Some studies have shown that influenza vaccination is associated with a lower risk of SARS-CoV-2 infection; in patients with COVID-19 infection, admission to intensive care is reduced, with less need for mechanical ventilation, shorter hospital stays, and reduced mortality. This study aimed to determine if a history of annual influenza vaccination impacts the clinical course of SARS-CoV-2 infection during hospitalization.
MethodsThis was an observational, prospective, cohort study of patients older than 65 admitted to the COVID-19 unit from January to June 2021. The history of influenza vaccination over the last 5 years was assessed in each patient during hospitalization. We measured the length of hospital stay, the need for admission to the intensive care unit (ICU), the patient's oxygen requirements, complications during hospitalization, and outcome (medical discharge or death). Patients with a history of vaccination against SARS-CoV-2 were not included.
ResultsWe analyzed 125 patients, 50.4% (n=63) with history of influenza vaccination and 49.6% (n=62) without a history of influenza vaccination. In-hospital mortality was 44.8%, higher in the unvaccinated (54.8%) population (p=0.008). ICU admission was 27% higher in vaccinated (35%) patients (p=0.05). Patients without a history of influenza vaccination had a higher prevalence of cardiac (8% vs. 5%, p=0.04) and renal complications (29% vs. 13%, p=0.02). Patients with a history of vaccination had a greater need for invasive mechanical ventilation (25.4%, p=0.02).
ConclusionIn this study, a history of influenza vaccination in older adults with SARS-CoV-2 infection was related to lower in-hospital mortality.
Algunos estudios han demostrado que la vacunación contra la influenza se asocia con un menor riesgo de infección por SARS-CoV-2; en pacientes con infección por COVID-19, se reduce el ingreso a cuidados intensivos, con menor necesidad de ventilación mecánica, estancias hospitalarias más cortas y reducción de la mortalidad. Este estudio tuvo como objetivo determinar si el antecedente de vacunación anual contra la influenza afecta el curso clínico de la infección por SARS-CoV-2 durante la hospitalización.
MétodosEstudio observacional, prospectivo, de cohorte en adultos mayores de 65 años ingresados en la unidad COVID-19 de enero a junio de 2021. Se evaluó en cada paciente el antecedente de vacunación contra la influenza durante los últimos 5 años durante la hospitalización. Medimos la duración de la estancia hospitalaria, la necesidad de ingreso en la unidad de cuidados intensivos (UCI), los requerimientos de oxígeno del paciente, las complicaciones durante la hospitalización y el desenlace (alta médica o muerte). No se incluyeron pacientes con antecedentes de vacunación contra el SARS-CoV-2.
ResultadosSe analizaron 125 pacientes, el 50,4% (n=63) con antecedente de vacunación contra influenza y el 49,6% (n=62) sin antecedente de vacunación contra influenza. La mortalidad hospitalaria fue del 44,8%, mayor en la población no vacunada (54,8%) (p=0,008). El ingreso en la UCI fue un 27% mayor en los pacientes vacunados (35%) (p=0,05). Los pacientes sin antecedentes de vacunación contra la influenza tuvieron una mayor prevalencia de complicaciones cardíacas (8 vs. 5%; p=0,04) y renales (29 vs. 13%; p=0,02). Los pacientes con antecedente de vacunación tuvieron mayor necesidad de ventilación mecánica invasiva (25,4%; p=0,02).
ConclusiónEn este estudio, el antecedente de vacunación contra influenza en adultos mayores con infección por SARS-CoV-2 se relacionó con una menor mortalidad hospitalaria.
