To evaluate the effect of drug interactions with chronic direct oral anticoagulants (DOAC) on mortality in older atrial fibrillation (AF) patients during the Coronavirus disease 2019(COVID-19) pandemic.
MethodsWe followed a total of 601 elderly patients (65 years of age) from the NOEL-Drug Registry cohort who were referred to a tertiary outpatient clinic between 9 March 2020 and 1 March 2021. We recorded clinical characteristics and medications for the last 3 months. In addition, all drug interactions were identified using Lexicomp®. Finally, we recorded retrospectively all death events, COVID-19 diagnosis, and relevant deaths from the database at the end of the study. According to logistic regression, we performed propensity score (PS) matching to reduce potential bias. Factors associated with total mortality in the 12 months were analyzed using multivariable Cox proportion hazard analysis.
ResultsThe mean age [standard deviation (SD)] was 74.5 (±6.9), and the male/female ratio was 337/264. The prevalence of total mortality was 16.9% (n=102). A total of 4472 drugs were analyzed for DOAC interaction. 81.8% of older AF patients were not at risk in terms of potential interaction. In the Cox proportional hazard model after PS-matching, previous DOAC use with class X interaction was associated with significantly higher mortality risk (adjusted hazard ratio: 2.745, 95% confidence interval: 1.465–5.172, p=0.004).
ConclusionsOur study showed that while most co-medications do not have significant interactions with DOACs, few serious drug interactions contribute to mortality in elderly patients with AF during the pandemic.
Evaluar el efecto de las interacciones farmacológicas con anticoagulantes orales directos (ACOD) crónicos sobre la mortalidad en pacientes mayores con fibrilación auricular (FA) durante la pandemia de la enfermedad por coronavirus 2019 (COVID-19).
MétodosSeguimos a un total de 601 pacientes ancianos (65años) de la cohorte NOEL-Drug Registry que fueron remitidos a una consulta externa de tercer nivel entre el 9 de marzo de 2020 y el 1 de marzo de 2021. Registramos las características clínicas y los medicamentos durante los últimos tres meses. Además, todas las interacciones medicamentosas se identificaron utilizando Lexicomp®. Finalmente, registramos retrospectivamente todos los eventos de muerte, el diagnóstico de COVID-19 y las muertes relevantes de la base de datos al final del estudio. De acuerdo con la regresión logística, realizamos un emparejamiento por puntaje de propensión (PP) para reducir el posible sesgo. Los factores asociados con la mortalidad total en los 12 meses se analizaron mediante análisis de riesgo de proporción de Cox multivariable.
ResultadosLa edad media (desviación estándar [DE]) fue de 74,5 (±6,9) y la relación hombre/mujer, de 337/264. La prevalencia de mortalidad total fue del 16,9% (n=102). Se analizaron un total de 4.472 fármacos para determinar la interacción con ACOD. El 81,8% de los pacientes mayores con FA no estaban en riesgo en términos de interacción potencial. En el modelo de riesgos proporcionales de Cox después del emparejamiento por PP, el uso previo de ACOD con interacción X clase se asoció con un riesgo de mortalidad significativamente mayor (índice de riesgo ajustado: 2,745; intervalo de confianza del 95%: 1,465-5,172; p=0,004).
ConclusiónNuestro estudio mostró que, si bien la mayoría de los medicamentos concomitantes no tienen interacciones significativas con los ACOD, pocas interacciones farmacológicas graves contribuyen a la mortalidad en pacientes de edad avanzada con fibrilación auricular durante la pandemia.
Coronavirus disease 2019 (COVID-19) is characterized by vascular inflammation and endothelial dysfunction, with a hypercoagulable state leading to thrombotic events. Studies have shown that thrombosis is common in hospitalized COVID-19 patients;1 its incidence has been found between 4 and 31% in recent studies, especially in the geriatric population.1,2
Atrial fibrillation (AF) is the most frequent arrhythmia seen in older patients. For instance, the prevalence of AF increases dramatically with age, 5% in patients aged over 65 years and approximately 10% over 80 years.3 In addition, AF also leads to ischemic stroke, which increases mortality prevalence. Moreover, data from several studies suggest that the mortality rate was 20% in COVID-19 patients with a history of atrial fibrillation (AF) before the infection.1 Consequently, aging and AF are both expected to contribute to the increasing trend in mortality in the COVID pandemic. On the other hand, theoretically chronic direct oral anticoagulant (DOAC) use could alleviate the death risk of AF and COVID-19 with related thrombotic complications. However, previous cross-sectional studies have conflicting results about the effects of previously taking DOACs on mortality during the pandemic.4–6
Furthermore, comorbid diseases and polypharmacy are more frequent in elderly AF patients. Additionally, no previous research has examined drug interactions with DOACs in older AF patients during the COVID-19 outbreak. Therefore, we hypothesized that despite the advantageous profile of DOACs, co-medications might interact and reduce their effectiveness in the cases mentioned above. Therefore, this study aimed to interpret whether drug interactions could increase mortality in older AF patients during the COVID-19 pandemic.
