There is still limited evidence on the role of glucagon-like peptide-1 receptor agonists in heart failure. We analyzed the efficacy in terms of health status, and the change in body weight of once-weekly semaglutide in patients with heart failure with preserved ejection fraction, obesity and type 2 diabetes.
Patients and methodsThis prospective, real-world study included patients with heart heart failure with preserved ejection fraction, obesity and type 2 diabetes treated with once-weekly 1.00mg semaglutide (Sema-Preserved Group) and patients not treated with glucagon-like peptide-1 receptor agonists (Control-Preserved Group). A 1:1 propensity score matching analysis was performed. The primary outcome was the heart failure status defined as the ≥5 point difference in the Spanish version of the Kansas City Cardiomyopathy Questionnaire total symptom score, and the change of body weight at 24 months.
ResultsAfter matching, 203 patients were included in each group. A primary outcome event occurred in 123 patients (60.6%) in the Sema-Preserved Group and 36 (17.7%) in the Control-Preserved Group (odds ratio: 3.99; 95% confidence interval: 1.69–6.28; p<0.01), and the mean change in body weight was −12.9±4.2kg in patients with semaglutide and −2.5±1.1kg in control patients (p<0.01). There were also significant declines in the heart failure events, and in all-cause hospitalizations.
ConclusionsOnce-weekly 1.00mg semaglutide was associated with improved heart failure health status, and weight loss in patients with heart failure with preserved ejection fraction, obesity, and type 2 diabetes. Further research on glucagon-like peptide-1 receptor agonists in heart failure is needed.
La evidencia sobre el papel de los agonistas del receptor del péptido similar al glucagón tipo 1 en pacientes con insuficiencia cardíaca es limitada. Analizamos la eficacia de semaglutida semanal con relación al estado de salud y el cambio del peso corporal en pacientes con insuficiencia cardíaca con fracción de eyección preservada, obesidad y diabetes mellitus tipo 2.
Pacientes y métodosEstudio prospectivo en vida real en pacientes con insuficiencia cardíaca preservada, obesidad y diabetes tipo 2 tratados con semaglutida 1mg/semana (Grupo Sema-Preservada) y no tratados con agonistas del receptor del péptido similar al glucagón tipo 1 (Grupo Sema-Control). Se realizó un análisis de puntuaciones de propensión 1:1. El objetivo primario fue el estado de insuficiencia cardíaca (diferencia≥5 puntos en la puntuación del cuestionario de cardiomiopatía Kansas City) y el cambio del peso corporal a los 24 meses.
ResultadosTras la propensión, 203 pacientes fueron incluidos en cada grupo. El episodio primario ocurrió en 123 pacientes (60,6%) en el Grupo Sema-Preservada y 36 (17,7%) en el Grupo Sema-Control (odds ratio: 3,99; intervalo de confianza del 95%: 1,69-6,28; p<0,01) y la media del cambio del peso fue de −12,9±4,2kg en los pacientes con semaglutida y −2,5±1,1kg en los controles (p<0,01). Los episodios de insuficiencia cardíaca y las hospitalizaciones por cualquier motivo también disminuyeron.
ConclusionesSemaglutida 1,00mg/semana se asoció con una mejoría del estado de salud y pérdida de peso en pacientes con insuficiencia cardíaca preservada, obesidad y diabetes mellitus tipo 2. Se precisa mayor investigación de los agonistas del receptor del péptido similar al glucagón tipo 1 en la insuficiencia cardíaca.
The heart failure with preserved ejection fraction (HFpEF) is a multifactorial heart disease that includes cardiovascular, metabolic, renal, and geriatric conditions.1 The presence of comorbidities such as hypertension, T2D, obesity, pulmonary hypertension, chronic kidney disease, and coronary artery disease increases the risk of developing HFpEF mediated by structural and functional heart changes, and systemic inflammation.2,3 Obesity has been described as a relevant risk factor for HFpEF. Its associations with arterial stiffness, left ventricular hypertrophy, and hypertension, which is a nuclear factor in the physiopathology of the HFpEF, contribute to the diastolic left ventricular dysfunction.4,5
In recent years, sodium–glucose cotransporter 2 inhibitors (SGLT-2i) have been associated with significant cardiovascular and renal and heart failure (HF) benefits demonstrated in patients with T2D.6,7 These benefits have been extended to HF patients with reduced and preserved left ventricular ejection fraction (LVEF) regardless of the presence of T2D.8–11 Furthermore, glucagon-like peptide-1 receptor agonists (GLP-1ra) have also showed significant reductions in hospitalizations due to HF in patients with T2D in a meta-analysis of pivotal cardiovascular clinical trials,12 although these benefits were not achieved in each individual clinical trial.7,12 Recently, a placebo-controlled trial (STEP-HFpEF DM Trial) has shown benefits of the GLP-1ra 2.4mg semaglutide in patients with HF with preserved ejection fraction (HFpEF), obesity, and T2D: it showed larger reductions in HF-related symptoms and physical limitations than placebo at 1 year.13 The benefits of 2.4mg semaglutide were previously shown in patients with HFpEF, and obesity without T2D (STEP-HFpEF Trial), also reducing symptoms and physical limitations and improving exercise function.14
On the other hand, the evidence provided by observational studies is quite limited.15 Recently, once-weekly semaglutide has been shown to be efficacious in terms of HF health status improvement and other adverse outcomes in obese patients with T2D and HF in a real-world observational study.16
Due to the complex interplay between comorbidities in HFpEF and their reciprocal associations, and the limited evidence on the role of GLP-1ra in patients with HF, we analyzed the efficacy in terms of health status, the change of body weight, and cardiovascular/renal outcomes of once-weekly semaglutide in patients with HFpEF, obesity, and T2D in real-world clinical practice. We hypothesize that the use of subcutaneous semaglutide would be efficacious in terms of clinical outcomes, and safe in patients with HFpEF, obesity, and T2D.