In December 2019, a severe pneumonia outbreak began in Wuhan, Hubei Province, China.1 The International Committee on Taxonomy of Viruses gave it the name severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single positive-stranded RNA virus.2,3 This virus causes COVID-19, characterized by respiratory distress, fever, cough, fatigue, pneumonia, and muscle pain. SARS-CoV-2 spread rapidly internationally, leading the World Health Organization to declare a pandemic.3–5 COVID-19 is classified as mild, moderate, severe, or critical, depending on the degree of respiratory compromise.6 In the United States, mortality was higher among older people, with 80% of deaths occurring in persons 65 years and over.7 Advanced age, chronic diseases, complications, and demographic variables, among others, are risk factors for a fatal outcome.8,9
Influenza is a virus of the RNA Orthomixoviridae family that evolves rapidly due to frequent antigenic variation. Its most important transmission routes are inhalation by aerosols and direct contact with infected people and objects.10 The international recommendation is annual vaccination in individuals older than 6 months.11
Several studies have shown that influenza vaccination is associated with a lower risk of SARS-Cov-2 infection. In patients with COVID-19 infection, admission to intensive care is reduced, with less need for mechanical ventilation, shorter hospital stays, and reduced mortality.12–14 In an Italian study, the regional influenza vaccination coverage rate was inversely associated with SARS-CoV-2 shedding rates and clinical consequences in populations 65 years and older. This study estimated that a 1% increase in the influenza vaccination coverage rate among subjects 65 years or older would reduce SARS-CoV-2 infection of 78,560 subjects, 2512 hospitalized patients with symptoms, and 1989 deaths.15
A retrospective analysis of a cohort of epidemiological surveillance systems conducted in Mexico reported that influenza immunization was an independent protective factor against COVID-19 mortality, this appears greater in patients older than 60.16 Current data on older adults in Mexico regarding hospitalization, complications, and mortality from COVID-19 and its relationship with influenza vaccination is insufficient. Based on this, our aim was to evaluate if annual influenza vaccination impacts the clinical course of SARS-CoV-2 infection in hospitalized patients 60 years old and older.
