94%, PPV 78%, NPV 69%), and 3 risk groups were estimated.
ConclusionsThere are clinical and paraclinical factors that can predict the risk of chronicity for pITP in the adult population; these findings must be confirmed by prospective studies and external validation of the multivariate model proposed in the present investigation.
La trombocitopenia inmune primaria (TIP) es un desorden hematológico, caracterizado por la destrucción de las plaquetas y mediado por un fenómeno inmunológico. El principal objetivo terapéutico es el aumento rápido y sostenido del recuento plaquetario para disminuir el riesgo de sangrado. Existen discrepancias en torno a los factores pronósticos en la población infantil y en los pacientes adultos.
ObjetivoDeterminar los factores pronósticos (clínicos, de laboratorio, sociodemográficos) para el desarrollo de la TIP persistente-crónica en adultos, en 2 instituciones de tercer nivel del Departamento de Antioquia en el periodo 2012–2018.
Materiales y métodosEstudio observacional, analítico, diseño longitudinal a partir de la información obtenida de una cohorte de pacientes. Se utilizaron técnicas de análisis bivariado y multivariado (regresión logística) para estudio de predictores y desarrollo de modelo.
ResultadosLa TPI persistente-crónica correspondió al 65% de los pacientes, con predomino de mujeres (82%). Se encontró asociación con cronicidad entre las variables tiempos de síntomas mayor de 15 días (OR: 5,45; IC 95%: 2,3–12,5), tratamiento con dexametasona (OR: 0,074; IC 95%: 0,023–0,23), o tratamiento con prednisolona. Se desarrolló un modelo predictivo en el que se incluyeron las variables tiempo de síntomas y tratamiento, con sensibilidad de 87,5%, especificidad de 52,94% y valores predictivos positivo de 78% y negativo de 69%. Se estimaron tres grupos de riesgo a partir del modelo desarrollado.
ConclusionesExisten factores clínicos y paraclínicos que pueden predecir el riesgo de cronicidad para TIP en la población adulta, estos hallazgos tienen que ser confirmados mediante estudios prospectivos y validación externa del modelo multivariado propuesto en la presente investigación.
Primary immune thrombocytopenia (pITP) is a hematologic disorder characterized by the destruction of platelets and mediated by an immunologic phenomenon.1 It is defined as a platelet count less than 100 × 109/L and the absence of conditions that could explain the decrease in the count.1–3 It can be classified according to the stage of the disease: newly diagnosed (within 3 months after diagnosis), persistent (3–12 months), and chronic (greater than one year).2 The disease is characterized by a predisposition to hemorrhagic events that might be mild, such as petechiae, or severe, such as bleeding from the gastrointestinal tract and central nervous system.4–6
Data on the epidemiology of the disease in Colombia are limited. The incidence in adults in the United Kingdom has been estimated at 3.9 cases per 100,000 people/year, being higher in women (4.5 cases per 100,000 people/year); likewise, the incidence of chronic ITP increases in older groups—prevalence among adults in the United States. The prevalence rate in the U.S. is approximately 3.3 cases per 100,000 adults.7–10 In relation to clinical manifestations such as bleeding, in those over 60 years, the annual rate of ITP with fatal bleeding is 0.13 events per year/person, while for non-fatal bleeding, it is 0.719.11
The ITP has a variable clinical course according to age: pediatric patients respond better to first-line treatment and reach global responses close to 80%, in contrast to adults, whose response is around 63%, have a trend towards chronicity, higher relapses and need for long steroid cycles to achieve stable platelet counts12–16; however, adverse effects associated with chronic steroid use have been documented, as well as deterioration in multiple domains of quality of life (work, reproductive health, functionality, mental health, etc.). Therefore, predicting the initial response to treatment or the risk of subsequent relapse is difficult. Some research, primarily in pediatrics, has been done to determine potential predictive factors,17–19 but information in adults is limited. The proposed research hypothesis was that there are differences in the prognostic factors of patients who develop newly diagnosed ITP compared to those who develop persistent and chronic ITP.