Materials and methodsStudy design and patientsWe performed a prospective, multicenter, real-world study on patients with with HFpEF, obesity, and T2D treated with once-weekly 1.00mg semaglutide (Sema-Preserved Group) and patients not treated with semaglutide or another GLP-1ra (Control-Preserved Group) and followed-up on for 24 months from June 2019 to June 2024. The diagnosis of HF was established according to the 2021 European Society of Cardiology Guidelines.17 In accordance with these guidelines, patients with symptoms and signs of HF, with evidence of structural and/or functional heart abnormalities and/or increase of natriuretic peptides, and with a left ventricular ejection fraction (LVEF) of 50% or higher were defined as patients with HFpEF.
Patients in the Sema-Preserved Group started a once-weekly 0.25mg dose of semaglutide for four weeks that could be increased to 0.50mg for the following four weeks until they reached the maintenance dose of 0.50mg or 1.00mg if the healthcare professionals considered it advisable according to their clinical judgment. During the follow-up, all patients received general recommendations on a healthy diet and physical activity according to their functional class. Treatments with lipid-lowering drugs, antihypertensive agents, and diuretics were modified, if necessary, as per the healthcare professionals’ judgment.
Follow-up was conducted every 3–4 months following the routine clinical practice. Data on anthropometric (body weight, body mass index [BMI], and waist circumference), sociodemographic, clinical (T2D duration and treatment, principal cause of HF, HF duration, left ventricular ejection fraction, previous medical history, and medication), and laboratory variables (serum creatinine, estimated glomerular filtration rate measured using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulae,18 basal fasting blood glucose (BG), glycated hemoglobin (HbA1c), LDL cholesterol, HDL cholesterol and total cholesterol, triglycerides, uric acid, hematocrit, N-terminal pro-brain natriuretic peptide (NT-proBNP), and urinary albumin/creatinine ratio) were collected at each evaluation. The total symptom score on the Spanish version of the Kansas City Cardiomyopathy Questionnaire (KCCQ)19 was used to estimate HF heath status. Adverse drug reactions, the need to discontinue semaglutide due to adverse events, the HF events (emergency department visit due to HF decompensation, hospitalizations due to HF, and unplanned outpatient visits); all-cause hospitalizations, and mortality (all-cause and cardiovascular) were also recorded.
Measured outcomesThe primary outcome was the HF health status defined as the ≥5 point difference in the Spanish version of the KCCQ total symptom score, and the change of body weight at 24 months. Secondary outcomes included the number of HF events (defined as a composite of emergency department visits due to HF decompensation, HF hospitalizations, and unplanned outpatient visits), cardiovascular death, all-cause death, all-cause hospitalizations, and new or worsening nephropathy (persistent macroalbuminuria, persistent doubling of the serum creatinine level and a creatinine clearance of <45ml/min/1.73m2, or the need for continuous renal-replacement therapy). The glycemic control, determined by a reduction in glycated hemoglobin, was also evaluated.
Statistical analysesThe characteristics of patients included in this study were analyzed using descriptive statistics. Continuous and categorical variables were expressed as means (standard deviation) and as absolute values and percentages, respectively. The differences between groups were determined using the two-sample Student's t-test or the Mann–Whitney–Wilcoxon rank-sum test for continuous variables and Pearson's chi-square for categorical variables. The Pearson correlation coefficient was calculated to estimate the linear correlations.
We grouped patients according to the use of once-weekly semaglutide. In order to match each patient who started on the semaglutide with a patient on the control group in a 1:1 manner, a propensity score matching (PSM) with a caliper of 0.2 and a greedy matching algorithm were used. The probability of starting the semaglutide was estimated using a logistic regression model that included variables that could have affected treatment assignment or outcomes as independent variables (sex, age, anthropometric characteristics, previous medical history, T2D and HF characteristics, and laboratory findings). The adequacy of PSM was assessed using the standardized difference of patient characteristics after matching. A significant imbalance in the group was considered to be present if there was a standardized difference >10% between baseline variables.