Patients and methodsStudy designWe conducted an observational, prospective, cohort study approved by the local Research and Ethics Committee with registration GE21-00001. Verbal informed consent was obtained from patients who met the inclusion criteria.
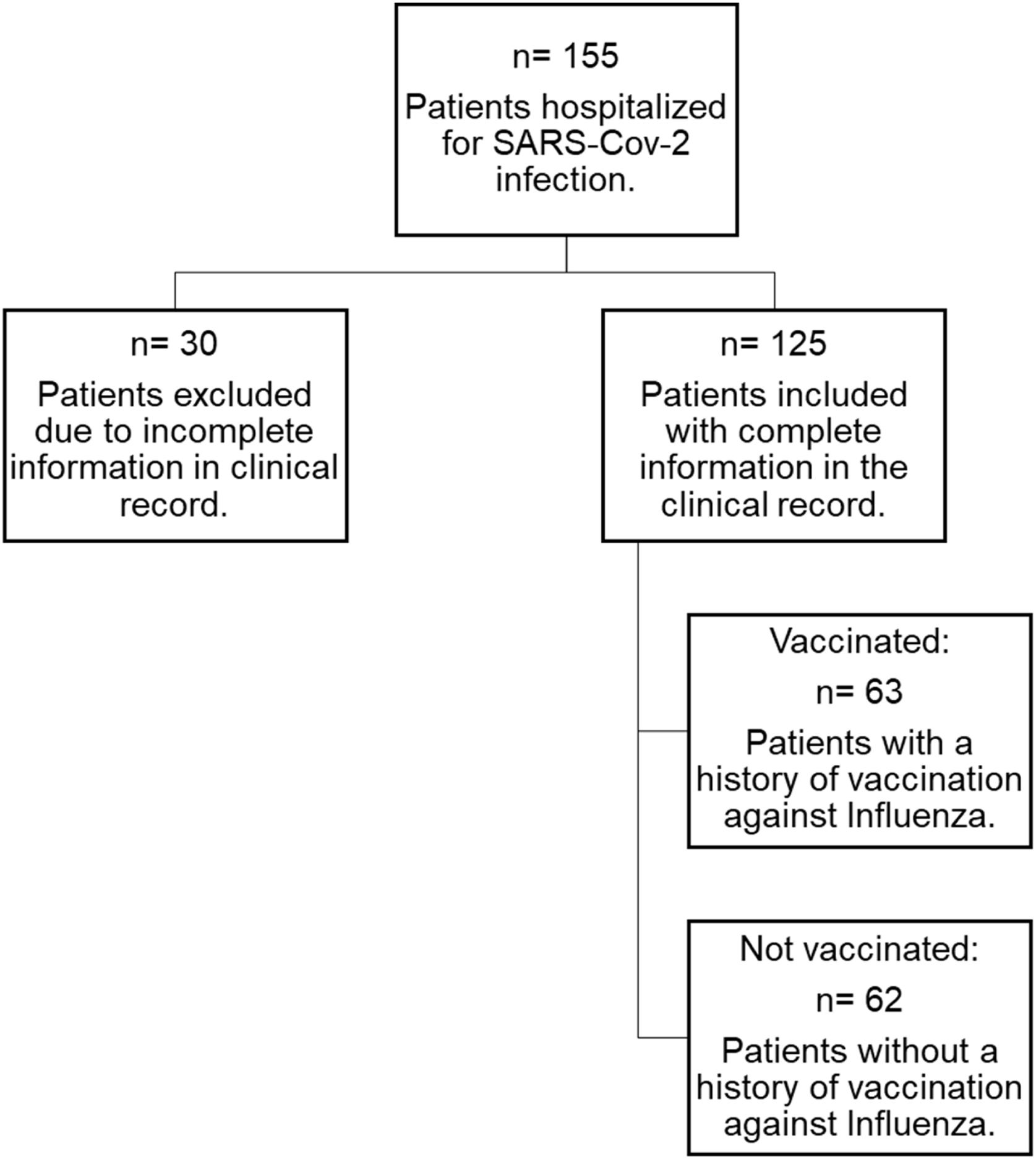
ParticipantsAdult patients admitted to the Acute Respiratory Infections Diagnostic Unit were recruited. One hundred fifty-five patients were assessed between January and July 2021. The inclusion criteria were adults over 65 years with a diagnosis of SARS-CoV-2 confirmed by RT-PCR (Reverse Transcriptase Polymerase Chain Reaction) or antigen from a nasopharyngeal swab or another respiratory specimen. Patients with a history of SARS-CoV-2 vaccination and patients with incomplete clinical records and/or inadequate follow-up information were excluded (Fig. 1).
Data collectionThe history of influenza vaccination was obtained by direct and indirect questioning within the first three hours of hospital admission. The patients were asked about the number of annual influenza vaccine applications (vaccination during the 2019–2020 season and the number of applications in the last five years). The demographic characteristics, comorbidities, clinical course, and frequency of complications were obtained during hospitalization by reviewing the clinical record of each patient.
Data analysisData were analyzed using SPSS Statistics version 23.0 (IBM Corp., Armonk, NY). Categorical variables were summarized using frequencies and percentages. Normally distributed continuous data were presented as means and standard deviations. Non-normally distributed continuous variables were presented as medians and interquartile ranges (IQR). The Mann–Whitney U test was used for continuous variables, and the χ2 test or Fisher's exact test for categorical variables. p-Values<0.05 were considered statistically significant.
ResultsOf the 155 screened, 125 with complete information were included, 50.4% (n=63) had a history of vaccination against influenza, and 49.6% (n=62) did not (Fig. 1). The majority of patients were male. The median age was 73 years (IQR 69–79). Regarding the length of hospital stay, this varied from 1 to 5 days (78.4%). Almost all had comorbidity.
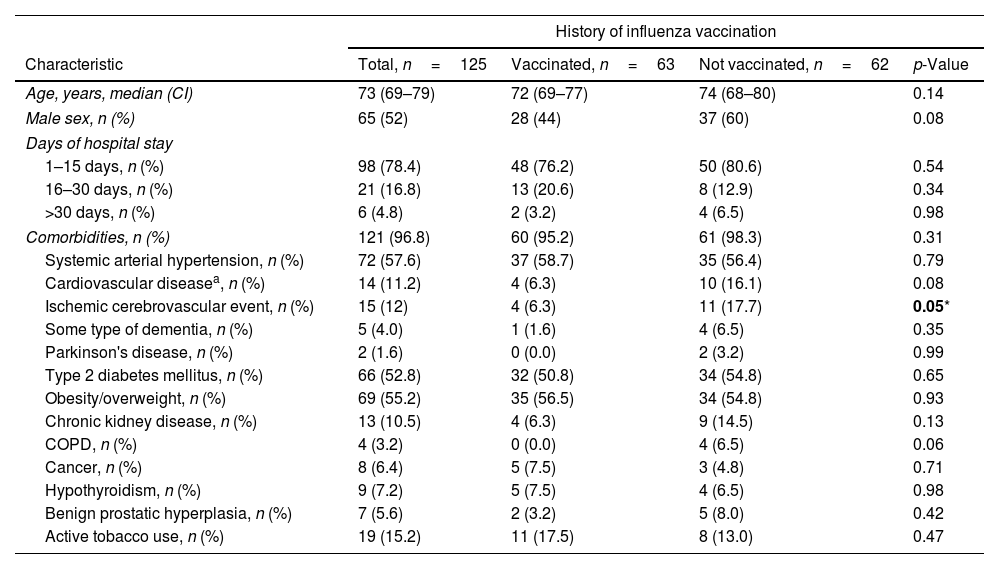
No significant differences were found between the groups regarding demographic characteristics and comorbidities, except in the history of cerebrovascular disease, which was more prevalent in unvaccinated patients (Table 1). In-hospital mortality was 44.8% (n=56) and slightly higher in non-vaccinated patients (55% vs. 37%, p=0.06).
