Regular physical activity is associated with a low risk of severe community-acquired infections. However, the hypothesis that a physical inactivity pattern is associated with a higher risk for severe COVID-19 has not been completely proven, especially with severe pneumonia.
ObjectiveThe goal of this study was to confirm the link between physical activity patterns and severe SARS-CoV-2 pneumonia.
DesignCase–control study.
MethodsThis study involved 307 patients who developed SARS-CoV-2 severe pneumonia and were hospitalized in an intensive care unit. Age- and sex-matched controls (307) were selected from the same population: patients with mild to moderate forms of COVID-19 who were not hospitalized. Physical activity patterns were assessed using the short version of the International Physical Activity Questionnaire.
ResultsThe mean physical activity levels were lower in the SARS-CoV-2 severe pneumonia group as compared to the control group: 1576±2939 vs 2438±2999, metabolic equivalent of task (MET-min/week), p<0.001. A high or moderate physical activity level was more common in the control group, and a low physical activity level was more observed in the case group (p<0.001). Obesity was also associated with severe SARS-CoV-2 pneumonia (p<0.001). Multivariable analysis showed that a low physical activity level was associated with a higher risk for severe SARS-CoV-2 pneumonia, independent of nutritional status (CI 3.7; 2.24–5.99), p<0.001).
ConclusionA higher and moderate level of physical activity is linked to a lower risk of SARS-CoV-2 severe pneumonia.
La actividad física regular se asocia con un bajo riesgo de infecciones graves adquiridas en la comunidad. Sin embargo, la hipótesis de que un patrón de inactividad física se asocia con un mayor riesgo de COVID-19 grave no ha sido completamente probada, especialmente con neumonía grave. El objetivo de este estudio fue confirmar el vínculo entre los patrones de actividad física y la neumonía grave por SARS-CoV-2.
Material y métodosEstudio de casos y controles. Este estudio involucró a 307 pacientes que desarrollaron neumonía grave por SARS-CoV-2 y fueron hospitalizados en una unidad de cuidados intensivos. Se seleccionaron controles emparejados por edad y sexo (307) de la misma población: pacientes con formas leves a moderadas de COVID-19 que no fueron hospitalizados. Los patrones de actividad física se evaluaron utilizando la versión corta del Cuestionario Internacional de Actividad Física.
ResultadosLos niveles medios de actividad física fueron menores en el grupo de neumonía grave por SARS-CoV-2 en comparación con el grupo control: 1576±2939 vs 2438±2999, equivalente metabólico de la tarea (MET-min/semana), p<0.001. Un nivel de actividad física alto o moderado fue más común en el grupo control, y un nivel de actividad física bajo fue más observado en el grupo de casos (p<0,001). La obesidad también se asoció con neumonía grave por SARS-CoV-2 (p<0,001). El análisis multivariable mostró que un bajo nivel de actividad física se asoció con un mayor riesgo de neumonía grave por SARS-CoV-2, independientemente del estado nutricional (IC 3,7; 2,24-5,99), p<0,001).
