Retinal vein occlusion (RVO) is mostly a consequence of vascular risk factors (VRF). COVID-19 vaccines have been related to vascular and thrombotic events (VTE).
ObjectiveTo assess the RVO incidence in the general population in our health area and the possible relation with COVID-19 infection and vaccination.
MethodsDemographic features, classic VRF, thrombophilia data, COVID-19 status, and Framingham risk score were collected prospectively.
Results472 consecutive patients studied over 13 years with RVO were included (Valdecilla Cohort). Classic VRFs were present in 90%, antiphospholipid syndrome in 12.3%, and genetic thrombophilia in 13.5%. Ninety-one percent of RVO patients were vaccinated and 6.8% suffered COVID-19 infection. In the cohort, no patient had a new RVO after vaccination or infection. In the general population, 20 subjects had RVO after receiving the vaccine (0.006%). Overall, 8 cases occurred in the first-month post-vaccination and 12 after 30 days. In the early and late groups, there are 3 and 4 patients respectively, with a low-intermediate risk Framingham score. Twenty-nine patients in the cohort suffered SARS-CoV-2 infection, twenty-seven of them had RVO before infection. Two patients with low-risk Framingham scores had RVO after infection, one of them early (<1 month).
ConclusionsVaccination and COVID-19 might be involved in the development of RVO in some cases, mainly in patients without VRF, thrombophilia, or chronic inflammatory conditions and with a lower Framingham score, especially in the first month after vaccination or infection.
La oclusión venosa retiniana (OVR) es principalmente una consecuencia de los factores de riesgo vascular (FRV). Las vacunas contra la COVID-19 se han relacionado con eventos vasculares y trombóticos (EVT).
ObjetivoEvaluar la incidencia de OVR en la población general de nuestra área de salud y su posible relación con la COVID-19 y la vacunación.
MétodosSe recopilaron prospectivamente las características demográficas, FRV clásicos, datos sobre trombofilia, padecimiento de la COVID-19 y puntuación de riesgo de Framingham.
ResultadosSe incluyeron 472 pacientes consecutivos con OVR, estudiados durante 13 años (Cohorte Valdecilla). Los FRV clásicos estaban presentes en el 90%, el síndrome antifosfolípido en el 12,3% y la trombofilia genética en el 13,5% de los casos. El 91% de los pacientes con OVR recibieron la vacuna frente a la COVID-19 y el 6.8% sufrió la infección. En la cohorte, ningún paciente tuvo una nueva OVR después de la vacunación o de la infección. En la población general, 20 sujetos presentaron OVR después de recibir la vacuna (0,006%). En general, 8 casos ocurrieron en el primer mes después de la vacunación y 12 después de 30 días. En los grupos precoz y tardío, 3 y 4 pacientes respectivamente, presentaban una puntuación de Framingham de riesgo bajo o intermedio. Veintinueve pacientes de la cohorte sufrieron infección por SARS-CoV-2 y 27 de ellos tuvieron una OVR antes de ésta. Dos pacientes con puntuaciones de Framingham de bajo riesgo presentaron una OVR después de la infección, uno de ellos precozmente (<1 mes).
ConclusionesLa vacunación y la COVID-19 podrían estar involucradas en el desarrollo de OVR en algunos casos, principalmente en pacientes sin FRV, trombofilia o procesos inflamatorios crónicos y con una puntuación de Framingham más baja, especialmente en el primer mes después de la vacunación o de la infección.
The classic vascular risk factors (VRF), mainly hypertension and aging, represent the core pathogenic factors for retinal vein occlusion (RVO).1–5 In this sense, RVO may be considered a manifestation of atherosclerosis. Although thrombophilia seems to play a minor role in the pathogenesis of RVO, antiphospholipid syndrome (APS) and hyperhomocysteinemia have been associated with this ophthalmological disorder.6,7 In young patients or those without VRF, genetic thrombophilia has greater importance.8 Depending on the location of the venous blockage, RVO is classified as central (CRVO) or peripheral/branch (BRVO), which is three times more frequent than the central type.1–3 On the other hand, glaucoma has been implicated as a local factor for the development of RVO, especially in cases with central involvement.9
The World Health Organization declared in March 2020, the pandemic coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2. The spectrum of the disease varies widely, from asymptomatic patients to pneumonia and acute respiratory distress syndrome.10 As a result of the hyperinflammatory response which develops in severe cases, COVID-19 has also been associated with thromboinflammation and thrombosis, causing artery and vein occlusion, microinfarcts, and multiple organ failure.11 Nevertheless, COVID-19 is a very infrequent cause of RVO with scarce cases reported in the literature.12–15
Vaccination has proven to be an effective strategy to reduce the burden of the SARS-CoV-2 pandemic. The vaccines approved in the European Union mRNA-based: BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna); and adenoviral vector-based: ChAdOx1-S (Astra- Zeneca) and Ad26.COV2-S (Janssen) are highly effective, without a counterbalancing safety signal in randomized trials.16,17
In patients who had received adenoviral vector-based vaccines, a small number of thrombotic events have been described, mainly the so-called vaccine-induced immune thrombotic thrombocytopenia (VITT) and the immune thrombocytopenic purpura (ITP). VITT consists of thrombotic complications, such as cerebral vein thrombosis, associated with thrombocytopenia. It is thought that antibodies against platelet factor 4 (PF4; CXCL4) play a pivotal role in the pathophysiology of this condition.18 VITT usually develops 2–4 weeks after vaccination (range 5–48 days). On the other hand, the pathophysiology of ITP is quite similar to heparin-induced thrombocytopenia and usually appears during the first 3 weeks after vaccination. Moreover, cases of deep vein thrombosis (DVT) and pulmonary thromboembolism (PE) have been reported after the Ad26.COV2-S vaccine.