Demographic characteristics and comorbidities of the study population.
| History of influenza vaccination | ||||
|---|---|---|---|---|
| Characteristic | Total, n=125 | Vaccinated, n=63 | Not vaccinated, n=62 | p-Value |
| Age, years, median (CI) | 73 (69–79) | 72 (69–77) | 74 (68–80) | 0.14 |
| Male sex, n (%) | 65 (52) | 28 (44) | 37 (60) | 0.08 |
| Days of hospital stay | ||||
| 1–15 days, n (%) | 98 (78.4) | 48 (76.2) | 50 (80.6) | 0.54 |
| 16–30 days, n (%) | 21 (16.8) | 13 (20.6) | 8 (12.9) | 0.34 |
| >30 days, n (%) | 6 (4.8) | 2 (3.2) | 4 (6.5) | 0.98 |
| Comorbidities, n (%) | 121 (96.8) | 60 (95.2) | 61 (98.3) | 0.31 |
| Systemic arterial hypertension, n (%) | 72 (57.6) | 37 (58.7) | 35 (56.4) | 0.79 |
| Cardiovascular diseasea, n (%) | 14 (11.2) | 4 (6.3) | 10 (16.1) | 0.08 |
| Ischemic cerebrovascular event, n (%) | 15 (12) | 4 (6.3) | 11 (17.7) | 0.05* |
| Some type of dementia, n (%) | 5 (4.0) | 1 (1.6) | 4 (6.5) | 0.35 |
| Parkinson's disease, n (%) | 2 (1.6) | 0 (0.0) | 2 (3.2) | 0.99 |
| Type 2 diabetes mellitus, n (%) | 66 (52.8) | 32 (50.8) | 34 (54.8) | 0.65 |
| Obesity/overweight, n (%) | 69 (55.2) | 35 (56.5) | 34 (54.8) | 0.93 |
| Chronic kidney disease, n (%) | 13 (10.5) | 4 (6.3) | 9 (14.5) | 0.13 |
| COPD, n (%) | 4 (3.2) | 0 (0.0) | 4 (6.5) | 0.06 |
| Cancer, n (%) | 8 (6.4) | 5 (7.5) | 3 (4.8) | 0.71 |
| Hypothyroidism, n (%) | 9 (7.2) | 5 (7.5) | 4 (6.5) | 0.98 |
| Benign prostatic hyperplasia, n (%) | 7 (5.6) | 2 (3.2) | 5 (8.0) | 0.42 |
| Active tobacco use, n (%) | 19 (15.2) | 11 (17.5) | 8 (13.0) | 0.47 |
COPD, chronic obstructive pulmonary disease.