Because patients who develop persistent and chronic ITP require a timely approach and rapid decision-making regarding the change of therapy in order to avoid severe complications, the objective of this work was to explore the prognostic factors (clinical, paraclinical, and sociodemographic) for the development of chronic and persistent immune thrombocytopenia in the adult population of 2 health institutions in the Department of Antioquia (Colombia), and build a predictive model that allows identifying the population at risk.
Materials and methodsAn observational, longitudinal, analytical, follow-up study of a cohort of patients diagnosed with ITP, in which 2 groups were compared according to the outcome: recently diagnosed ITP (ITPr) and persistent (ITPp). The source of information was secondary; patients entered the cohort at the time of initial diagnosis of ITP established at the consultation.
Two measurements were performed on all the patients who made up the cohort: the first one, at the time of admission to the cohort, allowed to identify the recent diagnosis of ITP and collect information about sociodemographic aspects, laboratory tests, and established treatment. The second measurement was the follow-up recorded in the clinical history at 6 months of diagnosis of all patients. The evolution of each patient made it possible to classify ITP as newly diagnosed, in the case of sustaining a complete response 6 months after the initial event, without relapse episodes or the need to continue chronic pharmacological treatment. In contrast, the second group was classified as persistent-chronic ITP because it did not meet the abovementioned criteria.
The sample consisted of patients over 18 years old with newly diagnosed, persistent, and chronic ITP who attended two third-level institutions in the Department of Antioquia between 2012 and 2018. Patients with HIV-associated ITP, hepatitis C and B, systemic lupus erythematosus, and oncology diagnosis were excluded. No imputation technique was performed. As a dependent variable, the presence of newly diagnosed ITP or persistent-chronic ITP was established; the group of persistent ITP and chronic ITP was encoded in a single category (ITPp), allowing the dichotomous analysis of the outcome variable. The Charlson comorbidity index was calculated from the variables age, diabetes, liver disease, malignancy, AIDS, heart failure, chronic kidney failure, myocardial infarction, chronic obstructive pulmonary disease (COPD), peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia, connective tissue disease, and peptic ulcer, and was encoded into 2 categories (0: no comorbidity; 1: more than one comorbidity).
A univariate, bivariate, and multivariate analysis was carried out between the independent and dependent variables. In the univariate analysis for variables of a qualitative nature, absolute and relative frequencies (proportions) were calculated. For variables of quantitative nature, the distribution was established by means of the Shapiro–Wilk test, and measures of central tendency and dispersion were calculated. In the bivariate analysis, to determine the association of the variable of interest with qualitative variables, the Chi-square test (χ2) of independence and the Student’s T test or the Mann–Whitney U test was used, according to the normality test for quantitative variables.
In the multivariate analysis, a binary logistic regression analysis was performed, and an ENTER model was constructed with each of the variables previously identified as potential predictors. Likewise, the multivariate model also included variables according to the odds ratio (OR) values, statistical significance, Hosmer–Lemeshow test (p < 0.2), and clinical relevance. The calibration of the model was evaluated by the Hosmer–Lemeshow goodness-of-fit test and the discriminatory capacity of the model using the ROC curve. The internal validation test and the overfitting by Bootstrap resampling techniques were carried out with 100 samples without changes in the coefficients.20,21 To facilitate potential clinical use, the probability of presenting persistent-chronic ITP was estimated using the logistic regression equation, which uses the coefficients of the variables included in the final model. The result allowed us to estimate three risk groups; the analyses were carried out with the SPPS® statistical software and Stata 14®.