The BNT162b2 vaccine has been rarely related to thrombotic events, which usually occurred a few days after its administration, including five RVO cases.19–21 Besides, 3 patients with central RVO, one following the mRNA-1273 vaccine, and 2 who had received the ChAdOx1-S vaccine have also been reported.22-24 Furthermore, an increased risk of hematological and vascular events that led to hospital admission or death was observed for short time intervals after the first doses of COVID-19 vaccines. However, the risk of most of these events was substantially higher and more prolonged after SARS-CoV-2 infection than after vaccination in the same population.25
On these bases, we aimed to assess the incidence and characteristics of RVO in the general population in our health area after COVID-19 vaccination or infection and their relationship with VRF and thrombophilia. Besides, demographic variables, VRF, thrombophilia, and vascular events in a large cohort of patients with RVO were also reported.
Patients and methodsStudy participantsThis is a prospective cohort study (“Valdecilla Cohort”) that includes all the patients diagnosed with RVO from December 2008 to November 2021, at the University Hospital Marqués de Valdecilla, a tertiary-care center that serves as a reference hospital for a population of 350,000 inhabitants in Cantabria, an autonomous community in Northern Spain. A cohort of 472 patients diagnosed with RVO by the Ophthalmology Division, according to clinical, funduscopic, and angiographic criteria, was included. They were referred to our Internal Medicine outpatient clinic for overall clinical assessment.
All the RVO patients were followed up in our outpatient clinic until the goal for high blood pressure, dyslipidemia, or diabetes mellitus was achieved, according to the updated Clinical Practice Guidelines. Those patients with folic acid, vitamin B12 deficiency, or serum hyperhomocysteinemia were treated with oral folic acid and vitamin B12 supplements. RVO patients were usually treated with aspirin. Nevertheless, for those with thrombophilia, we recommended low-molecular-weight heparin prophylaxis in high-risk thrombotic situations. Besides, in patients with thrombophilia younger than 50 years or without VRF or who had suffered from vascular or thrombotic events, anticoagulation with acenocoumarol was prescribed. Those patients diagnosed with thrombophilia have been indefinitely followed up, and they attended our outpatient clinic at least once a year. Those without thrombophilia who achieved the goals for controlling VRF according to the clinical guidelines were followed up by electronic chart review at least once a year before completing the study.
This study represents a real-world assessment of patients with RVO in the outpatient setting. The Valdecilla cohort study was approved by the Ethics Committee of Cantabria (internal code: 2019.340) and the participants gave written informed consent.
Data collectionDemographic, clinical, laboratory, and supra-aortic trunks ultrasound data were collected and stored in a computerized database.
Demographic and clinical variablesThe following variables were collected: age, sex, current tobacco use, high blood pressure (blood pressure greater than or equal to 140/90mmHg or taking antihypertensive drugs), dyslipidemia (serum cholesterol or triglyceride levels greater than 230 and 150mg/dl respectively, in at least 2 measurements after 12-h fasting, or taking lipid-lowering drugs), diabetes mellitus (according to the American Diabetes Association criteria)26 and type of RVO (central or branch).