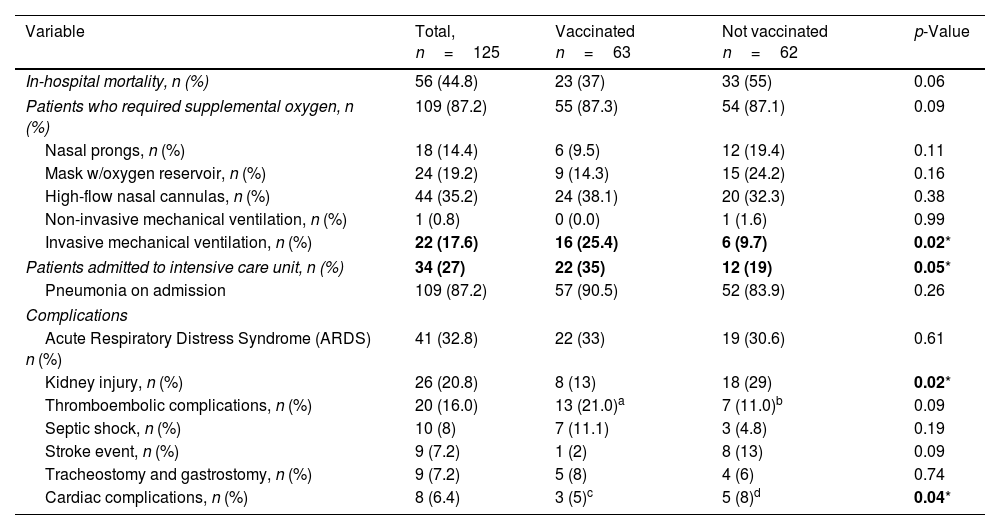
Regarding clinical evolution and in-hospital complications, most patients required supplemental oxygen. Invasive mechanical ventilation and admission to the ICU were more frequent in vaccinated patients; this group also had a lower incidence of renal and cardiac complications (Table 2).
Clinical course and in-hospital complications.
| Variable | Total, n=125 | Vaccinated n=63 | Not vaccinated n=62 | p-Value |
|---|---|---|---|---|
| In-hospital mortality, n (%) | 56 (44.8) | 23 (37) | 33 (55) | 0.06 |
| Patients who required supplemental oxygen, n (%) | 109 (87.2) | 55 (87.3) | 54 (87.1) | 0.09 |
| Nasal prongs, n (%) | 18 (14.4) | 6 (9.5) | 12 (19.4) | 0.11 |
| Mask w/oxygen reservoir, n (%) | 24 (19.2) | 9 (14.3) | 15 (24.2) | 0.16 |
| High-flow nasal cannulas, n (%) | 44 (35.2) | 24 (38.1) | 20 (32.3) | 0.38 |
| Non-invasive mechanical ventilation, n (%) | 1 (0.8) | 0 (0.0) | 1 (1.6) | 0.99 |
| Invasive mechanical ventilation, n (%) | 22 (17.6) | 16 (25.4) | 6 (9.7) | 0.02* |
| Patients admitted to intensive care unit, n (%) | 34 (27) | 22 (35) | 12 (19) | 0.05* |
| Pneumonia on admission | 109 (87.2) | 57 (90.5) | 52 (83.9) | 0.26 |
| Complications | ||||
| Acute Respiratory Distress Syndrome (ARDS) n (%) | 41 (32.8) | 22 (33) | 19 (30.6) | 0.61 |
| Kidney injury, n (%) | 26 (20.8) | 8 (13) | 18 (29) | 0.02* |
| Thromboembolic complications, n (%) | 20 (16.0) | 13 (21.0)a | 7 (11.0)b | 0.09 |
| Septic shock, n (%) | 10 (8) | 7 (11.1) | 3 (4.8) | 0.19 |
| Stroke event, n (%) | 9 (7.2) | 1 (2) | 8 (13) | 0.09 |
| Tracheostomy and gastrostomy, n (%) | 9 (7.2) | 5 (8) | 4 (6) | 0.74 |
| Cardiac complications, n (%) | 8 (6.4) | 3 (5)c | 5 (8)d | 0.04* |
SD, standard deviation.
9 patients diagnosed with pulmonary thromboembolism (PTE) by quantitative D-dimer and chest angio-tomography; 4 patients obtained a suspected diagnosis of PTE with an elevated quantitative D-dimer but chest angio-tomography due to the patient's clinical situation; however, medical treatment was started.
2 patients were diagnosed with PTE by quantitative D-dimer and chest angio-tomography, and 5 patients had a suspected diagnosis of PTE with an elevated quantitative D-dimer but without chest angio-tomography due to the patient's clinical situation; however, medical treatment was started.
All patients in this group had a diagnosis of acute myocardial infarction (AMI) without specifying the ST segment.
Patients who received influenza vaccination at least once in the last 5 years had an in-hospital mortality of 30.8%, vs. 54.8% in those with no history of influenza vaccination (p=.008) (Table 3). Additionally, an analysis was carried out on patients who received influenza vaccination during the 2019–2020 period, finding an in-hospital mortality of 45% (n=18/40), compared to 44.7% (n=38/85) of the group without history of influenza vaccination (p=0.97). The patients that received the vaccination in the last 5 years, 36.5% were vaccinated once, 7.7% were vaccinated twice and 55.7% were vaccinated 3 times or more.