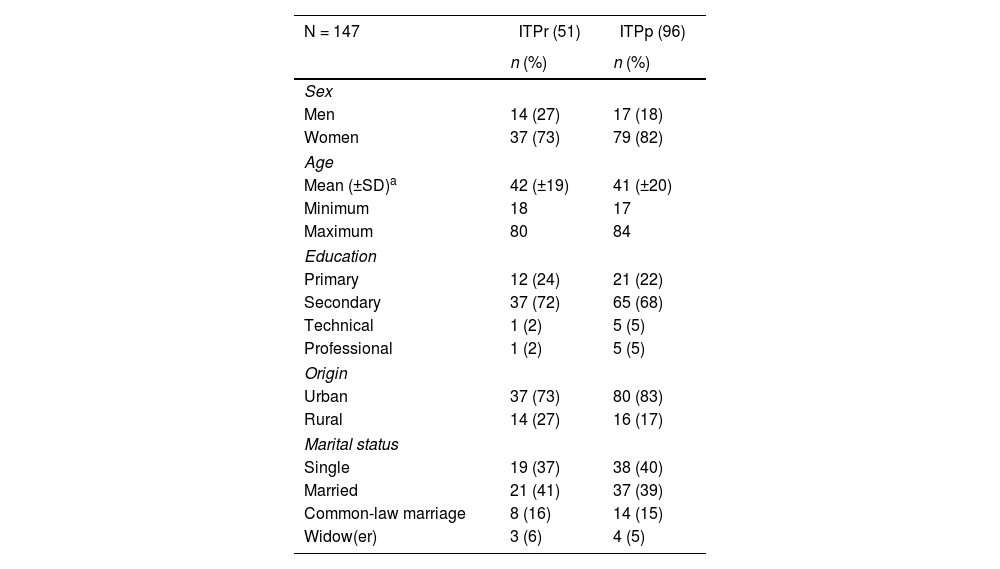
ResultsData were collected from 147 patients with the characteristics of interest; of the total patients diagnosed with ITP, 65% (96) presented persistent-chronic ITP. Regarding sociodemographic characteristics, the average age of patients with newly diagnosed ITP and persistent-chronic ITP was similar (ITPp 42 years and ITPp 41 years). Likewise, the highest proportion of patients had secondary education (ITPp 72% and ITPp 68%) and came from the urban area (ITPp 72% and ITPp 83%) (Table 1).
Sociodemographic characteristics of patients with primary immune thrombocytopenia, 2012–2018.
| N = 147 | ITPr (51) | ITPp (96) |
|---|---|---|
| n (%) | n (%) | |
| Sex | ||
| Men | 14 (27) | 17 (18) |
| Women | 37 (73) | 79 (82) |
| Age | ||
| Mean (±SD)a | 42 (±19) | 41 (±20) |
| Minimum | 18 | 17 |
| Maximum | 80 | 84 |
| Education | ||
| Primary | 12 (24) | 21 (22) |
| Secondary | 37 (72) | 65 (68) |
| Technical | 1 (2) | 5 (5) |
| Professional | 1 (2) | 5 (5) |
| Origin | ||
| Urban | 37 (73) | 80 (83) |
| Rural | 14 (27) | 16 (17) |
| Marital status | ||
| Single | 19 (37) | 38 (40) |
| Married | 21 (41) | 37 (39) |
| Common-law marriage | 8 (16) | 14 (15) |
| Widow(er) | 3 (6) | 4 (5) |
ITPp: persistent-chronic immune thrombocytopenia; pITPr: recently diagnosed primary immune thrombocytopenia.
Regarding clinical characteristics, a frequency of symptoms greater than 15 days was found in 72% (68) of patients with persistent-chronic ITP (p < 0.05), no deaths associated with bleeding were identified in the study participants, and clinical manifestations such as petechiae and mucosal bleeding were the most frequent (close to 80% in both groups).