MethodsPatient populationIn this cross-sectional study, we included 601 non-valvular AF patients who take at least three months of any DOAC (Apixaban, Dabigatran, Edoxaban, or Rivaroxaban) from the cohort consisting of the NOEL-Drug Registry7 aged 65 or older and alive through 9 March 2020 (the date of the first confirmed COVID-19 case in Turkey).8 In brief, the NOEL drug registry is a study for detecting a novel drug interaction index with DOACs before the pandemic. Therefore, we followed up with patients during the COVID-19 outbreak between 9 March 2020 and 1 March 2021 in a university medical center (Afyonkarahisar Health Sciences University Hospital, Turkey).
Criteria for selecting the subjects were1: aged 65 and over2; diagnosed AF4; taking one DOAC for at least 3 months before the assessment4; medication list including last three months. There were no exclusion criteria, except for patients’ refusal to participate. However, no patient refused to attend. In addition, all the patients gave their verbal informed consent before inclusion in the study.
Follow-up and outcomes measurementsThe investigators invited patients in the cohort to participate in the study. And all participants were recruited from their routine outpatient clinic visits, not a hospital or emergency admissions. Therefore, we recorded current echocardiographic measurements and laboratory test results from the local hospital database (Nucleus, Hospital Management System, Ankara,Turkey) within the last three months of the admission. At the same time, a detailed registration paper is also recorded from medications near the patient, the local hospital database, and, if communication is limited, from the patient's relatives. Polypharmacy was defined as taking five or more different medications at the last control visit.9,10 All prescribed DOACs were assessed for an appropriate dose based on the following factors: renal function, weight, and age, as indicated.11–13 Inappropriate DOAC dosage was defined as under- and overdosing by drug-specific dose criteria (e.g., renal function).
Finally, all death events (regardless of the cause) and dates were recorded from the local hospital database retrospectively at the end of the study (1 March 2021). In addition, we recorded the COVID-19 diagnosis and relevant deaths from the Public Health Management System (PHMS/Turkish abbreviation-HSYS). COVID-19 diagnosed patients were based on positive polymerase chain reaction (PCR) results with a combined oral and nasopharyngeal swab. We also obtained anti-COVID agents from HSYS (see Data Supplement).
Drug interaction measurementAll co-medications were checked for drug interaction using Lexicomp® (Wolters Kluwer, Hudson, Ohio) program.14 The program operates with the latest database and interprets each interaction with possible clinical consequences based on pharmacodynamics. Drug interaction was defined as a potential pharmacological interaction between two individual compounds. Risk scoring of each DOAC medication for possible drug-drug interactions was performed using the online Lexicomp® program. The classification of this program is as follows: (A) evidence of no interactions in literature, (B) known interactions but no action needed, (C) monitor therapy, (D) consider therapy modification, (X) avoid combination.
There are currently several methods for assessing “clinically relevant drug interactions”. In this study, we used this term in reference to Lexicomp's recommendations to monitor therapy or take action when a drug interaction, such as a class C, D, and X interaction, is identified.
The researchers critically reviewed all annotated drug interactions. The critical review aimed to exclude confusions originating from the drug's formulation, like considering low-dose aspirin as an antiaggregant, not non-steroid anti-inflammatory co-medications were classified according to the Anatomical, Therapeutic, Chemical (ATC) system.
Statistical analysesAll statistical tests were performed in R version 4.1.2. Continuous variables were expressed as mean±standard deviation (SD) or median and interquartile range (IQR) as appropriate according to the Kolmogorov–Smirnov test for normality of the distribution. The categorical data were summarized as the percentage frequency. Comparison tests were performed using the Kruskal–Wallis test for continuous variables and the chi-square test for categorical variables.
The primary endpoint was all-cause mortality. Patients were followed up until death or censorship on 1 March 2021. The comparison of the continuous variables according to all-cause mortality was carried out with the Students t-test and the Mann–Whitney test. For categorical variables, we used the chi-square test. The predictors [age, sex, hypertension, chronic renal disease, X class interaction, COVID-19 test positiveness, inappropriate DOAC dosage, chronic renal disease, left ventricular ejection fraction (LV EF), CHA2DS2VASC and HASBLED scores, clinically relevant interactions, Class X interactions, N-terminal prohormone of brain natriuretic peptide (NT-ProBNP), and Troponin I (TnI)] for the primary endpoint included in the model.