In order to evaluate the association between treatment and study outcomes, mixed effect logistic regressions were used and adjusted for confounding variables (sociodemographic and clinical characteristics) that were included in the models. We also performed univariate and multivariate logistic regression models adjusted with confounding variables in order to estimate the treatment effect using the totality of the data, as a sensitivity analysis. The change in body weight was evaluated with the use of analysis of covariance, with the change in the endpoint at month 24 as the dependent variable. The regression analysis values were expressed as odds ratio (OD) and 95% confidence interval (95% CI). Statistical significance was defined as p<0.05. Statistical analyses were performed using SPSS Statistics for Windows, version 22.0 (IBM SPSS Statistics for Windows, IBM Corporation, Armonk, New York).
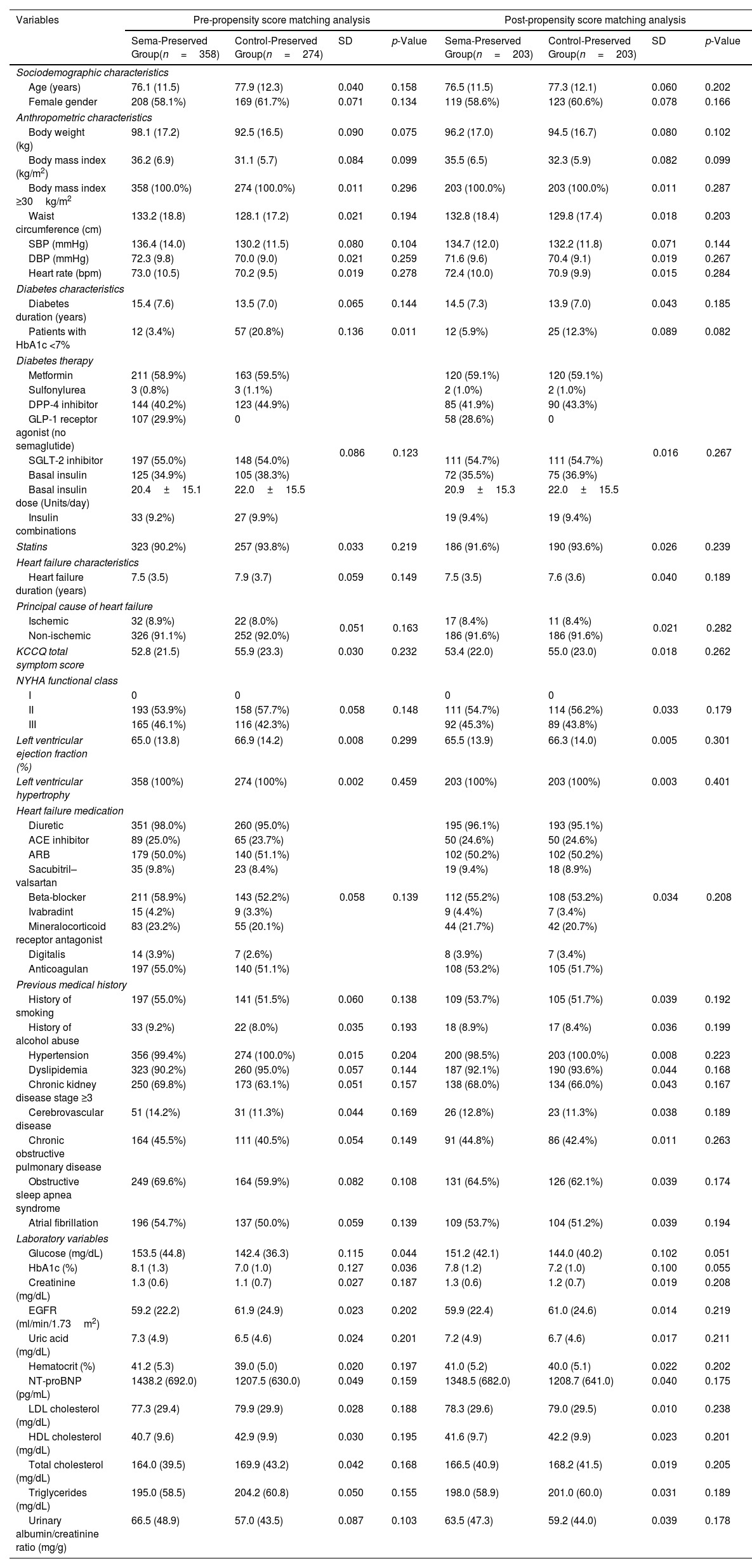
ResultsA total of 632 patients with HFpEF, obesity, and T2D were included in this study. From these patients, 358 patients who received once-weekly semaglutide (Sema-Preserved Group) and 274 patients who did not receive any GLP-1ra (Control-Preserved Group) completed 24 months of follow-up. After PSM, 203 patients were included in each group. At 24 months, a total of 180 patients (86.1%) had 1.00mg of semaglutide.