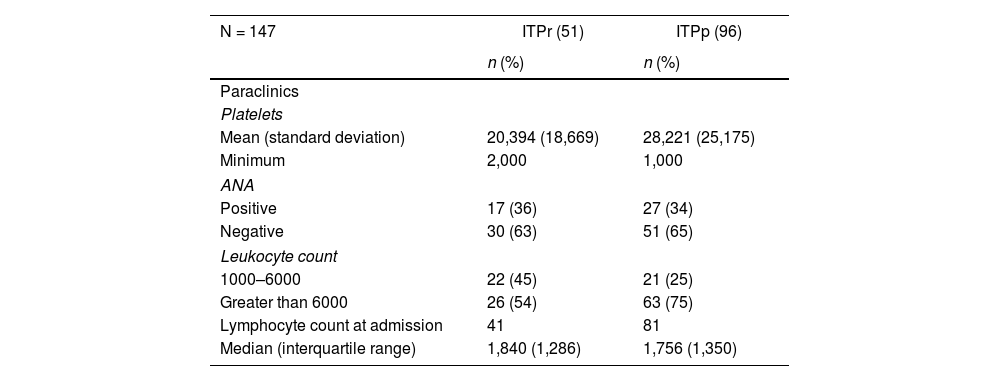
Regarding the paraclinical variables, in the group of patients with persistent-chronic ITP, a higher average platelet count was observed at the time of diagnosis concerning the recently diagnosed ITP patient group, as well as a higher proportion of negative antinuclear antibody (ANA) result in both groups. 45% of patients with newly diagnosed ITP had a leukocyte count between 1,000 and 6,000, compared to 25% of patients with persistent-chronic ITP (Table 2). Of the 96 patients with persistent and chronic ITP, only 14% (14) had a gastric biopsy report, with a positive result for Helicobacter Pylori (H. pylori) in 10 patients.
Distribution of paraclinical variables of patients with primary immune thrombocytopenia, 2012–2018.
| N = 147 | ITPr (51) | ITPp (96) |
|---|---|---|
| n (%) | n (%) | |
| Paraclinics | ||
| Platelets | ||
| Mean (standard deviation) | 20,394 (18,669) | 28,221 (25,175) |
| Minimum | 2,000 | 1,000 |
| ANA | ||
| Positive | 17 (36) | 27 (34) |
| Negative | 30 (63) | 51 (65) |
| Leukocyte count | ||
| 1000–6000 | 22 (45) | 21 (25) |
| Greater than 6000 | 26 (54) | 63 (75) |
| Lymphocyte count at admission | 41 | 81 |
| Median (interquartile range) | 1,840 (1,286) | 1,756 (1,350) |
ANA: antinuclear antibodies; ITPp: persistent-chronic immune thrombocytopenia; pITPr: recently diagnosed primary immune thrombocytopenia.
Regarding treatment, of the total patients who entered the cohort, 70% (103) received some corticosteroid treatment protocol as the only initial therapy; among these, 64 patients received prednisone, and 39 received dexamethasone treatment. Of the total patients receiving initial prednisone treatment, 72% (46) progressed to chronic ITP, while of the patients receiving dexamethasone treatment, 33% (13) progressed to chronicity. Of the total patients who entered the cohort, 14% (20) received initial treatment with erythropoietin + corticosteroid analogs, and of these, 80% (16) progressed to chronicity. Other treatments initially given to patients were: splenectomy + steroid (5%), corticosteroids + immunoglobulin G (3%), and 4% of patients did not receive any initial treatment.