The main candidate for the predictor was at least one serious (Class X) interaction with any DOAC. The primary analysis method was to compare the time to death of patients who had a serious interaction with DOACs by a multivariable Cox proportional hazards model. Therefore, we included candidate predictors in the model according to clinically plausible and known death-related variables. Since the basal characteristics of the surviving and the deceased patients differed, we performed propensity score (PS) matching to reduce potential bias. The PS was obtained by logistic regression of 10 variables [age, sex, hypertension, diabetes, heart failure, vascular disease, cerebrovascular disease, chronic renal disease, CHA2DS2VASC, and HASBLED scores]. Taking the estimated PS of each patient, a 1:2 match analysis was performed using the nearest-neighbor matching with a caliper distance of 0.02 without replacement was performed using the MatchIt package in R.15 The adjusted hazard ratio (HR) quantified the associations between candidate predictors and all-cause death with a 95% confidence interval (CI). All statistical tests were two-tailed. A p-value of less than 0.05 was considered to show statistically significant results.
Ethical considerationsThe Ethics Committee of Afyonkarahisar Health Sciences University, Turkey, approved the study (no: 2021/13). The study was conducted in compliance with the good clinical practices protocol and Declaration of Helsinki principles.
ResultsIn our study, 601 patients were enrolled. The mean age (SD) was 74.5 (±6.9) years, and there were 337 men (56%) and 264 women (44%). The most frequent comorbid diseases were hypertension (60.2%), coronary artery disease (45.3%), and diabetes mellitus (29.8%). The mean CHA2DS2VASC score was 4.02±1.56, and the mean HASBLED score was 3.97±0.88. Thus, 129 patients were taking inappropriate DOAC dosage (21.5%). Moreover, when we look at the laboratory parameters, TnI [0.016 (0.008–0.064 IQR) vs. 0.0225 (0.0128–0.600 IQR), p=0.012], and NT-Pro BNP [1393.0 (400.9–3327.0 IQR) vs. 4049.0 (1518.0–10262.0 IQR), p<0.001] were significantly higher in the deceased group. However, LV EF and other routine laboratory parameters were not different in the two groups. Furthermore, the overall median follow-up time was 1063.0 days (732.8–1309.8 IQR). The median follow-up time in the deceased group was shorter than survived group [939.0 (604.1–883.4 IQR) vs. 1088.2 (790.5–1318.6 IQR)] (Table 1).
Basal characteristics of the study population.
| Variables | Survived (n=499) | Non-survived (n=102) | p-Value |
|---|---|---|---|
| Age (years) (SD) | 73.8±6.72 | 78.0±6.9 | <0.001 |
| Sex (F/M) | 285/214 | 52/50 | 0.274 |
| Concomitant diseases, n (%) | |||
| Diabetes mellitus | 144 (28.9) | 35 (34.3) | 0.272 |
| Hypertension | 299 (59.9) | 63 (61.8) | 0.729 |
| Coronary artery disease | 225 (45.1) | 46 (45.1) | 0.903 |
| Heart failure | 146 (29.3) | 38 (37.3) | 0.110 |
| Stroke/TIA | 87 (17.5) | 19 (18.6) | 0.780 |
| Hyperlipidemia | 111 (22.3) | 20 (19.6) | 0.550 |
| Chronic renal disease | 40 (8.0) | 15 (14.7) | 0.033 |
| Chronic hepatic disease | 19 (3.8) | 4 (3.9) | 0.956 |
| Chronic obstructive pulmonary disease/asthma | 89 (17.8) | 26 (25.5) | 0.073 |
| Positive COVID-19 test, n (%) | 113 (22.6) | 36 (35.3) | 0.007 |
| CHA2DS2VASC score (SD) | 3.96±1.55 | 4.31±1.59 | 0.036 |
| HASBLED score (SD) | 3.98±0.78 | 3.92±0.79 | 0.524 |
| DOAC duration, (days) (SD) | 910.7±443.4 | 850.4±428.4 | 0.216 |
| Inappropriate DOAC dosage, n (%) | 94 (18.8) | 35 (34.3) | 0.001 |
| LV EF (%) (SD) | 54.0±14.3 | 49.3±18.1 | 0.281 |
| Hemoglobin (g/dL) (SD) | 13.27±1.90 | 12.67±2.06 | 0.431 |
| Platelets (×109/L) (SD) | 230.00±75.94 | 215.72±65.51 | 0.172 |
| WBC (×103/mm2) (SD) | 7.89±2.46 | 8.00±2.84 | 0.254 |
| GFR (ml/min) (SD) | 65.70±19.25 | 56.23±19.19 | 0.950 |
| AST (U/L) (SD) | 20.43±9.26 | 22.45±11.6 | 0.240 |
| ALT (U/L) (SD) | 16.90±9.12 | 15.30±7.22 | 0.160 |
| CRP (mg/L) (IQR) | 1.17 (0.40–3.30) | 1.30 (0.53–3.55) | 0.210 |
| Troponin I (ng/mL) (IQR) | 0.016 (0.008–0.064) | 0.0225 (0.0128–0.600) | 0.012 |
| NT-Pro BNP (pg/mL) (IQR) | 1393.0 (400.9–3327.0) | 4049.0 (1518.0–10262.0) | <0.001 |
| Follow-up time (days) (IQR) | 1088.2 (790.5–1318.6) | 939.0 (604.1–883.4) | 0.001 |
F: female; M: male; COVID-19: coronavirus disease 2019; DOAC: direct oral anticoagulant; TIA: transient ischemic attack; WBC: white blood cells; GFR: glomerular filtration rate; AST: aspartate transaminase; ALT: alanine transaminase; CRP: C-reactive protein; NT-ProBNP: N-terminal prohormone of brain natriuretic peptide; SD: standard deviation; IQR: interquartile range.