The baseline sociodemographic, clinical, and therapeutic characteristics of patients are shown in Table 1. The proportion of patients with glycated hemoglobin (HbA1c) <7% was higher in the Control-Preserved Group before the PSM (20.8% vs 3.4%, p=0.011). No other differences between groups were found, and both groups were well-balanced after the PSM.
Sociodemographic, clinical, and therapeutic characteristics at baseline: pre- and post-propensity score matching analysis.
| Variables | Pre-propensity score matching analysis | Post-propensity score matching analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Sema-Preserved Group(n=358) | Control-Preserved Group(n=274) | SD | p-Value | Sema-Preserved Group(n=203) | Control-Preserved Group(n=203) | SD | p-Value | |
| Sociodemographic characteristics | ||||||||
| Age (years) | 76.1 (11.5) | 77.9 (12.3) | 0.040 | 0.158 | 76.5 (11.5) | 77.3 (12.1) | 0.060 | 0.202 |
| Female gender | 208 (58.1%) | 169 (61.7%) | 0.071 | 0.134 | 119 (58.6%) | 123 (60.6%) | 0.078 | 0.166 |
| Anthropometric characteristics | ||||||||
| Body weight (kg) | 98.1 (17.2) | 92.5 (16.5) | 0.090 | 0.075 | 96.2 (17.0) | 94.5 (16.7) | 0.080 | 0.102 |
| Body mass index (kg/m2) | 36.2 (6.9) | 31.1 (5.7) | 0.084 | 0.099 | 35.5 (6.5) | 32.3 (5.9) | 0.082 | 0.099 |
| Body mass index ≥30kg/m2 | 358 (100.0%) | 274 (100.0%) | 0.011 | 0.296 | 203 (100.0%) | 203 (100.0%) | 0.011 | 0.287 |
| Waist circumference (cm) | 133.2 (18.8) | 128.1 (17.2) | 0.021 | 0.194 | 132.8 (18.4) | 129.8 (17.4) | 0.018 | 0.203 |
| SBP (mmHg) | 136.4 (14.0) | 130.2 (11.5) | 0.080 | 0.104 | 134.7 (12.0) | 132.2 (11.8) | 0.071 | 0.144 |
| DBP (mmHg) | 72.3 (9.8) | 70.0 (9.0) | 0.021 | 0.259 | 71.6 (9.6) | 70.4 (9.1) | 0.019 | 0.267 |
| Heart rate (bpm) | 73.0 (10.5) | 70.2 (9.5) | 0.019 | 0.278 | 72.4 (10.0) | 70.9 (9.9) | 0.015 | 0.284 |
| Diabetes characteristics | ||||||||
| Diabetes duration (years) | 15.4 (7.6) | 13.5 (7.0) | 0.065 | 0.144 | 14.5 (7.3) | 13.9 (7.0) | 0.043 | 0.185 |
| Patients with HbA1c <7% | 12 (3.4%) | 57 (20.8%) | 0.136 | 0.011 | 12 (5.9%) | 25 (12.3%) | 0.089 | 0.082 |
| Diabetes therapy | ||||||||
| Metformin | 211 (58.9%) | 163 (59.5%) | 0.086 | 0.123 | 120 (59.1%) | 120 (59.1%) | 0.016 | 0.267 |
| Sulfonylurea | 3 (0.8%) | 3 (1.1%) | 2 (1.0%) | 2 (1.0%) | ||||
| DPP-4 inhibitor | 144 (40.2%) | 123 (44.9%) | 85 (41.9%) | 90 (43.3%) | ||||
| GLP-1 receptor agonist (no semaglutide) | 107 (29.9%) | 0 | 58 (28.6%) | 0 | ||||
| SGLT-2 inhibitor | 197 (55.0%) | 148 (54.0%) | 111 (54.7%) | 111 (54.7%) | ||||
| Basal insulin | 125 (34.9%) | 105 (38.3%) | 72 (35.5%) | 75 (36.9%) | ||||
| Basal insulin dose (Units/day) | 20.4±15.1 | 22.0±15.5 | 20.9±15.3 | 22.0±15.5 | ||||