Besides, in patients receiving COVID-19 vaccines, we analyzed: the date of COVID-19 vaccination (first, second and third doses), type of manufactured vaccine (BNT162b2, mRNA-1273, ChAdOx1- S, Ad26.COV2-S, and its combinations), development of post-vaccination RVO (date). We have distinguished between RVO that occurred before or after 30 days after COVID-19 infection or post-vaccination (the vast majority of thrombotic events described in the literature occurred during the first-month post-vaccination).18
In the patients vaccinated against COVID-19 who developed an RVO after SARS-CoV-2 infection or post-vaccination, the Framingham risk score (ATP III, 2002) was also calculated. Risk categories are low risk <10%; intermediate risk 10–20% and high risk >20%.27
Laboratory parametersBlood samples were obtained from the antecubital vein after 12h of overnight fasting. Routine biochemical parameters were measured in an ADVIA 2400 autoanalyzer (Siemens) and total cholesterol and high-density lipoprotein were included.
Regarding coagulation studies. Anticardiolipin and anti-β2 glycoprotein I antibodies were determined by ELISA and AESKULISA. Lupus anticoagulant (LA): neutralizing activated partial thromboplastin time (aPTT)<10s; dilute Russell viper venom time (dRVVT): R≤1.4. The remainder of the hypercoagulability study included platelet count, prothrombin time (PT), aPTT, fibrinogen, protein C, protein S, antithrombin, FV Q506 (FV Leiden), and prothrombin 20210A mutation. Normal values were established according to 100 control patients of the same age range and gender and were as follows: antithrombin, 85–140%; protein C, 85–140%; protein S, 70–120%.
The diagnosis of antiphospholipid syndrome (APS) was established according to the International Society on Thrombosis and Haemostasis Guidelines.28 In patients with an initial positive result for LA or antiphospholipid antibodies, but whose second test was negative, we perform the third test at 12 weeks to confirm the diagnosis.
A genetic thrombophilia study was performed on all patients from December 2008 to December 2015. Thereafter, since genetic thrombophilia had been found to play a minor role in RVO pathogenesis, it was determined only in patients younger than 50 years or in those without vascular risk factors at the time of RVO diagnosis 6.
The diagnosis of SARS-CoV-2 infection was made by polymerase chain reaction (qRT-PCR; EDX SARS- CoV-2. Standard. Exat Diagnostics, Bio-Rad).
Imaging testA Doppler ultrasound of the supra-aortic trunks was used to evaluate the presence of atheromatous plaques. The study was conducted with a B-mode, color Doppler, and spectral mode ultrasound of the carotid and vertebral arteries (General Electric, Logic® model) by the same section of Vascular Radiology of our center.
Statistical analysisQuantitative variables were expressed as mean± standard deviation and qualitative variables as percentages. The Student's t-test was used to compare quantitative variables. IBM SPSS Statistics for Windows, Version 28.0. (Armonk, NY: IBM Corp) was used to perform the statistical analyses. A p-value <0.05 was considered significant in all the calculations.
ResultsGeneral characteristics, VRF, and thrombophilia data in the Valdecilla CohortBetween December 2008 and November 2021, 472 patients with RVO were assessed. RVO was peripheral in 320 (67.8%) cases (temporal in 314 and nasal in 6) and central in 152 (32.2%). Some 52.8% (n=249) of patients were men and 47.2% (n=223) were women. The mean age was 67.4±12.8 years (range, 18–96 years). Forty-six patients had died before the onset of the SARS-CoV-2 pandemic in March 2020, and 8 during the pandemic, due to diseases unrelated to COVID-19. The Valdecilla Cohort study represents, to our knowledge, the prospective cohort study of RVO patients, with the largest follow-up (up to 13 years, to date).
Overall, 90% of the cases presented some of these VRFs. Thus, 338 patients had arterial hypertension (71.6%), 309 (65.5%) dyslipidemia, and 112 (23.7%), diabetes mellitus. Sixty-seven (14.2%) were active smokers and 143 (30.3%) were ex-smokers. Atherosclerotic involvement of the supra-aortic trunks was present in 224 patients, 52.5% of the 427 in whom carotid Doppler ultrasound was performed.
Concerning thrombophilia, 58 RVO patients (12.3%) met the criteria for antiphospholipid syndrome (APS). In these cases, at least one of the three antiphospholipid antibodies was persistently positive, either lupus anticoagulant (LA); n=33/460 (7.2%), anti-cardiolipin (ACL), or anti- β2glycoprotein I (anti-β2GPI) (7.7% either of the ACL or anti-β2GPI; 36/468 cases). Thirty-five of 258 (13.5%) patients in whom the genetic study was carried out, had a genetic alteration: factor V Leyden (n=4); factor II mutation (n=11); protein C deficiency (n=5); protein S deficiency (n=10) and antithrombin deficiency (n=8). In 3 cases, more than one genetic alteration was present. Two patients had genetic thrombophilia and positive antiphospholipid antibodies simultaneously, so there is a difference between the number of thrombophilia alterations and the number of patients with thrombophilia. In this sense, only 6.4% (n=30) of our patients did not present any classic VRFs or thrombophilia.