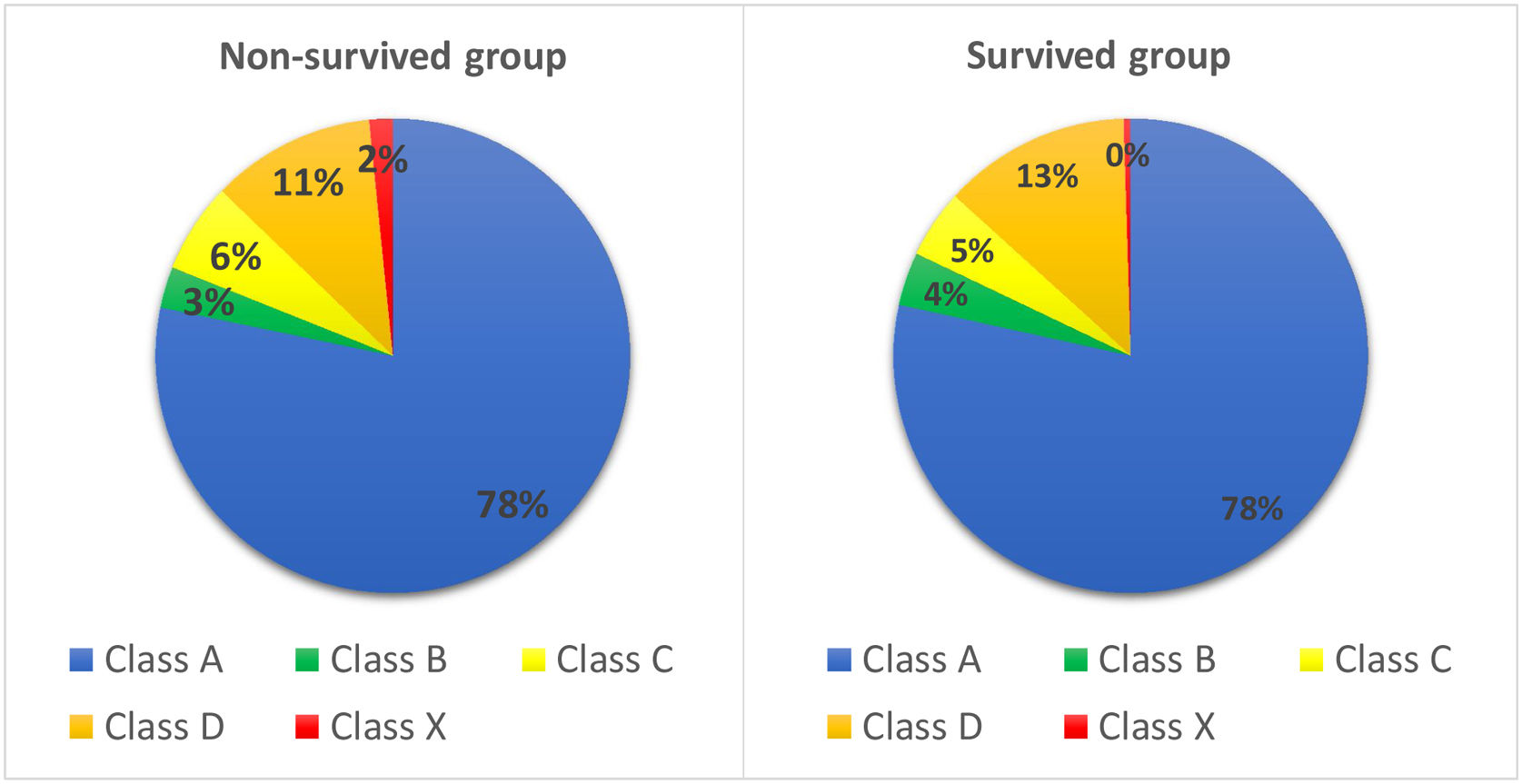
A total of 4472 drugs were analyzed for potential drug interactions with DOACs. While 81.8% of older AF patients are not at risk in terms of potential interaction (class A and B), 4.9% of patients should be followed in terms of interaction (class C interaction risk score). Furthermore, 12.5% of patients have clinically significant interaction, considering therapy modification (class D interaction risk score). Finally, 30 patients (0.06%) were detected as they have class X interaction risk that is considered as avoid combination (Figure 1). The most used concomitant drug groups in the total study population were beta-blockers (61.2%), non-steroid anti-inflammatory drugs (NSAIDs) (54.1%), and calcium channel blockers (CCB) (36.6%). The mean medication number per day was 7.41 (±3.66). A striking result was that we found a very high polypharmacy level (80.4%, n=483) in our study group. In summary, there was no significant difference between groups of survived and deceased in terms of drug categories (Table 2).