| Insulin combinations | 33 (9.2%) | 27 (9.9%) | 19 (9.4%) | 19 (9.4%) | ||||
| Statins | 323 (90.2%) | 257 (93.8%) | 0.033 | 0.219 | 186 (91.6%) | 190 (93.6%) | 0.026 | 0.239 |
| Heart failure characteristics | ||||||||
| Heart failure duration (years) | 7.5 (3.5) | 7.9 (3.7) | 0.059 | 0.149 | 7.5 (3.5) | 7.6 (3.6) | 0.040 | 0.189 |
| Principal cause of heart failure | ||||||||
| Ischemic | 32 (8.9%) | 22 (8.0%) | 0.051 | 0.163 | 17 (8.4%) | 11 (8.4%) | 0.021 | 0.282 |
| Non-ischemic | 326 (91.1%) | 252 (92.0%) | 186 (91.6%) | 186 (91.6%) | ||||
| KCCQ total symptom score | 52.8 (21.5) | 55.9 (23.3) | 0.030 | 0.232 | 53.4 (22.0) | 55.0 (23.0) | 0.018 | 0.262 |
| NYHA functional class | ||||||||
| I | 0 | 0 | 0.058 | 0.148 | 0 | 0 | 0.033 | 0.179 |
| II | 193 (53.9%) | 158 (57.7%) | 111 (54.7%) | 114 (56.2%) | ||||
| III | 165 (46.1%) | 116 (42.3%) | 92 (45.3%) | 89 (43.8%) | ||||
| Left ventricular ejection fraction (%) | 65.0 (13.8) | 66.9 (14.2) | 0.008 | 0.299 | 65.5 (13.9) | 66.3 (14.0) | 0.005 | 0.301 |
| Left ventricular hypertrophy | 358 (100%) | 274 (100%) | 0.002 | 0.459 | 203 (100%) | 203 (100%) | 0.003 | 0.401 |
| Heart failure medication | ||||||||
| Diuretic | 351 (98.0%) | 260 (95.0%) | 0.058 | 0.139 | 195 (96.1%) | 193 (95.1%) | 0.034 | 0.208 |
| ACE inhibitor | 89 (25.0%) | 65 (23.7%) | 50 (24.6%) | 50 (24.6%) | ||||
| ARB | 179 (50.0%) | 140 (51.1%) | 102 (50.2%) | 102 (50.2%) | ||||
| Sacubitril–valsartan | 35 (9.8%) | 23 (8.4%) | 19 (9.4%) | 18 (8.9%) | ||||
| Beta-blocker | 211 (58.9%) | 143 (52.2%) | 112 (55.2%) | 108 (53.2%) | ||||
| Ivabradint | 15 (4.2%) | 9 (3.3%) | 9 (4.4%) | 7 (3.4%) | ||||
| Mineralocorticoid receptor antagonist | 83 (23.2%) | 55 (20.1%) | 44 (21.7%) | 42 (20.7%) | ||||
| Digitalis | 14 (3.9%) | 7 (2.6%) | 8 (3.9%) | 7 (3.4%) | ||||
| Anticoagulan | 197 (55.0%) | 140 (51.1%) | 108 (53.2%) | 105 (51.7%) | ||||
| Previous medical history | ||||||||
| History of smoking | 197 (55.0%) | 141 (51.5%) | 0.060 | 0.138 | 109 (53.7%) | 105 (51.7%) | 0.039 | 0.192 |
| History of alcohol abuse | 33 (9.2%) | 22 (8.0%) | 0.035 | 0.193 | 18 (8.9%) | 17 (8.4%) | 0.036 | 0.199 |
| Hypertension | 356 (99.4%) | 274 (100.0%) | 0.015 | 0.204 | 200 (98.5%) | 203 (100.0%) | 0.008 | 0.223 |
| Dyslipidemia | 323 (90.2%) | 260 (95.0%) | 0.057 | 0.144 | 187 (92.1%) | 190 (93.6%) | 0.044 | 0.168 |
| Chronic kidney disease stage ≥3 | 250 (69.8%) | 173 (63.1%) | 0.051 | 0.157 | 138 (68.0%) | 134 (66.0%) | 0.043 | 0.167 |
| Cerebrovascular disease | 51 (14.2%) | 31 (11.3%) | 0.044 | 0.169 | 26 (12.8%) | 23 (11.3%) | 0.038 | 0.189 |
| Chronic obstructive pulmonary disease | 164 (45.5%) | 111 (40.5%) | 0.054 | 0.149 | 91 (44.8%) | 86 (42.4%) | 0.011 | 0.263 |