In-hospital mortality in patients vaccinated against influenza on at least one occasion in the last 5 years.
| Total, n=125 | Group of patients vaccinated in the last 5 years, n=52 | Group of patients not vaccinated in the last 5 years, n=73 | p-Value | |
|---|---|---|---|---|
| In-hospital mortality, n (%) | 56 (44.8) | 16 (30.8) | 40 (54.8) | 0.008* |
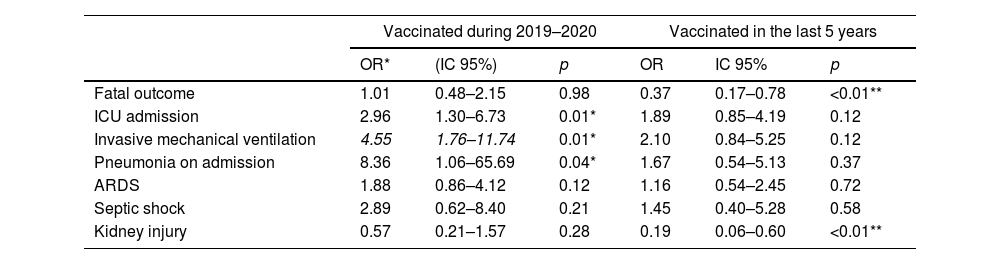
Table 4 reports the logistic regression analysis that evaluated the risk of in-hospital complications in patients vaccinated against influenza. A history of vaccination during the year prior to the study (2019–2020) was associated with an increased risk in ICU admission, requirement for invasive mechanical ventilation, and pneumonia upon admission, with no increased risk of complications such as fatal outcome, ARDS, septic shock, or kidney injury. When performing the multivariate analysis adjusted to the significant outcomes, no increased risk was identified in any of the complications reported above. A history of vaccination in the last 5 years was associated with a reduced risk of fatal outcome and kidney injury in the univariate analysis. When performing the multivariate analysis, the reduction in risk of fatal outcome persisted with an OR of 0.43 (95% CI 0.20–0.94, p=0.03) and of kidney injury with an OR of 0.23 (95% CI 0.07–0.73, p=0.01).
Logistic regression for the risk of in-hospital complications in patients vaccinated against influenza.
| Vaccinated during 2019–2020 | Vaccinated in the last 5 years | |||||
|---|---|---|---|---|---|---|
| OR* | (IC 95%) | p | OR | IC 95% | p | |
| Fatal outcome | 1.01 | 0.48–2.15 | 0.98 | 0.37 | 0.17–0.78 | <0.01** |
| ICU admission | 2.96 | 1.30–6.73 | 0.01* | 1.89 | 0.85–4.19 | 0.12 |
| Invasive mechanical ventilation | 4.55 | 1.76–11.74 | 0.01* | 2.10 | 0.84–5.25 | 0.12 |
| Pneumonia on admission | 8.36 | 1.06–65.69 | 0.04* | 1.67 | 0.54–5.13 | 0.37 |
| ARDS | 1.88 | 0.86–4.12 | 0.12 | 1.16 | 0.54–2.45 | 0.72 |
| Septic shock | 2.89 | 0.62–8.40 | 0.21 | 1.45 | 0.40–5.28 | 0.58 |
| Kidney injury | 0.57 | 0.21–1.57 | 0.28 | 0.19 | 0.06–0.60 | <0.01** |
When performing the multivariate analysis adjusted to the significant variables, none of the outcomes were statistically significant in patients who were vaccinated during 2019–2020.
When performing multivariate analysis adjusted to the significant variables, patients who were vaccinated in the last 5 years persisted with an OR of 0.43 (95% CI 0.20–0.94, p=0.03) for fatal outcome and an OR of 0.23 (95%CI 0.07–0.73, p=0.01) for kidney injury. ICU, intensive care unit; ARDS, Acute Respiratory Distress Syndrome.