Co-medication patterns and interaction characteristics classes of study population.
| Variables | Survived (n=499) | Non-survived (n=102) | p-Value |
|---|---|---|---|
| Drug interaction class, n (%) | |||
| A | 2920 (78.4) | 587 (78.2) | 0.758 |
| B | 135 (3.6) | 21 (2.8) | 0.227 |
| C | 173 (4.6) | 46 (6.1) | 0.121 |
| D | 476 (12.7) | 84 (11.2) | 0.224 |
| X | 18 (0.4) | 12 (1.6) | <0.001 |
| Drug number per day (SD) | 7.43±3.66 | 7.31±3.64 | 0.834 |
| Polypharmacy, n (%) | 400 (80.2) | 83 (81.3) | 0.779 |
| Concomitant drug use, n (%) | |||
| ARB | 164 (32.9) | 26 (25.5) | 0.141 |
| ACEinh | 128 (25.7) | 36 (35.3) | 0.051 |
| CCB | 180 (36.1) | 39 (38.2.4) | 0.679 |
| Beta-blockers | 300 (60.1) | 64 (62.7) | 0.621 |
| Mineralocorticoids | 104 (20.8) | 17 (16.7) | 0.338 |
| Alfa-blockers | 40 (8.0) | 11 (10.8) | 0.361 |
| Aspirin | 96 (19.2) | 23 (22.5) | 0.445 |
| P2Y12 inhibitors | 21 (4.2) | 7 (6.9) | 0.246 |
| Loop diuretic | 148 (29.7) | 35 (34.3) | 0.352 |
| Statin | 98 (19.6) | 19 (18.6) | 0.439 |
| Metformin | 99 (19.8) | 25 (24.5) | 0.288 |
| Insulin | 43 (8.6) | 13 (12.7) | 0.191 |
| Other glucose-lowering drugs | 61 (12.2) | 18 (17.7) | 0.352 |
| Antidepressants/antipsychotics | 66 (13.2) | 10 (9.8) | 0.343 |
| Glucocorticoid inhalants | 32 (6.4) | 10 (9.8) | 0.221 |
| Anti-cholinergic inhalants | 42 (8.4) | 15 (14.7) | 0.058 |
| Oral glucocorticoids | 21 (4.2) | 1 (1.0) | 0.150 |
| NSAIDs | 240 (48.5) | 46 (45.1) | 0.491 |
| Opioids | 6 (1.2) | 1 (1) | 0.849 |
SD: standard deviation; ARB: angiotensin receptor blockers; ACEinh: angiotensin-converting enzyme inhibitors; CCB: calcium channel blockers; P2Y12: purinergic G protein-coupled receptor 12; NSAID: non-steroid anti-inflammatory drugs.
Of the 102 patients who died, 12 had serious DOAC-drug interaction (Class X interaction), and 90 had no serious interaction (the ratio of serious interactions in the non-surviving group to all participants was 12/30=40%, the ratio of non-serious interactions in the non-surviving group to all participants was 90/571=16%, unadjusted hazard ratio was=2.538, with 95% CI: 1.574–4.091, p<0.001). In the adjusted multivariable Cox proportional hazard model, we found that age [adjusted HR: 1.089, 95% CI: 1.056–1.123, p<0.001], COVID-19 test positivity [adjusted HR: 1.943, 95% CI: 1.220–3.073, p=0.005], hypertension [adjusted HR: 1.894, 95% CI: 1.076–3.321, p=0.027], CHA2DS2VASC score [adjusted HR: 1.138, 95% CI: 1.005–1.302, p=0.046], inappropriate DOAC dosing [adjusted HR: 1.706, 95% CI: 1.021–2.832, p=0.004], clinical relevant interaction [adjusted HR: 2.563, 95% CI: 1.175–5.634, p=0.019], Tn I [adjusted HR: 1.052, 95% CI: 1.016–1.0114, p=0.016] and Class X interaction [adjusted HR: 3.442, 95% CI: 1.560–7.612, p=0.001] associated with higher mortality events (Table 3).
Cox proportional hazard model in selected predictors and mortality before-after propensity score matching analysis.