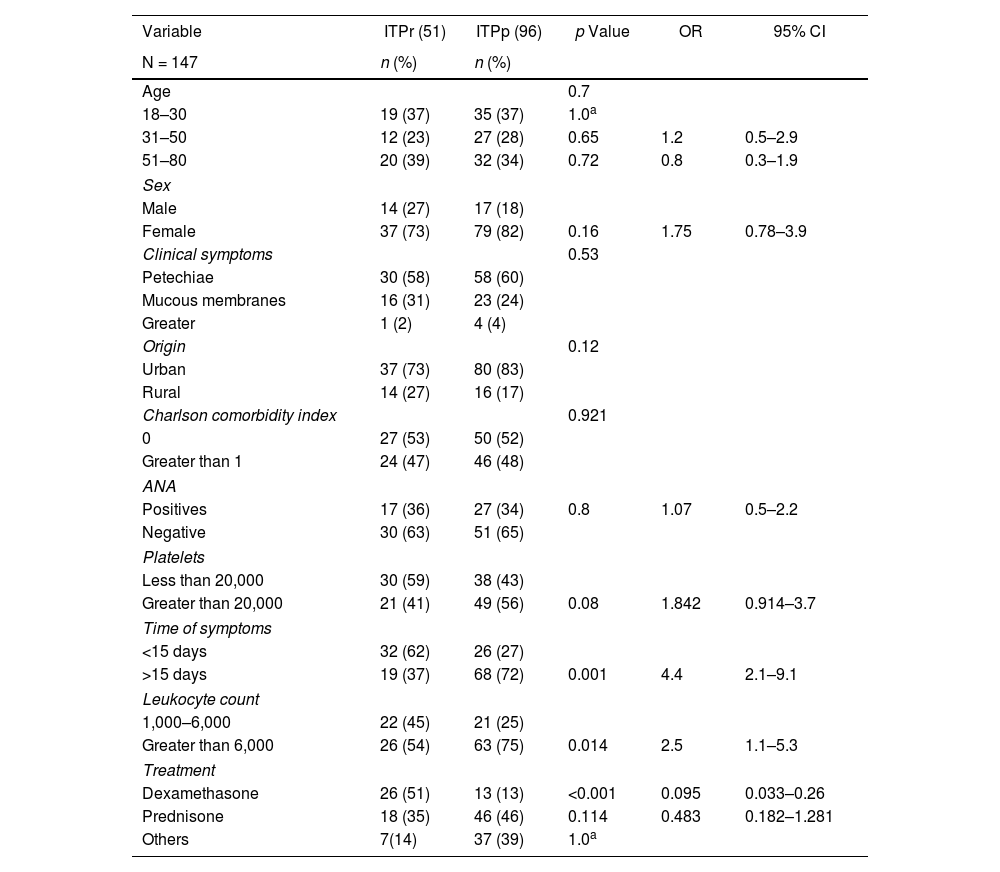
The bivariate analysis found a significant association of the dependent variable with leukocyte count, time of symptoms, and treatment (p < 0.05). There was no association for other variables (origin, age, marital status, Charlson comorbidity index, clinical manifestations, ANA positive, platelet count, and lymphocyte count) (Table 3).
Factors associated with chronicity of patients with primary immune thrombocytopenia, 2012–2018.
| Variable | ITPr (51) | ITPp (96) | p Value | OR | 95% CI |
|---|---|---|---|---|---|
| N = 147 | n (%) | n (%) | |||
| Age | 0.7 | ||||
| 18–30 | 19 (37) | 35 (37) | 1.0a | ||
| 31–50 | 12 (23) | 27 (28) | 0.65 | 1.2 | 0.5–2.9 |
| 51–80 | 20 (39) | 32 (34) | 0.72 | 0.8 | 0.3–1.9 |
| Sex | |||||
| Male | 14 (27) | 17 (18) | |||
| Female | 37 (73) | 79 (82) | 0.16 | 1.75 | 0.78–3.9 |
| Clinical symptoms | 0.53 | ||||
| Petechiae | 30 (58) | 58 (60) | |||
| Mucous membranes | 16 (31) | 23 (24) | |||
| Greater | 1 (2) | 4 (4) | |||
| Origin | 0.12 | ||||
| Urban | 37 (73) | 80 (83) | |||
| Rural | 14 (27) | 16 (17) | |||
| Charlson comorbidity index | 0.921 | ||||
| 0 | 27 (53) | 50 (52) | |||
| Greater than 1 | 24 (47) | 46 (48) | |||
| ANA | |||||
| Positives | 17 (36) | 27 (34) | 0.8 | 1.07 | 0.5–2.2 |
| Negative | 30 (63) | 51 (65) | |||
| Platelets | |||||
| Less than 20,000 | 30 (59) | 38 (43) | |||
| Greater than 20,000 | 21 (41) | 49 (56) | 0.08 | 1.842 | 0.914–3.7 |
| Time of symptoms | |||||
| <15 days | 32 (62) | 26 (27) | |||
| >15 days | 19 (37) | 68 (72) | 0.001 | 4.4 | 2.1–9.1 |
| Leukocyte count | |||||
| 1,000–6,000 | 22 (45) | 21 (25) | |||
| Greater than 6,000 | 26 (54) | 63 (75) | 0.014 | 2.5 | 1.1–5.3 |
| Treatment | |||||
| Dexamethasone | 26 (51) | 13 (13) | <0.001 | 0.095 | 0.033–0.26 |
| Prednisone | 18 (35) | 46 (46) | 0.114 | 0.483 | 0.182–1.281 |
| Others | 7(14) | 37 (39) | 1.0a | ||
ANA: antinuclear antibodies; 95% CI: 95% confidence interval; OR: odds ratio; ITPp: persistent-chronic immune thrombocytopenia; pITPr: recently diagnosed primary immune thrombocytopenia.