This study evaluated the impact of influenza vaccination on adult patients older than 65 hospitalized for COVID-19 pneumonia. The effect of non-pharmacological interventions to control SARS-CoV-2 transmission, such as using a face mask, suggests that these measures, combined with influenza vaccination, could also be important public health tools during influenza season.17,18 The findings in our study were (1) In-hospital mortality from COVID-19 pneumonia in adults older than 65 years was 44.8%, which was significantly lower when at least one influenza vaccination was reported in the last 5 years before infection; (2) there is a greater tendency of cardiovascular, thromboembolic, and cerebrovascular events and the development of acute kidney disease in patients who do not have a history of vaccination against seasonal influenza; (3) there is a greater requirement for invasive mechanical ventilation and admission to the ICU in patients with no history of influenza vaccination.
Influenza vaccination keeps the immune system active through toll-like receptor 7 (TLR-7). This receptor significantly binds to single-stranded RNA respiratory viruses, such as SARS-CoV-2, due to the similarity in their structures. Immune system stimulation by these vaccines could occur through early activation (cross-reactivity), facilitating early detection of SARS-CoV-2 infection.12,15,19 Influenza is a leading infectious cause of morbidity and mortality in older adults,20 with about 4 million cases yearly in the United States and accounting for more than three-quarters of vaccine-preventable diseases in people 65 and older. It is also responsible for half of the healthcare costs of vaccine-preventable diseases.21
Mortality of 35.6% has been reported in patients admitted to the ICU due to COVID-19 infection, with 18.4% belonging to the 65–74-year age group and 48.2% being older than 75.22 A history of influenza vaccination reduces the risk of hospitalization and death in patients with COVID-19, according to various studies. Eldanasory et al. found that a 10% increase in influenza vaccination coverage was associated with a 28% reduction in death from COVID-19 in older adults. In a pre-print study of 92,664 COVID-19 patients in which about one-third received influenza vaccination, there was 17% less death among influenza-vaccinated patients.12 A study in Brazil that estimated the association between influenza vaccination status and COVID-19 mortality found that influenza vaccination was associated with a 35% reduction in death among COVID-19 patients.14 Candelli et al. showed that influenza vaccination was independently associated with a lower risk of death from COVID-19 at 60 days.23
Finally, in a study in Mexico, a mortality of 7% was reported in patients with no history of influenza vaccination compared to 3.5% in patients with a history of vaccination (p≤0.0001).16 In our study, there was a mortality of 44.8% in individuals>65 years, with 37% in vaccinated patients and 55% in unvaccinated patients. Although this difference was not statistically significant, we found that mortality was 30.8% compared to 54.8%, respectively (p=0.008) in the sub-analysis of patients vaccinated at least once in the last 5 years, which was also reflected by the reduction in mortality risk with an OR of 0.43 (95% CI 0.20–0.94, p=0.03). This finding complements previous studies that suggest that an annual influenza vaccination strategy reduces the number of intrahospital deaths in elderly COVID-19 patients.
Patients with a history of vaccination in our study had a lower prevalence of cardiovascular disease, ischemic cerebrovascular events, chronic kidney disease, and COPD at admission. Although this difference was not significant in all cases, the lower tendency of these diseases could be a potential cause of the lower incidence of cardiac and renal complications, regardless of the vaccination history.