ConclusiónUn nivel de actividad física más alto y moderado se relaciona con un menor riesgo de neumonía grave por SARS-CoV-2.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel betacoronavirus, originated in Wuhan, China, in December 2019.1 The World Health Organization (WHO) declared COVID-19 a global pandemic in March 2020.2 Currently, COVID-19 has become a global threat, with 662 million confirmed cases and over 6.7 million deaths reported globally as of January 15, 2023 (https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---19-january-2023). Although in the last few months the pandemic has decreased worldwide, a new wave of COVID has arisen in the last few months in some countries with SARS-CoV-2 Omicron subvariants, especially BQ.1, BQ.1, and XBB.3
Infection with SARS-CoV-2 is silent or a benign upper respiratory disease in 80% of cases and causes pneumonia in 20% of cases.4,5 Approximately half of these patients develop hypoxemic pneumonia, requiring hospitalization and, sometimes, UTI admission and mechanical ventilation. Pneumonia represents the typical presentation of COVID-19 and is the main mortality cause.6,7 SARS-CoV-2 pneumonia can lead to acute hypoxic respiratory failure, multiorgan failure, and death.8,9 SARS-CoV-2 leads to diffuse alveolar damage and micro- and macro-thrombi in pulmonary arterial vessels, resulting in profound oxygen desaturation and respiratory distress.10
Social distancing has been used as the best option to reduce the spread of COVID-19.11 However, quarantine and isolation have resulted in a change in the physical activity pattern.12 Physical activity has a crucial role in the prevention of non-communicable diseases and contributes to reducing the risk of severe community-acquired infections and mortality.13,14 Some studies have shown that physical activity influences pneumonia prognosis15 and that individuals with a lower physical activity pattern have an increased risk for COVID-19 hospitalization.16,17 However, other studies did not find differences between physical activity levels and COVID-19 outcomes.18 Two recent systematic reviews showed that physical activity lowers the risk of incident pneumonia and COVID-19 mortality risk,19,20 but authors pointed out some limitations of the studies reviewed: different definitions to determine physical activity levels; only leisure-time activities collected; potential sources of bias such as social status and vaccination status; and confounders associated with severe COVID-19 (i.e., obesity, diabetes, and hypertension) not reported.
The hypothesis that a physical inactivity pattern is associated with a higher risk for severe COVID-19 has not been completely proven, especially with severe pneumonia. Based on all this, this case–control study verified the association between physical activity pattern and the risk of SARS-CoV-2 severe pneumonia.
Patients and methodsThis matched case–control study was performed in Recife, Pernambuco State, Brazil. Patients admitted to the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), Recife, and Hospital Dom Malan (HDM), Petrolina, were screened. Patients were selected from a total of 1,034 consecutive patients hospitalized with COVID-19 between April 2020 and February 2022 at IMIP and HDM.
This study followed the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained previously from each participant. This study was previously approved by the Research Ethical Committee of IMIP (CAAE: 40857920.0.0000.5201). This research was funded by CNPq (Brazilian National Council for Scientific and Technological Development), grant number 401907/2020-1. The funding organization did not participate in the collection of data, their analysis, interpretation, and publication of the finished manuscript.
Individuals over the age of 18 who were hospitalized with COVID-19 severe pneumonia were considered case patients. When a patient complained of shortness of breath, low SpO2, and pulmonary alteration on a CT image, severe pneumonia was considered. The real-time reverse transcriptase polymerase chain reaction (RT-PCR) of pharyngeal and nasal swab samples was used to confirm the COVID-19 diagnosis. For every case patient admitted to the study, one patient control with mild or moderate COVID-19 was selected, always matched by gender and age (± 5 years). Controls were selected from the same hospitals and attended by the same team at the same time as long as they had no indication of hospitalization.
The sample size calculation was based on the following information: (1) significance level of 0.05; (2) =80%; (3) proportion of those who were exposed to the protection factor (physically active) versus those who were not exposed; a difference of 20% in the outcome (severe COVID-19 pneumonia); the value of the minimum odds ratio to be detected (0.2); and a case-to-control ratio of one case to one control. A total of 307 cases and 307 controls were calculated.
Physical activity was evaluated by the International Physical Activity Questionnaire (IPAQ), short form. The IPAQ assesses physical activity undertaken across a set of domains, including work-related and transport-related activities, leisure time, domestic activities, and gardening including work-related and transport-related activities, leisure time, domestic activities, and gardening. The frequency and duration of physical activity were evaluated in three ranges of intensity: vigorous physical activity (VPA=8.0 metabolic equivalents [METs]), moderate physical activity (MPA=4.0 METs), and low physical activity (LPA=3.3 METs). Based on their questionnaire responses, individuals were categorized into three groups according to their physical activity levels: High physical activity level—meeting any one of the following criteria: 3 or more days of vigorous activity of at least 1500METmin/week or 7 or more days of any combination of activities from three intensity ranges of at least EE 3000METmin/week; Moderate physical activity level: 3 or more days of vigorous activity of at least 20min/day, 5 or more days of moderate- or low-intensity activity of at least 30min/day, or 5 or more days of any combination of low-, moderate-, or vigorous activity of at least EE 600METmin/week; Low physical activity level: no physical activity reported or some physical activity reported but insufficient to meet the Moderate physical activity level criteria.