The annual incidence rate of RVO was 36.3 patients/year, over the 13 years of follow-up. The highest frequency of RVO occurred in 2021 (60 cases). There were differences between the first 2 years of the pandemic (2020–2021) and the previous ones (33.7±9.8 vs 50.5±13.5 patients/year; p<0.0001).
Vaccination scheme in the study populationThe first COVID-19 vaccine was administered in our area (Cantabria) in January 2021. By the end of November 2021, 484,914 subjects had been vaccinated (92.5% of those ≥12 years; 83.2% of the entire population) and 481,642 immunized (91.9% of those ≥12 years; 82.6% of the total). Subjects who have received more than one dose of vaccine are considered immunized. The distribution of the 1001.596 vaccines administered in Cantabria according to the manufacturer was: BNT162b2, 751,405 doses; mRNA-1273, 107,665 doses; ChAdOx1-S, 120.352 doses; and Ad26.COV2-S, 22,174 doses 29. In our area, considering a population of 350,000 inhabitants, there would be about 323,750 vaccinated subjects (321.650 immunized). In our cohort, overall 91.1% (n=389) of our 426 RVO patients alive at the start of the pandemic, received any of the manufactured COVID-19 vaccines by the end of November 2021 BNT162b2, 79.7% (n=310); mRNA-1273, 4.9% (n=19); ChAdOx1-S, 10% (n=39); Ad26.COV2-S, 