Acute kidney injury (AKI) during COVID-19 infection has been significantly associated with increased mortality, which has been reported as high as 91.7% compared to 8.8% in patients without kidney injury. The prevalence of AKI varied from 3% to 29%, depending on the presence of other comorbidities and a history of pre-existing chronic kidney disease. Some mechanisms involved include (1) downregulation of angiotensin-converting enzyme (ACE) expression with the subsequent increase in angiotensin II (AGII), which participates as a proinflammatory marker and generates increased vascular permeability; (2) a direct cytotoxic effect of SARS-CoV-2 in the proximal tubule generating a collapsing glomerulopathy and proteinuria in nephrotic ranges; (3) the elevation of inflammatory markers that trigger a cytotoxic storm at the renal level; (4) renal ischemia secondary to hypoperfusion and septic shock; (5) lung–kidney syndrome associated with acute respiratory failure and the need for invasive mechanical ventilation; (6) the development of a pro-thrombotic hypercoagulable state; (7) the paradoxical effect of treatment of patients at the time of hospitalization, such as the use of ACE inhibitors and ARBs, and finally (8) rhabdomyolysis reported in some case series due to the muscular cytotoxic effect of the virus.24 In our study, a history of vaccination, especially in the last 5 years, was associated with a lower incidence of acute kidney injury, 13% vs. 29% in non-vaccinated patients (p=0.02). This lower incidence likely impacted the reduced mortality in our patients, associated with an attenuation of the dysregulation of the immune system caused by COVID-19 infection, as suggested by some authors.24,25
An increase in major cardiovascular diseases, particularly acute myocardial infarction, atrial fibrillation, and stroke, has been found in influenza infection and COVID-19. Influenza during the 21 days before a cardiovascular event is associated with an increase in mortality from cardiovascular disease of 2.3% (95% CI 0.7–3.9%) and mortality between 2.4% (95% CI 1.1–3.36%) and 6.9%. (95% CI 4.0–9.9%) from ischemic heart disease in adults>65 years.26 COVID-19 infection has been associated with increased susceptibility to atrial fibrillation during the infective stages and presumably post-recovery, increasing the risk of death, stroke, and length of hospitalization.24 A meta-analysis by Lee et al. showed that influenza-vaccinated patients had a decreased risk of developing stroke (OR 0.82; 95% CI 0.75–0.91; p<0.001).27 This increased risk of cardiovascular disease is associated with mechanisms such as influenza-induced systemic proinflammatory cytokine storm and a hypercoagulable state, which precipitate vulnerable plaque rupture, accelerated endothelial injury, alteration of the beta-adrenergic response of myocytes, adverse myocardial remodeling, and increased oxygen demands.25 In our patients, the incidence of cardiac complications and stroke was lower in vaccinated patients, although the incidence of acute myocardial infarction and atrial fibrillation were not documented individually. These potential mechanisms might have been associated with a reduction in mortality compared to patients with no vaccination history.
According to Fink et al., vaccination is associated with 8% lower odds of intensive care unit admission and 18% lower odds of requiring mechanical ventilation.14 In contrast, our patients with a history of influenza vaccination had more thromboembolic complications, mainly pulmonary thromboembolism (PTE), which could explain a greater requirement for invasive mechanical ventilation and ICU admission. A possible associated finding might be tobacco consumption, which was more prevalent in this group of patients, as suggested by Vickers et al., who reported a higher risk of venous thromboembolism in the first 10 days after influenza vaccination in patients with a history of smoking, compared with those with no active smoking.28 Another point to mention is that, in the vaccinated group, there was more history of cancer, which was also associated with a procoagulable state.29 Although the prevalence of smoking, cancer, and other conditions, such as obesity and overweight, did not reach statistical significance, we feel that the greater tendency toward the prevalence of these factors, either individually or combined, could have influenced the higher incidence of pulmonary thromboembolism with a greater requirement for invasive mechanical ventilation and ICU admission.
Among the strengths of our study, it is worth mentioning that this was the first observational, prospective study in Mexico that evaluated the evolution of SARS-CoV-2 disease in hospitalized older adults with a history of influenza vaccination. Our population had homogeneous characteristics between both groups, with a similar basal prevalence of systemic arterial hypertension, type 2 diabetes mellitus, obesity/overweight, cardiovascular diseases, cerebrovascular diseases, and chronic kidney disease. This fact gave us confidence in measuring the study variables. Among the main limitations are a small sample size, which may explain some of the results obtained, and the information regarding the history of seasonal influenza vaccination was obtained from the clinical history. We currently have specific vaccines against COVID-19, which have shown efficacy in preventing hospitalization, ICU admission, and severe disease.30,31 Unfortunately, our results were obtained before the availability of these vaccines, so we do not know if the combination of vaccination against influenza and against COVID-19 could generate other results. More studies and a larger sample size are needed to continue assessing the benefits of worldwide vaccination.
ConclusionsThis study highlights lower in-hospital mortality from COVID-19 in older adults with a history of influenza vaccination at least once in the last 5 years. Maintaining an annual vaccination strategy against influenza seems to be a determining factor in preventing complications and mortality in patients with COVID-19; however, further studies are required.
Ethical considerationsVerbal informed consent was obtained, not written.
FundingThe authors did not receive support from any organization for the submitted work.
Conflict of interestThe authors have no competing interests to declare relevant to the content of this article.
We thank Dr. Hazel Badillo-Rodríguez for obtaining data for the manuscript and Dulce F. Von Hartz-Hernández for preparation and final review.