Continuous variables are presented as means standard deviations (SDs), while categorical variables are presented as frequencies and percentages. Demographic and clinical variables of the case and control groups were compared using the chi-square test or Student's t-test. Multivariable logistic regression was used to assess the independent association between physical activity pattern and SARS-CoV-2 severe pneumonia after adjustment for categorical and continuous variables. Results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical analyses were performed using STATA version 12.1 (USA). A p value of 0.05 was considered statistically significant.
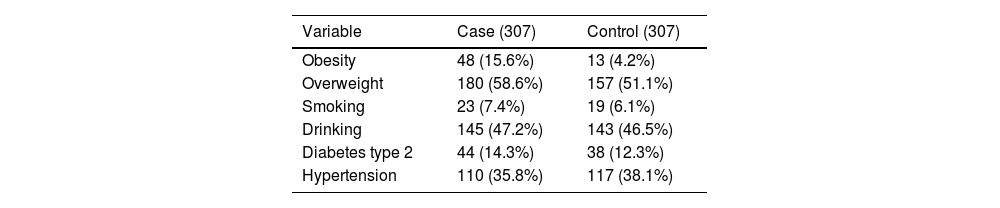
ResultsThere were 307 case patients hospitalized with severe COVID-19 pneumonia, all PCR positive for SARS-CoV-2, and 307 matched controls for age and gender. There was no difference in the age, mean difference between groups of – 0.5 years (95% CI −1.4–2.4) and gender (307 vs. 307), respectively, in the cases and control participants. Case patients had a higher prevalence of obesity but were not overweight. Type 2 diabetes, hypertension, smoking, and drinking habits showed no differences between cases and controls (Table 1).
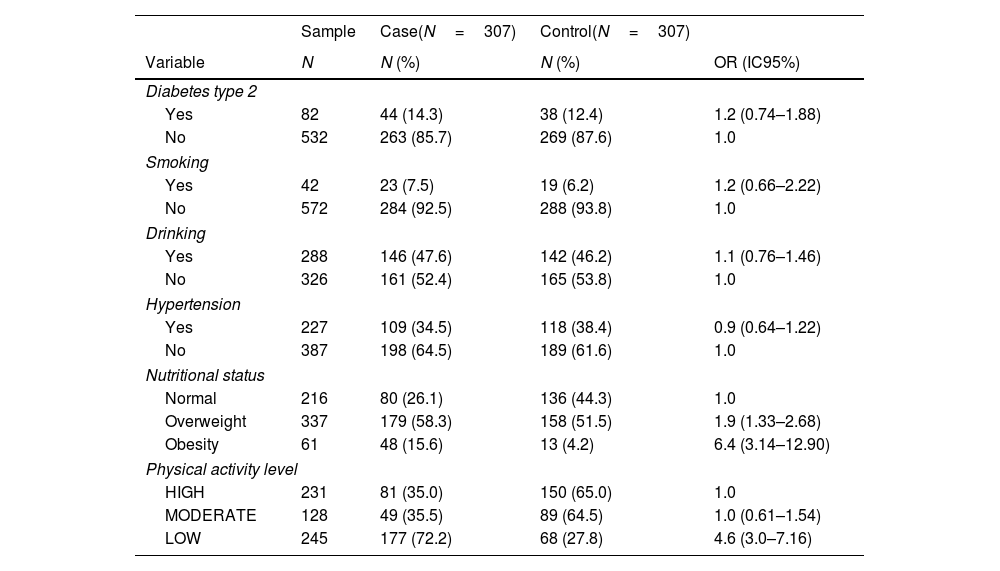
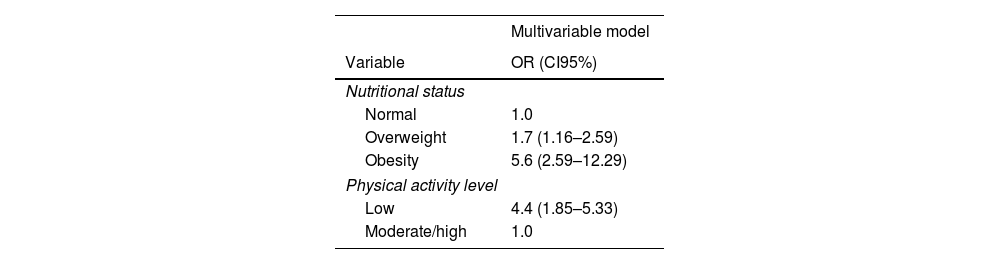
The mean physical activity level was lower in case patients as compared to control patients: a mean difference – 862 MET-min/week (95% CI −1332.637 to −391.3631). A high or moderate physical activity level was more common in the control group, and a low physical activity level was more observed in the case group (p<0.001) (Table 2). Obesity was also linked to severe SARS-CoV-2 pneumonia (p<0.001). Multivariable analysis showed that a low physical activity level was independently associated with a higher risk for SARS-CoV-2 severe pneumonia, CI 3.7 (2.24–5.99), p<0.001 (Table 3).