| Obstructive sleep apnea syndrome | 249 (69.6%) | 164 (59.9%) | 0.082 | 0.108 | 131 (64.5%) | 126 (62.1%) | 0.039 | 0.174 |
| Atrial fibrillation | 196 (54.7%) | 137 (50.0%) | 0.059 | 0.139 | 109 (53.7%) | 104 (51.2%) | 0.039 | 0.194 |
| Laboratory variables | ||||||||
| Glucose (mg/dL) | 153.5 (44.8) | 142.4 (36.3) | 0.115 | 0.044 | 151.2 (42.1) | 144.0 (40.2) | 0.102 | 0.051 |
| HbA1c (%) | 8.1 (1.3) | 7.0 (1.0) | 0.127 | 0.036 | 7.8 (1.2) | 7.2 (1.0) | 0.100 | 0.055 |
| Creatinine (mg/dL) | 1.3 (0.6) | 1.1 (0.7) | 0.027 | 0.187 | 1.3 (0.6) | 1.2 (0.7) | 0.019 | 0.208 |
| EGFR (ml/min/1.73m2) | 59.2 (22.2) | 61.9 (24.9) | 0.023 | 0.202 | 59.9 (22.4) | 61.0 (24.6) | 0.014 | 0.219 |
| Uric acid (mg/dL) | 7.3 (4.9) | 6.5 (4.6) | 0.024 | 0.201 | 7.2 (4.9) | 6.7 (4.6) | 0.017 | 0.211 |
| Hematocrit (%) | 41.2 (5.3) | 39.0 (5.0) | 0.020 | 0.197 | 41.0 (5.2) | 40.0 (5.1) | 0.022 | 0.202 |
| NT-proBNP (pg/mL) | 1438.2 (692.0) | 1207.5 (630.0) | 0.049 | 0.159 | 1348.5 (682.0) | 1208.7 (641.0) | 0.040 | 0.175 |
| LDL cholesterol (mg/dL) | 77.3 (29.4) | 79.9 (29.9) | 0.028 | 0.188 | 78.3 (29.6) | 79.0 (29.5) | 0.010 | 0.238 |
| HDL cholesterol (mg/dL) | 40.7 (9.6) | 42.9 (9.9) | 0.030 | 0.195 | 41.6 (9.7) | 42.2 (9.9) | 0.023 | 0.201 |
| Total cholesterol (mg/dL) | 164.0 (39.5) | 169.9 (43.2) | 0.042 | 0.168 | 166.5 (40.9) | 168.2 (41.5) | 0.019 | 0.205 |
| Triglycerides (mg/dL) | 195.0 (58.5) | 204.2 (60.8) | 0.050 | 0.155 | 198.0 (58.9) | 201.0 (60.0) | 0.031 | 0.189 |
| Urinary albumin/creatinine ratio (mg/g) | 66.5 (48.9) | 57.0 (43.5) | 0.087 | 0.103 | 63.5 (47.3) | 59.2 (44.0) | 0.039 | 0.178 |
Continuous data are shown as means (standard deviations) and qualitative data as absolute value and percentage.
ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; DBP: diastolic blood pressure; DPP-4: dipeptidyl peptidase-4; EGFR: estimated glomerular filtration rate; GLP-1: glucagon-like peptide-1; HbA1c: glycated hemoglobin; HF: heart failure; KCCQ: Kansas City Cardiomyopathy Questionnaire; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; SBP: systolic blood pressure; SD: standardized difference; SGLT-2: sodium–glucose cotransporter 2.
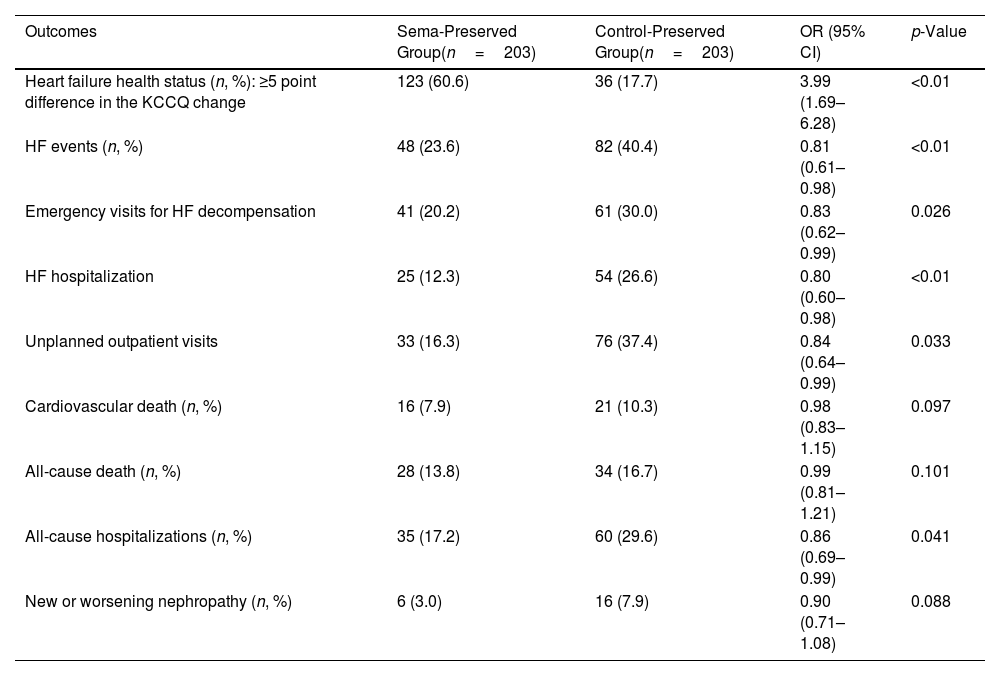
A primary outcome event (HF health status) occurred in 123/203 patients (60.6%) in the Sema-Preserved Group and 36/203 patients (17.7%) in the Control-Preserved Group (OR: 3.99; 95% CI: 1.69–6.28; p<0.01) (Table 2). At 24 months, patients with semaglutide had an improvement in the KCCQ total symptom score of 21.5 (4.2) points versus 7.3 (1.6) points for patients in the Control-Preserved Group (p<0.01). The mean change in body weight was −12.9 (4.2) kg in patients with semaglutide and −2.5 (1.1) kg in control patients (p<0.01).