| Variables | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||
| Age | 1.089 | 1.056 | 1.123 | <0.001 | 1.071 | 1.033 | 1.111 | <0.001 |
| Sex | 0.664 | 0.425 | 1.965 | 0.079 | – | – | – | |
| COVID-19 test positiveness | 1.943 | 1.220 | 3.073 | 0.005 | 1.33 | 0.873 | 2.021 | 0.180 |
| Hypertension | 1.894 | 1.076 | 3.321 | 0.027 | – | – | – | – |
| CHA2DS2VASC score | 1.138 | 1.005 | 1.302 | 0.046 | – | – | – | – |
| Inappropriate DOAC dosage | 1.706 | 1.021 | 2.832 | 0.004 | 1.97 | 1.198 | 3.239 | 0.007 |
| Clinically relevant interaction | 2.563 | 1.175 | 5.634 | 0.019 | – | – | – | – |
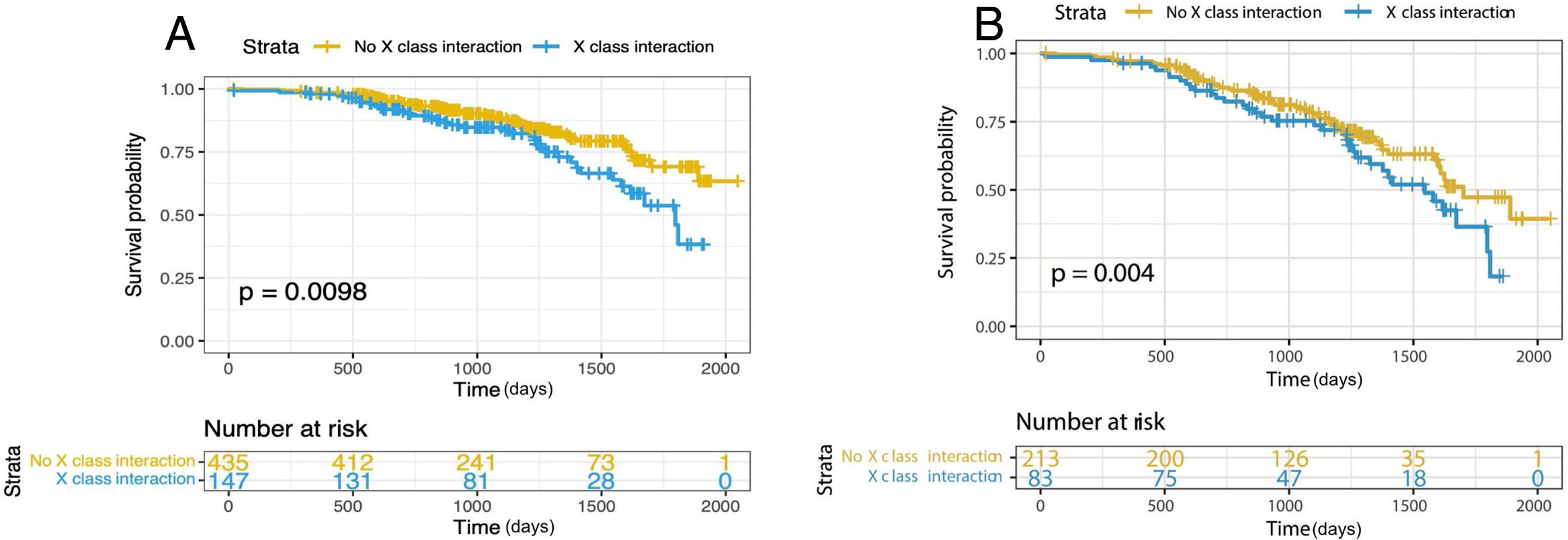
| Class X interaction | 3.442 | 1.560 | 7.612 | 0.001 | 2.745 | 1.465 | 5.172 | 0.004 |
| Troponin I | 1.052 | 1.016 | 1.0114 | 0.016 | 1.032 | 1.006 | 1.069 | 0.020 |
COVID-19: coronavirus disease 2019; PSM: propensity score matching; HR: hazard ratio; CI: confidence interval; DOAC: direct oral anticoagulant.