3.6% (n=14); and combinations of different vaccines, 1.8% (n=7, in all of them the first dose with Ad26.COV2-S and the second one with mRNA-1273). Twelve patients (3.1%) received one dose of the vaccine, 182 (46.8%) received two, and 195 (50.1%) three doses. The percentage of vaccination and the distribution of the type of vaccine by the manufacturer were similar in the general population of our region and the Valdecilla Cohort.
New RVO cases in the general population and the Valdecilla cohortIn our healthcare area, there were 30,000 COVID-19 cases and a cumulative incidence of 8.4%.29 In our cohort, this figure was 6.8% (29 cases). Twenty patients from the general population of our healthcare area (0.006%) had RVO after the administration of the COVID-19 vaccine. No patient included in the Valdecilla cohort experienced a relapsing RVO after vaccination or infection. Overall, 8 of 20 patients had RVO within the first 30 days of receiving any of the doses of the vaccine, and 12 after the first month of vaccination. Only one patient with RVO after vaccination had SARS-CoV-2 infection and it occurs after the RVO episode.
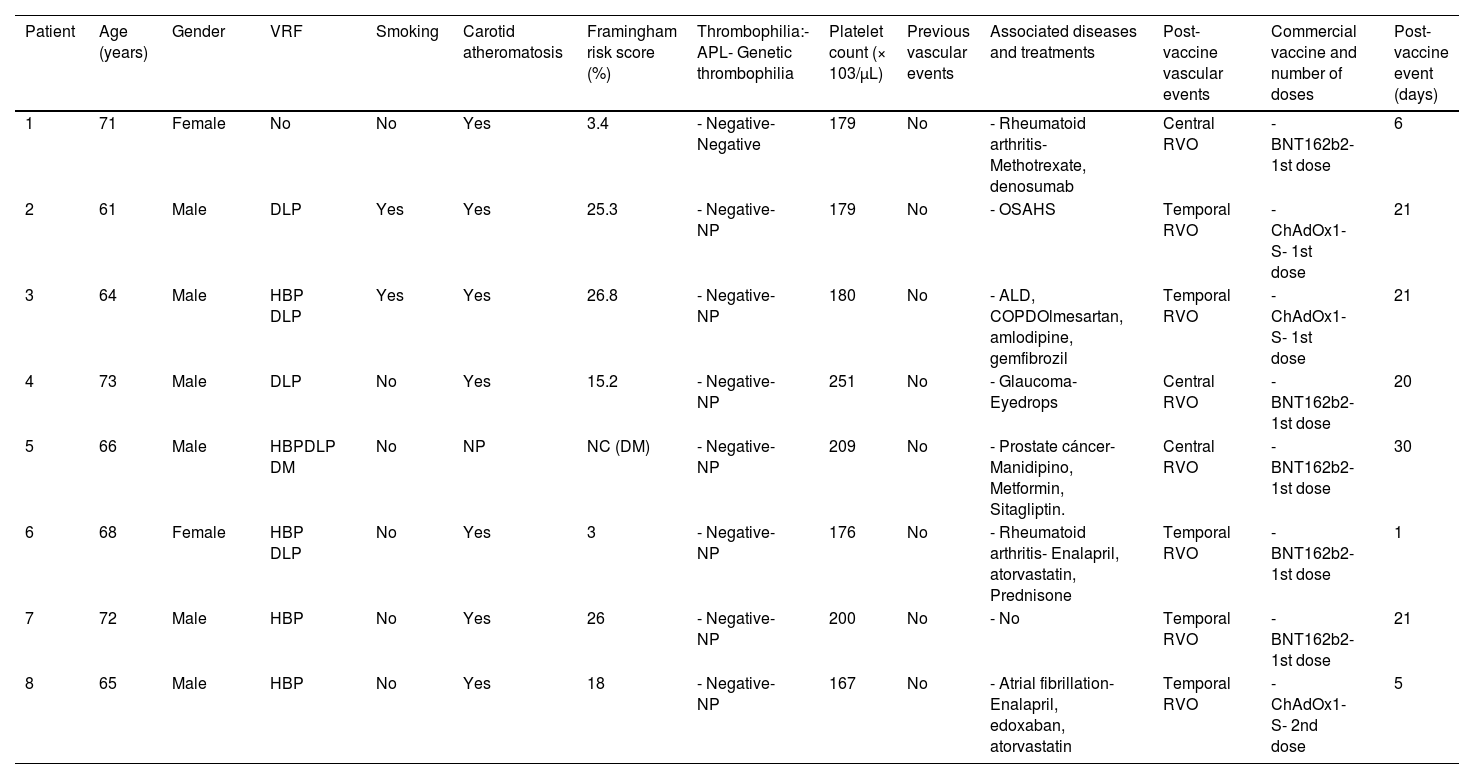
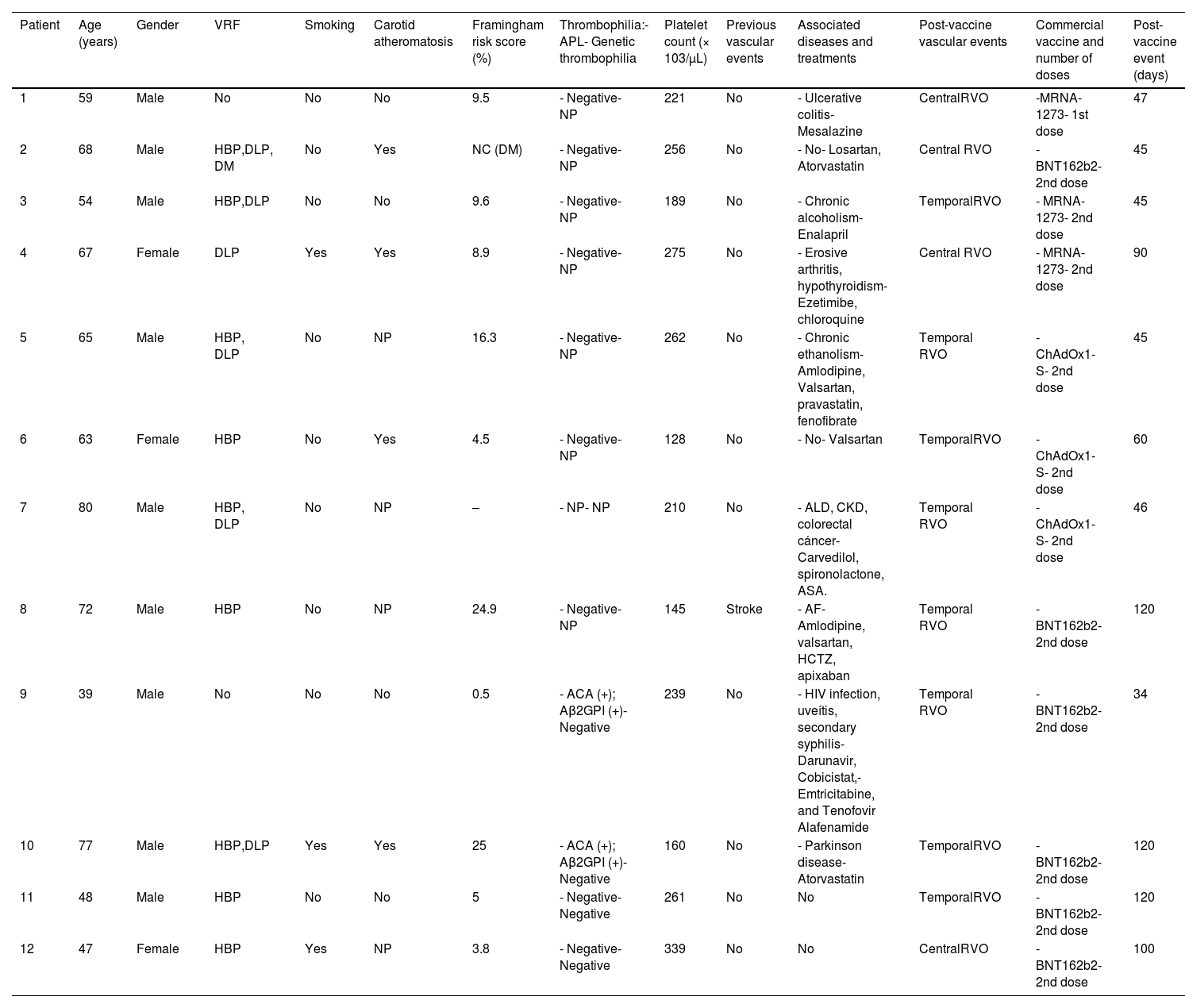
The main characteristics of these cases, including the Framingham score, are shown in Tables 1 and 2.