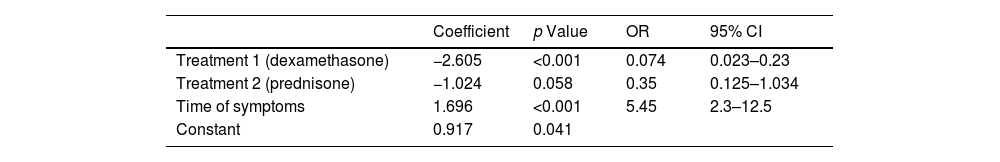
The following variables were included for multivariate analysis in the initial model: leukocyte count, platelet count, time of symptoms, treatment, and presence of positive ANA; subsequently, positive ANA variables, platelet count, and leukocyte count were excluded since they lost statistical significance in the model. For the final model, the time of symptoms and treatment variables were included (Table 4). Sensitivity, specificity, positive predictive value, and negative predictive value tests were performed (Table 5).
The probability of presenting chronic ITP was estimated to facilitate potential clinical use using the logistic regression equation, which uses the coefficients of the variables included in the final model. The result allowed estimating of three risk groups for chronic ITP (Table 6).
Risk groups for chronic ITP.
| A risk group. | Probability |
|---|---|
| Low risk: use of dexamethasone with symptom time less than 15 days | 15.6% |
| Intermediate risk: use of dexamethasone with symptom time greater than or equal to 15 days | 50.19% |
| Intermediate risk: use of prednisone with symptom time less than 15 days | 47.32% |
| High risk: use of prednisone with symptom time greater than or equal to 15 days | 83.04% |
pITP: primary immune thrombocytopenia.
The epidemiology of ITP is mostly described in European studies,8–10,16,22 with great ignorance of the behavior of the disease in Latin America. This research makes an approximation to the behavior of this disease in the Antioquia Department (Colombia); a clinical prediction model with internal validation is developed to make an approximation to the clinical course of adult patients with chronic ITP, including prognostic, clinical, and treatment factors available in the care of this disease.
In the present study, chronic ITP was found in 65% of patients, coinciding with that reported by Moulis et al. (prevalence of 50.4%). Likewise, it was found that patients come predominantly from the urban area (greater than 70%), that the largest proportion of patients were women, and no statistically significant association was found between sex and chronicity, a result that coincides with the series of Moulis et al.22
Since studies in adults are limited, the results were compared with some in children, such as those described in the meta-analysis of Heitink-Pollé et al., in which insidious onset, absence of previous infection, mild bleeding, and higher platelet counts are the most important risk factors for chronic ITP.18 In the present study, the time variable of symptoms greater than or equal to 15 days is significantly associated with chronicity (OR: 5.45; 95% CI: 2.3–12.5), a variable that was not explored in the studies of Grimaldi-Bensouda et al. and Moulis et al. For the other variables, such as comorbidities, age, and clinical manifestations of bleeding, the present study found no statistically significant association with the development of chronic ITP. This result is consistent with the French cases series.16,22
In the studies of Grimaldi-Bensouda et al. and Moulis et al., the platelet count variable greater than 20 × 109/L has no statistical association with the development of chronic ITP, as in this study; on the contrary, in the child population, this variable was described as a strong predictor of chronicity in the meta-analysis of Heitink-Pollé et al., with OR: 2.15 and a 95% CI: 1.63–2.83).18 The present study evidences the low number of gastric biopsy reports in patients with ITP, which suggests underdiagnosis for this association. It should be noted that international series have reported the presence of H. pylori in up to 72% of patients with ITP.23
ANA positivity is considered a predictor for chronicity in children, as described by the meta-analysis of Heitink-Pollé et al. (OR: 2.87; 95% CI: 1.57–2.54).18 This variable can be controversial in the adult population since, in the series of Moulis et al., it has a significant association with the development of chronic ITP (OR: 2.89; 95% CI: 1.08–7.74)22 and, on the contrary, in this study, it is not statistically significant (OR: 1.07; p = 0.8). Despite being considered a biologically plausible relationship, the likely discrepancies lie in the cut-off values of the antibody titers in the different case series.