Univariable analysis of some variables between patients with (Cases) and without (Controls) severe SARS-CoV-19 pneumonia.
| Sample | Case(N=307) | Control(N=307) | ||
|---|---|---|---|---|
| Variable | N | N (%) | N (%) | OR (IC95%) |
| Diabetes type 2 | ||||
| Yes | 82 | 44 (14.3) | 38 (12.4) | 1.2 (0.74–1.88) |
| No | 532 | 263 (85.7) | 269 (87.6) | 1.0 |
| Smoking | ||||
| Yes | 42 | 23 (7.5) | 19 (6.2) | 1.2 (0.66–2.22) |
| No | 572 | 284 (92.5) | 288 (93.8) | 1.0 |
| Drinking | ||||
| Yes | 288 | 146 (47.6) | 142 (46.2) | 1.1 (0.76–1.46) |
| No | 326 | 161 (52.4) | 165 (53.8) | 1.0 |
| Hypertension | ||||
| Yes | 227 | 109 (34.5) | 118 (38.4) | 0.9 (0.64–1.22) |
| No | 387 | 198 (64.5) | 189 (61.6) | 1.0 |
| Nutritional status | ||||
| Normal | 216 | 80 (26.1) | 136 (44.3) | 1.0 |
| Overweight | 337 | 179 (58.3) | 158 (51.5) | 1.9 (1.33–2.68) |
| Obesity | 61 | 48 (15.6) | 13 (4.2) | 6.4 (3.14–12.90) |
| Physical activity level | ||||
| HIGH | 231 | 81 (35.0) | 150 (65.0) | 1.0 |
| MODERATE | 128 | 49 (35.5) | 89 (64.5) | 1.0 (0.61–1.54) |
| LOW | 245 | 177 (72.2) | 68 (27.8) | 4.6 (3.0–7.16) |
Multivariable analysis of some variables between patients with (Cases) and without (Controls) severe SARS-CoV-19 pneumonia.
| Multivariable model | |
|---|---|
| Variable | OR (CI95%) |
| Nutritional status | |
| Normal | 1.0 |
| Overweight | 1.7 (1.16–2.59) |
| Obesity | 5.6 (2.59–12.29) |
| Physical activity level | |
| Low | 4.4 (1.85–5.33) |
| Moderate/high | 1.0 |
This case–control study showed that a high or moderate physical activity pattern was associated with a lower risk of SARS-CoV-19 severe pneumonia. Inversely, a low physical activity pattern increased the risk of SARS-CoV-2-related severe pneumonia. However, we must highlight that our research has limitations because it lacks a randomized clinical trial design to assess the effects of physical activity on severe COVID-19. As such, we cannot guarantee that all confounding variables were equally distributed in both groups. As evaluators were not masked from the outcome COVID-19 status, we cannot guarantee they are free from evaluation bias.