Early COVID-19 post-vaccination RVO (≤1month post-vaccination).
| Patient | Age (years) | Gender | VRF | Smoking | Carotid atheromatosis | Framingham risk score (%) | Thrombophilia:- APL- Genetic thrombophilia | Platelet count (× 103/μL) | Previous vascular events | Associated diseases and treatments | Post-vaccine vascular events | Commercial vaccine and number of doses | Post-vaccine event (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Female | No | No | Yes | 3.4 | - Negative- Negative | 179 | No | - Rheumatoid arthritis- Methotrexate, denosumab | Central RVO | - BNT162b2- 1st dose | 6 |
| 2 | 61 | Male | DLP | Yes | Yes | 25.3 | - Negative- NP | 179 | No | - OSAHS | Temporal RVO | - ChAdOx1-S- 1st dose | 21 |
| 3 | 64 | Male | HBP DLP | Yes | Yes | 26.8 | - Negative- NP | 180 | No | - ALD, COPDOlmesartan, amlodipine, gemfibrozil | Temporal RVO | - ChAdOx1-S- 1st dose | 21 |
| 4 | 73 | Male | DLP | No | Yes | 15.2 | - Negative- NP | 251 | No | - Glaucoma- Eyedrops | Central RVO | - BNT162b2- 1st dose | 20 |
| 5 | 66 | Male | HBPDLP DM | No | NP | NC (DM) | - Negative- NP | 209 | No | - Prostate cáncer- Manidipino, Metformin, Sitagliptin. | Central RVO | - BNT162b2- 1st dose | 30 |
| 6 | 68 | Female | HBP DLP | No | Yes | 3 | - Negative- NP | 176 | No | - Rheumatoid arthritis- Enalapril, atorvastatin, Prednisone | Temporal RVO | - BNT162b2- 1st dose | 1 |
| 7 | 72 | Male | HBP | No | Yes | 26 | - Negative- NP | 200 | No | - No | Temporal RVO | - BNT162b2- 1st dose | 21 |
| 8 | 65 | Male | HBP | No | Yes | 18 | - Negative- NP | 167 | No | - Atrial fibrillation- Enalapril, edoxaban, atorvastatin | Temporal RVO | - ChAdOx1-S- 2nd dose | 5 |
ALD: alcoholic liver disease; APL: antiphospholipid antibodies; ASA: acetylsalicylic acid; COPD: chronic obstructive pulmonary disease; DLP: dyslipidemia; DM: diabetes mellitus; HBP: high blood pressure; HF: heart failure; NC: not calculated; NP: not performed; OSAHS: obstructive sleep apnea–hypopnea syndrome; PE: pulmonary embolism; retinal vein occlusion; VRF: vascular risk factors.
Late COVID-19 post-vaccination RVO (>1 month post-vaccination).