Regarding treatment, in the present study, the initial use of dexamethasone for the management of ITP suggests decreasing chronicity based on patients who required additional treatments. Initial corticosteroid use remains a point of contention, with dexamethasone research reporting sustained responses greater than 50% at 6 months.15 The meta-analysis of Mithoowani et al. highlights the equality of treatments between dexamethasone and prednisolone when comparing one to 3 cycles of dexamethasone (40 mg/for 4 days) with prednisolone (1 mg per kg/14–28 days), with no difference in the overall response at 6 months (54 vs. 43%; RR: 1.6; 95% CI: 0.79–1.71; p = 0.4).24 At 12 months follow-up, the study of Wei et al. reports a sustained response of 36.8% for dexamethasone vs. 33.0% for prednisolone.15 The previous studies were carried out in controlled settings, with strict dose monitoring and variability in treatment cycles; on the contrary, the findings of the present research reflect the reality of daily treatment of this disease in the region: variability in doses, difficulties in adherence to prednisolone therapy, variable cycles with dexamethasone.
In relation to other treatment options, the meta-analysis of Heitink-Pollé et al. reports risk of chronicity with the association of methylprednisolone and immunoglobulin (OR: 2.67; 95% CI: 1.44–4.96)18; this relationship was not reported in the studies of Grimaldi-Bensouda et al. and Moulis et al.16,22 In this study, it was found that of 4 patients who required corticosteroids and immunoglobulin, 3 presented as a chronicity outcome. It is important to highlight, given that the health institutions included in the study are reference centers of other municipalities, that the clinical course of the disease can vary towards refractoriness to treatment and need other additional lines to achieve clinical response.
A multivariate model was built from the results obtained, initially for predictive purposes to predict chronicity in patients with ITP in the absence of models in the literature in the adult population. The final model included the time of symptoms (less than 15 days or greater or equal to 15 days) and treatment (dexamethasone or prednisolone) and estimated the probability of chronic ITP in 3 risk groups (low, intermediate, and high). The characterization of patients for chronicity in these groups would help the initial approach to the problem under study, being a relevant tool for the doctor who provides care to patients with this condition.
Among the limitations of the study is that it is a retrospective design, which could favor the absence of data; information on some variables such as platelet volume, anticardiolipin antibodies, lupus anticoagulant, anti-DNA, or ANA titers was not always available. Patients between the ages of 15 and 18 were excluded due to the institutions’ ethics protocol. The study was carried out with data from patients who attended two third-level centers of the Department of Antioquia, which could lead to selection bias since there may be patients with less severity who attended institutions of different levels of complexity or were referred to other care centers.
ConclusionsThere are clinical and paraclinical factors that can predict the risk of chronicity for ITP in the adult population, such as initial treatment and time of symptoms. These findings have to be confirmed by prospective studies and external validation of the multivariate model proposed in the present research.
Ethical considerationsThe study respected the international norm of the Declaration of Helsinki and the Colombian norms decreed by Resolution 8430 of 1993. The study is classified as minimal risk. The information was collected from the medical records without contacting the patients. The Research Committee of Universidad CES and health institutions approved the research.
Conflict of interestsThe authors declare no conflict of interest.
To the Clínica Some (Rionegro) and the Hospital Manuel Uribe Ángel (Envigado).