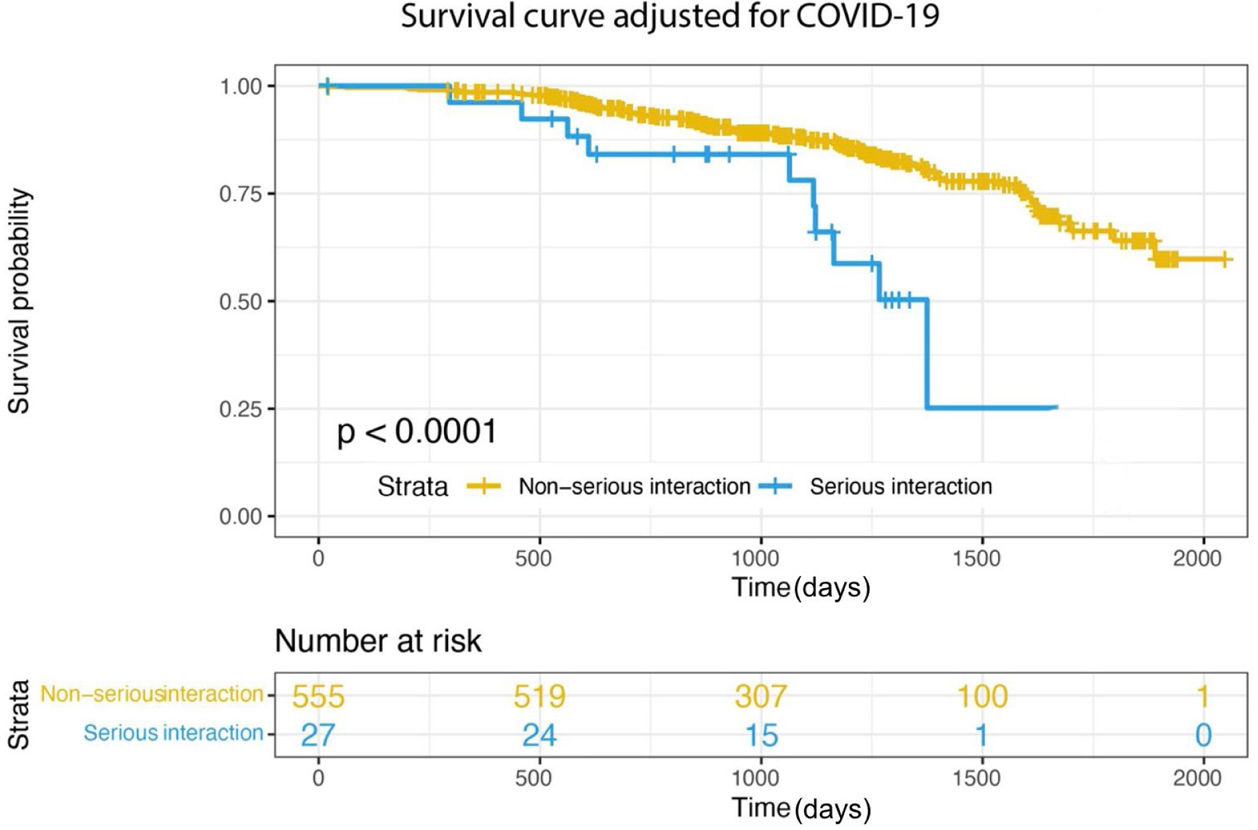
After the PS matching, 306 patients remained in the study (102 patients in the non-survived group, 204 in the survived group). We performed secondary analyzes to assess the relationship between class X interaction and mortality using both the multivariate Cox proportional hazards model (adjusted HR: 3.442, 95% CI: 1.560–7.612, p=0.001) (Figure 2a) and the PS-adjusted multivariable Cox by proportional hazards model from the matched population (adjusted HR: 2.745, 95% CI: 1.465–5.172, p=0.004) (Figure 2b). Furthermore, in the matched samples, age (adjusted HR: 1.071, 95% CI: 1.033–1.111, p<0.001), inappropriate DOAC dosing (adjusted HR: 1.97, 95% CI: 1.198–3.239, p=0.007), Tn I (adjusted HR: 1.032, 95% CI: 1.006–1.069, p=0.020) and Class X interaction (adjusted HR: 2.745, 95% CI: 1.465–5.172, p=0.004) were still associated with all-cause mortality (Table 3). In addition, when we adjusted according to the COVID-19 diagnosis, there is a strikingly clear trend of decreasing survival rates in Class X interaction (Figure 3). Taken together, these results suggest that there is an association between all-cause mortality and serious DOAC-drug interactions in elderly patients with AF that is even balanced by the COVID-19 diagnosis.
Finally, some additional material is given in the Data Supplement. It summarizes agents used for COVID-19 management and drug interactions with DOACs by mechanism. COVID-19 patients have been treated according to the Turkish Ministry of Health's treatment guidelines recommended and regularly updated by the National COVID-19 Scientific Committee in Turkey. More than half of our study population was treated with favipiravir (59.4%). Very few COVID-19 patients were treated with azithromycin (3.2%) and clarithromycin (0.9%), which have considerable DOAC–drug interactions. In summary, most COVID-19 therapeutics were in low interaction class with DOACs (Data Supplement Table S).
DiscussionThis cross-sectional follow-up study observed 601 elderly AF patients taking chronic oral anticoagulants and examined all-cause mortality during one year of the COVID-19 pandemic. And we found that 102 patients died, 36 of them deceased due to COVID-19. Moreover, our study was one of the first to demonstrate that serious drug interaction with DOACs significantly contributes to mortality in older AF patients during the outbreak. However, according to our analyses, we saw that majority of the elderly with AF were not at risk in terms of potential interaction. Further, we found that our study population has a high polypharmacy level; nevertheless, it did not contribute to mortality. Another important finding was that 21.5% of the study population took inappropriate DOAC doses. Additionally, consistent with expectations, this research found that a stronger contributor to the mortality was the COVID-19 diagnosis.