The heart failure health status and secondary outcomes.
| Outcomes | Sema-Preserved Group(n=203) | Control-Preserved Group(n=203) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Heart failure health status (n, %): ≥5 point difference in the KCCQ change | 123 (60.6) | 36 (17.7) | 3.99 (1.69–6.28) | <0.01 |
| HF events (n, %) | 48 (23.6) | 82 (40.4) | 0.81 (0.61–0.98) | <0.01 |
| Emergency visits for HF decompensation | 41 (20.2) | 61 (30.0) | 0.83 (0.62–0.99) | 0.026 |
| HF hospitalization | 25 (12.3) | 54 (26.6) | 0.80 (0.60–0.98) | <0.01 |
| Unplanned outpatient visits | 33 (16.3) | 76 (37.4) | 0.84 (0.64–0.99) | 0.033 |
| Cardiovascular death (n, %) | 16 (7.9) | 21 (10.3) | 0.98 (0.83–1.15) | 0.097 |
| All-cause death (n, %) | 28 (13.8) | 34 (16.7) | 0.99 (0.81–1.21) | 0.101 |
| All-cause hospitalizations (n, %) | 35 (17.2) | 60 (29.6) | 0.86 (0.69–0.99) | 0.041 |
| New or worsening nephropathy (n, %) | 6 (3.0) | 16 (7.9) | 0.90 (0.71–1.08) | 0.088 |
Data are shown as absolute values and percentages. In order to evaluate the association between treatment and study outcomes, mixed effect logistic regressions were used. The regression analysis values were expressed as odds ratio and 95% confidence interval. Values were considered to be statistically significant if p<0.05.
95% CI: 95% confidence interval; KCCQ: Kansas City Cardiomyopathy Questionnaire; OR: odds ratio.
Furthermore, there were significant declines in the HF events (OR: 0.81; 95% CI: 0.61–0.98; p<0.01) and in their individual components: emergency department visits for HF decompensation (OR: 0.83; 95% CI: 0.62–0.99; p=0.026), HF hospitalizations (OR: 0.80; 95% CI: 0.60–0.98; p<0.01), and unplanned outpatient visits (OR: 0.84; 95% CI: 0.64–0.99; p=0.033). There were also significant reductions in all-cause hospitalizations (OR: 0.86; 95% CI: 0.69–0.99; p=0.041). No significant reductions were observed for the cardiovascular death, the all-cause death, and the new or worsening nephropathy. HF health status and secondary outcomes results are shown in Table 2.
Patients in the Sema-Preserved Group had a bigger reduction in HbA1c (1.0 (0.3) vs 0.3 (0.1)%, p<0.01) than those patients without GLP-1ra. There were negative correlations between the KCCQ total symptom score and the body weight (r=−0.650, p<0.01) and HbA1c (r=−0.586, p<0.01).
With regards to safety, fewer serious adverse events occurred among patients who received semaglutide (24.1%), with all of them being gastrointestinal, and 19 patients (9.4%) discontinued semaglutide.
DiscussionThis study found that patients with HFpEF, obesity and T2D treated with once-weekly 1.00mg semaglutide were more likely to improve the HF health status (3.99-fold) and reduce body weight (mean difference: −10.4kg) than patients without GLP-1ra. There were also reductions in the HF events, including emergency department visits for HF decompensation, HF hospitalizations, unplanned outpatient visits; and all-cause hospitalizations. The tolerability and safety profiles were good, only showing fewer gastrointestinal adverse events.