| Patient | Age (years) | Gender | VRF | Smoking | Carotid atheromatosis | Framingham risk score (%) | Thrombophilia:- APL- Genetic thrombophilia | Platelet count (× 103/μL) | Previous vascular events | Associated diseases and treatments | Post-vaccine vascular events | Commercial vaccine and number of doses | Post-vaccine event (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Male | No | No | No | 9.5 | - Negative- NP | 221 | No | - Ulcerative colitis- Mesalazine | CentralRVO | -MRNA-1273- 1st dose | 47 |
| 2 | 68 | Male | HBP,DLP, DM | No | Yes | NC (DM) | - Negative- NP | 256 | No | - No- Losartan, Atorvastatin | Central RVO | - BNT162b2-2nd dose | 45 |
| 3 | 54 | Male | HBP,DLP | No | No | 9.6 | - Negative- NP | 189 | No | - Chronic alcoholism- Enalapril | TemporalRVO | - MRNA-1273- 2nd dose | 45 |
| 4 | 67 | Female | DLP | Yes | Yes | 8.9 | - Negative- NP | 275 | No | - Erosive arthritis, hypothyroidism-Ezetimibe, chloroquine | Central RVO | - MRNA-1273- 2nd dose | 90 |
| 5 | 65 | Male | HBP, DLP | No | NP | 16.3 | - Negative- NP | 262 | No | - Chronic ethanolism- Amlodipine, Valsartan, pravastatin, fenofibrate | Temporal RVO | - ChAdOx1-S- 2nd dose | 45 |
| 6 | 63 | Female | HBP | No | Yes | 4.5 | - Negative- NP | 128 | No | - No- Valsartan | TemporalRVO | - ChAdOx1-S- 2nd dose | 60 |
| 7 | 80 | Male | HBP, DLP | No | NP | – | - NP- NP | 210 | No | - ALD, CKD, colorectal cáncer- Carvedilol, spironolactone, ASA. | Temporal RVO | - ChAdOx1-S- 2nd dose | 46 |
| 8 | 72 | Male | HBP | No | NP | 24.9 | - Negative- NP | 145 | Stroke | - AF- Amlodipine, valsartan, HCTZ, apixaban | Temporal RVO | - BNT162b2- 2nd dose | 120 |
| 9 | 39 | Male | No | No | No | 0.5 | - ACA (+); Aβ2GPI (+)- Negative | 239 | No | - HIV infection, uveítis, secondary syphilis- Darunavir, Cobicistat,- Emtricitabine, and Tenofovir Alafenamide | Temporal RVO | - BNT162b2- 2nd dose | 34 |
| 10 | 77 | Male | HBP,DLP | Yes | Yes | 25 | - ACA (+); Aβ2GPI (+)- Negative | 160 | No | - Parkinson disease- Atorvastatin | TemporalRVO | - BNT162b2- 2nd dose | 120 |
| 11 | 48 | Male | HBP | No | No | 5 | - Negative- Negative | 261 | No | No | TemporalRVO | - BNT162b2- 2nd dose | 120 |
| 12 | 47 | Female | HBP | Yes | NP | 3.8 | - Negative- Negative | 339 | No | No | CentralRVO | - BNT162b2- 2nd dose | 100 |
Aβ2GPI: anti-β2-glycoprotein I antibodies; ACA: anticardiolipin antibodies; AF: atrial fibrillation; ALD: alcoholic liver disease; APLA: antiphospholipid antibodies; ASA: acetylsalicylic acid; CHD: coronary heart disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; DLP: dyslipidemia; DM: diabetes mellitus; HBP: high blood pressure; HCTZ: hydrochlorothiazide; MM: multiple myeloma; NC: not calculated; NP: not performed; OSAHS: obstructive sleep apnea–hypopnea syndrome; RVO: retinal vein occlusion; TSA: supra-aortic trunk; VRF: vascular risk factors.
Only two patients from the general population of our healthcare area had RVO after SARS-CoV-2 infection, both with low Framingham vascular risk scores (4.9, and 6.4, respectively). In the first patient, the ocular changes developed at 3 weeks and in the second at 5 months after the infection.
DiscussionWe have observed 20 patients with RVO after COVID-19 vaccination (0.006%) in our health area. Seven of them (3 early and 4 late post-vaccination cases) had low-intermediate Framingham vascular risk, and the remaining had high vascular risk, a prior vascular event, thrombophilia, or the RVO was diagnosed >100 days after vaccination. Moreover, in our healthcare area, we observed two patients with low Framingham scores, who had RVO after SARS-CoV-2 infection.
RVO is mainly considered a manifestation of the atherosclerotic process. Thus, the vast majority of patients with RVO had several VRF or thrombophilia, and only 6.4% do not have any of these predisposing prothrombotic factors. In this sense, our results confirm the association of RVO with VRF and to a lesser extent with thrombophilia 1–8. Furthermore, our RVO patients have a high incidence of carotid arteriosclerotic involvement of the supra-aortic trunks, an independent risk factor for VTE 4. It is worth mentioning that our RVO patients have an increased 10-year Framingham risk for cardiovascular disease.5
In the two last years (2020–2021) which include 20 months of the SARS-CoV-2 pandemic (March 2020–November 2021), we observed an increase in the incidence of RVO in our Valdecilla Cohort. We think that demographic changes over time do not explain the differences, since during the 13 years, the population in Cantabria did not change (584.657 inhabitants in 2009 and 584.507 in 2021). Nevertheless, there was a small increase in the population >64 years (18.4% in 2009 and 22.9% in 2021). It is difficult to ascertain whether this could be related to COVID-19 itself, the vaccination, or the impaired control of factors involved in RVO pathogenesis since in this pandemic period, face-to-face consultations for the diagnosis and treatment of VRF and glaucoma have been restricted.