Obesity was also associated with a higher risk of SARS-CoV-2 severe pneumonia, but the association between physical activity level and SARS-CoV-2 severe pneumonia was independent of the nutritional status of the patient. Our findings are in agreement with other studies that have approached the severity of illness and mortality in COVID-19.20 A case–control study in South Korea reported that higher levels of regular physical activity were associated with a lower risk of COVID-19 infection and mortality.16 These studies, however, did not look specifically at COVID-19 severe pneumonia. Septic shock and multi-organ failure have been reported as the most common immediate causes of death in COVID-19, often due to suppurative pulmonary infection. Respiratory failure due to diffuse alveolar damage presented as an immediate cause of death in fewer cases.21 Our study did not involve COVID-19 mortality but makes a specific contribution by showing a strong association between severe SARS-CoV-2 pneumonia and physical inactivity.
It has been hypothesized that physical activity improves both the immunosurveillance against pathogens and the cardiovascular and metabolic health, lowering the risk of chronic diseases.22,23 Physical activity increases the concentrations of CD4 T cell helpers and salivary immunoglobulin IgA.24 The pathophysiological mechanisms also included the ability of physical activity to decrease systemic inflammation.25,26 These effects on immunity have been supported by clinical studies. A recent meta-analysis showed that higher levels of physical activity were associated with a 31% lower risk of infectious disease and a 37% lower risk of infectious disease-related mortality.13
Specifically regarding COVID-19, there is some conflict, even though most studies pointed to the protective effect of physical activity. Low reported physical activity was reported as a risk factor for death in addition to age and low oxygen saturation.27 Other studies did not find a significant association between physical activity and susceptibility to the COVID-19 infection. Almansour et al.,17 in a retrospective matched case–control study with 142 participants in Arabia, observed that physical activity did not show any significant association with COVID-19 contraction (x2=0.254, p=0.614) or self-rated level of physical activity (x2=0.122, p=0.727). However, a small sample size was studied. Gao et al.28 studied 105 patients diagnosed with COVID-19, and 210 controls were included. They observed that, compared with the control group, the case group had higher proportions of increased physical activities, i.e., 5 times a week (56.2% vs. 32.9%, p<0.001).
A systematic review found that PA before infection may reduce severity and mortality in COVID-19 patients, particularly PA of 150min per week of moderate activity or 75min per week of vigorous activity.20 This meta-analysis included eighteen studies involving 1618 680 adults. PA significantly decreased the risk of death in COVID-19 patients (odds ratio [OR] 0.34; 95% confidence interval [CI], 0.19–0.62; p<0.001) and the risk of severe outcomes (OR 0.60; 95% CI, 0.48–0.76; p<0.001). However, the authors are sensitive to the difference in PA patterns and severity definitions among the included studies.
Our research has limitations. First, the physical activity was not assessed by an objective measurement; the assessment used was self-reported and dependent on memory and subjective perceptions. However, this questionnaire has been validated and is recommended by the WHO. Second, we could not control the vaccine response in case studies and control patients because, until the middle of the study, vaccines for COVID were not yet being applied in Brazil. Finally, we did not include cases of death. Our study design included patients at the intensive care unit, and they had to be able to respond to the IPAQ, which made it impossible to study patients who died. Thus, further studies are still needed to investigate the complete role of physical activity on the severity of COVID-19 pneumonia.
This finding emphasizes the protective effects of a physical activity pattern against the risks of severe COVID-19 pneumonia. This result has critical implications for public health, especially considering that COVID-19 has no specific treatment. Thus, it is mandatory to encourage the general population to incorporate physical activity into their everyday routine to reduce the risk of severe COVID-19 pneumonia, the main cause of COVID-19 mortality.
ConclusionPrevious studies have shown that patterns of physical activity are associated with the severity of illness and mortality in COVID-19 patients. This study adds that a previous pattern of high or moderate physical activity offers protection against severe COVID-19 pneumonia hospitalization.
Ethical considerationsThis study was previously approved by the Research Ethical Committee of Instituto de Medicina Integral Prof. Fernando Figueira (IMIP); CAAE: 40857920.0.0000.5201. Written informed consent was obtained previously from each participant.
FundingThis research was funded by CNPq (Brazilian National Council for Scientific and Technological Development). Grant number: 401907/2020-1.
Conflict of interestAll the authors declare no conflict of interest.