COVID-19 patients are prone to thromboembolism because of the possible direct action of the SARS-CoV-2 virus on coagulation, cells, such as endothelium and platelets, and the indirect immune activation further potentiated hypercoagulable state. Previous studies suggest that chronic anticoagulant treatment before the infection may protect against thrombotic events and COVID-19 related mortality.16,17 On the other hand, drug interactions generally reduce the beneficial effects of oral anticoagulant drugs. Although DOACs have less drug interaction potential than vitamin K antagonists, some recently published studies showed that drug interactions with DOACs cause morbidity and mortality in older AF patients.7,18–20 Our study also found that drugs such as carbamazepine, phenytoin, rifampicin, and enzalutamide, which have class X interactions with DOACs, increase mortality almost threefold. Most class X interactions emerged over Cytochrome P450 3A4 (CYP3A4) and P-glycoprotein (P-gp) induction. The concomitant use of strong inducers of P-gp and CYP3A4 may reduce the plasma concentration of DOACs.21 In addition, the most frequent interacting agents were diclofenac, dexketoprofen, naproxen, acetylsalicylic acid, clopidogrel, and propafenone in the moderate interaction group (class D). These results seem consistent with previous research, which found that phenytoin, NSAIDs, diltiazem, amiodarone, rifampicin, and verapamil were the most frequent agents interacting with DOACs.18 NSAIDs must be limited; antiplatelet agents should be discontinued on indications. However, some medications like carbamazepine could require long-term use.22 Therefore, it cannot be easily switched to alternative medicines. More attention should be paid to drug interactions in treating elderly patients with atrial fibrillation and epilepsy.
On whether COVID-19 management drug interaction affects mortality, we did not find a significant association (see Data Supplement). However, caution must be applied with a small COVID-19 positive sample size, as our findings might not mean drug interaction is trivial. Hence, a recent meta-analysis has shown that drug interactions between antiretroviral agents and anticoagulants result in poor outcomes.23 Furthermore, for the first time, European Heart Rhythm Association (EHRA) made recommendations on interaction with COVID-19 medications in the practical guide,24 and COVID-19 agents used in our country are generally in the no interaction category. So, DOAC drug interactions were not affecting mortality due to minor interaction patterns of the preferred COVID-19 medications.
Furthermore, we found that the polypharmacy level was 80.3%, too high. This finding was higher than previous studies conducted on elderly AF patients taking DOACs which vary from 40% to 53%.25,26 A prior report from Turkey showed that 55% of AF patients taking DOAC have polypharmacy.27 This inconsistency may be due to our study population's elderly, frail, comorbid patients compared to previous studies. Additionally, such polypharmacy in AF seems associated with certain risk factors such as HT, ischemic heart disease, heart failure, and diabetes. Indeed, polypharmacy can independently be associated with AF patients, particularly at high risk with multiple comorbidities. According to the previous studies, polypharmacy could increase mortality 2 or 3-fold in elders.25,28
Another important finding was that one-fifth of the study population took inappropriate DOAC doses. This finding was consistent with other international studies in which prescribing errors were 26% and 28% due to inappropriate drug choice.29,30 In addition, we found an association between inappropriate DOAC dosage and mortality. However, the multivariable regression analysis showed that these results were not statistically significant. The reasons for this remain unclear, but our results indicate that incorrect dosing could decrease the advantage of DOACs.
Study limitationsA note of caution is due here since total mortality rates increased across the globe during the COVID-19 pandemic compared to the previous years. So, we do not know every death was related to COVID-19. Besides, we recorded COVID-19 diagnoses and related deaths from the public health management system. However, total deaths could indirectly be associated with COVID-19 as far as our cohort has a higher mortality rate during the pandemic than the previous mortality data.7
The results of this study should be interpreted with some caution because of several limitations. The first and major limitation was that we could not confirm all death causes of the study population. For example, we do not know about cardiovascular mortality, thromboembolic or hemorrhagic related deaths. The second limitation was that data were collected in only one center. Furthermore, the study has a cross-sectional observational design that could affect bias and confounding. For this reason, our research should never be conclusive but generates hypotheses. Third, we examined drug interactions only from one online application (Lexicomp®). We did not measure DOAC plasma levels. Therefore, it was not a full pharmacokinetic and pharmacodynamic assessment. The final and important limitation was that we only assess interaction with DOACs, so we do not know about co-medications interactions with each other. So, this study does not fully consider the problems that can appear in real situations. However, it does demonstrate the need for further research.
ConclusionThe most obvious finding to emerge from this study is that potential serious drug interactions with DOACs affect mortality in older AF patients during the COVID-19 pandemic. However, DOAC interaction rates were low with most co-medications for COVID-19 management and chronic morbidities. Still, further, and larger studies are required to validate our findings.
Consequently, physicians should scrutinize DOACs by appropriate doses and drug interactions in elderly AF patients, even in serious pandemic conditions.
Author contributionsAccording to the International Committee of Medical Journal Editors (ICMJE), all authors of this manuscript meet the authorship criteria. In addition, all authors have seen and approved the manuscript being submitted and published.
Funding sourcesNone
Conflicts of interestThe authors report no conflicts of interest in this work.
The authors have no affiliation or endorsement from any company in this article. Therefore, this article did not require receiving specific grants from funding agencies in the public, commercial or non-profit sectors.