Eight of 20 new RVO cases had an early RVO, within the first month after COVID-19 vaccination. In this sense, VITT usually develops after 2 weeks of vaccination (range 5–48 days), and in most cases before 4 weeks post-vaccination 18. The potential association between vaccines and RVO has been previously reported. Thus, 4 cases of central RVO have been described after hepatitis B vaccination (adenovirus vaccine) in young patients without risk factors for RVO.30
Patients who developed an early-onset RVO after COVID-19 vaccination are the population in whom the association of RVO and vaccine is more plausible. In this group, antiphospholipid antibodies were negative, platelet count was normal, and, in seven of the eight in which supra-aortic trunk ultrasonography was performed, carotid atheromatosis was observed. The patient without previous classic VRF or thrombophilia and a low Framingham score is the one who most probably could have developed RVO after the COVID-19 vaccination. Nevertheless, she had been diagnosed with rheumatoid arthritis, a well-known condition related to increased cardiovascular risk, mainly mediated by inflammation, and besides, carotid atheromatosis was disclosed in the ultrasound study. The association of RVO and vaccination could be plausible in the cases fourth and sixth since they have a low-intermediate Framingham score, although also VRF (see Table 1). Besides, one of them suffered from rheumatoid arthritis and received corticosteroids. In the remaining patients with early-onset RVO after COVID-19 vaccination, this relationship seems to be less probable.
Eight cases with a possible relationship between RVO and COVID-19 vaccine have been reported in the literature to date. In all of them, the event postvaccination was early, 3 weeks after vaccination. Five patients received the BNT162b2 vaccine,19-22 another one the mRNA-1273 vaccine, and two were immunized with the ChAdOx1-S vaccine.23,24
Since RVO is mainly a consequence of arteriosclerosis and therefore, is associated with VRF and aging, we cannot be certain that this retinal disease is related to the vaccination, even if it occurs early after the administration of the vaccine. This probability is greater in patients without thrombophilia and lower VRF load and lower Framingham risk score. Overall, whether vaccination could act as a trigger for the development of RVO in these cases remains unproven.
In patients with late-onset RVO (>1 month), the probability of an association with COVID-19 vaccination decreases as the time interval between vaccine administration and RVO development increases. In this sense, this association could be plausible in the first, third, fifth, and sixth patients since they have low-intermediate Framingham scores, although the first one had a chronic inflammatory bowel disease (see Table 2). In this group, there was a 39-year-old man without VRF, carotid atheromatosis, or genetic thrombophilia who had a temporal RVO 34 days after the second dose of the COVID-19 vaccine. However, retinal disease occurs in the context of secondary syphilis with uveitis. The other patients have VRF, a high Framingham score or the RVO occurs more than 90 days after vaccination, and therefore, the association between RVO and vaccination is unlikely.
On the other hand, in the first patient who had an RVO after SARS-CoV-2 infection, the retinal disease was a probable consequence of COVID-19. She was a young patient with a low Framingham score, without VRF or thrombophilia and RVO occurred early after the infection.15 In the second patient, RVO occurred very late after infection, and then, the probability to be related to COVID-19 was low. Noteworthy, in patients with prior RVO, no new retinal abnormalities or worsening of RVO was observed after SARS-CoV-2 infection. In this sense, it should be noted that COVID-19 is an infrequent cause of RVO.13–15
Our study has the limitations of observational studies, and therefore, a causal relationship between RVO and COVID-19 vaccination cannot be established. The main strength of the study was the assessment of RVO, a scarcely studied event after COVID-19 vaccination, through the Valdecilla Cohort which includes all subjects diagnosed with RVO in our healthcare area and followed up for more than 13 years.
In summary, during the first 2-years of the COVID-19 pandemic, there has been an increase in the incidence of RVO in our area. Vaccination against COVID-19 with mRNA or adenovirus vector vaccines might be involved in the development of RVO in some cases, mainly in patients without VRF, thrombophilia, and lower Framingham scores, and no chronic inflammatory conditions, especially in the first month after vaccination.
FundingThis study did not receive any funding.
Ethical considerationsThe study was approved by the Ethics Committee of Cantabria (internal code: 2019.340) and the participants gave written informed consent.
Conflict of interestNone of the authors disclose any conflict of interest regarding the present study.