In recent years, SGLT-2i have shown enormous cardiovascular, renal, and HF benefits in patients with T2D at high risk for cardiovascular disease or established cardiovascular disease, with consistent reductions in hospitalizations for HF.6,7,20 These benefits were extended to patients with HF with reduced LVEF regardless of the presence of T2D after the results from the pivotal clinical trials with dapagliflozin (DAPA-HF trial) and empagliflozin (EMPEROR-Reduced trial).8,9 However, no treatment has been shown to convincingly reduce mortality and morbidity in patients with preserved LVEF until the recent results from two large SGLT-2i trials using dapagliflozin (DELIVER trial) and empagliflozin (EMPEROR-Preserved trial),10,11 showing for first time consistent cardiovascular/renal benefits in patients with HFpEF regardless of the presence of T2D. On the other hand, GLP-1ra have also demonstrated significant cardiovascular and renal benefits in patients with T2D,7,20 but they have not been demonstrated to clearly reduce hospitalizations for HF in their pivotal cardiovascular clinical trials.7,12 Nevertheless, GLP-1ra were indeed found to significantly reduce HF hospitalizations in a meta-analysis which included patients from all GLP-1ra’ trials, although this reduction was modest (0.91; 0.83–0.99; p=0.028).12 Recently, the use of efpeglenatide in placebo-controlled trial conducted in patients with T2D and either a history of cardiovascular disease or current kidney disease showed a significant reduction in HF hospitalizations (39%).21 To date, only the STEP-HFpEF DM and STEP-HFpEF Trials have specifically evaluated the impact of once-weekly 2.4mg semaglutide on HF outcomes in patients with HFpEF. Semaglutide showed reductions in symptoms and physical limitations and improvements in exercise function in patients with HFpEF and obesity with and without T2D.13,14
Furthermore, the evidence from observational studies is quite limited. In a systematic review including several observational studies, GLP-1ra were neutral for HF outcomes in some studies and beneficial in terms of reductions in HF hospitalization rates, in other studies.15
The HFpEF is a complex clinical entity that includes multisystem abnormalities, such as heart, pulmonary, vascular, kidney, systemic metabolic, hepatic, and skeletal muscle.2,3 Due to this complexity, the evidence of effective treatment has been limited. Currently, HFpEF fist-line therapy includes SGLT-2i, exercise, loop diuretics to maintain euvolemia, and weight loss for obese patients.22 Obesity has been described as one of the clinical phenotypes in HFpEF and it is recognized as a risk factor for new onset of HFpEF.23 Up to 75% of patients with HFpEF have obesity and it constitutes a major determinant of arterial stiffness, hypertension, and left ventricular hypertrophy with increased risk of diastolic left ventricular dysfunction.3 Additionally, the presence of obesity associates higher prevalence of obstructive sleep apnea syndrome (up to 4-fold), contributing to the pathogenesis of HFpEF.4 Several factors such as activation of the renin–angiotensin–aldosterone and sympathetic nervous systems, the inflammatory adipocitokines, and the presence of oxidative stress would lead to heart interstitial fibrosis, increasing the heart rigidity and the need for energy required for left ventricular diastolic relaxation.2–4 The GLP-1ra, additionally to the significant cardiovascular/renal benefits showed in patients with T2D,6,7 induce long-term body weight loss, are highly efficacious for glycemic control, and reduce insulin resistance.6 These facts in combination with the direct effects of GLP-1ra on endothelial function reducing pro-inflammatory mediators, their role in cardiac tissue ameliorating insulin resistance with the up-regulation of GLP-1 isoforms,24,25 and the reduction in hyperfiltration and increase in natriuresis modulating water and sodium homeostasis and reducing the activation markers of the renin–angiotensin system would contribute to the improvement of patients with T2D, obesity, and HFpEF.26 The epicardial adipose tissue may also have a role in the beneficial mechanism of GLP-1ra in patients with HF due to its involvement in adipokine production and energy homeostasis in the heart.27 In our study, the use of semaglutide was associated with 3.99-fold greater improvement in HF health status, and reductions in the HF events, and all-cause hospitalizations. In addition, semaglutide improved glycemic control and reduced body weight, correlating with the KCCQ total symptom scored. These benefits show the potential role of GLP-1ra in reducing adverse outcomes in patients with HFpEF. The implementation of structured treatment programs including the use of GLP-1ra in combination with improvements in quality of diet and exercise to achieve long-term body weight loss and an increase in lean mass could be established as an important goal in the management of patients with T2D, obesity, and HFpEF. All these potential benefits may also even be extrapolated to overweight/obese patients with HFpEF without T2D.28–30
The findings of the present study are important because this is the first study to provide valuable information on the role of once-weekly 1.00mg semaglutide on HF heart status and adverse outcomes in patients with HFpEF, obesity and T2D. However, these results should be considered within the context of several potential limitations. First of all, despite the PSM analysis and the similar baseline characteristics between treatment groups and the fact that the mixed effect logistic regressions were adjusted for confounding variables, due to the observational nature of our data, the possible effects of unmeasured confounding factors cannot be ruled out. The only difference in baseline characteristics was regarding the higher percentage of control patients with HbA1c <7% before PSM. The main reason for this difference is that semaglutide is only funded by Spain's national health system if patients have a HbA1c >7% with at least 1 glucose-lowering drug for 3 months, and a body mass index ≥30kg/m2. If patients do not meet these criteria, they have to cover the cost. Secondly, although there was a sufficient number of primary outcome events, the number of some secondary outcome events could be considered as low, so their relationship to the use of semaglutide could not be conclusively determined. Thirdly, as the medication for HF could be modified if deemed advisable according to healthcare providers’ judgment, the entirety of our findings cannot be strictly attributed to starting once-weekly semaglutide. In addition, these results could have also been influenced by the general recommendations about a healthy diet and physical activity suitable to the patient's functional class given during the follow-up. Finally, the only GLP-1ra evaluated was once-weekly semaglutide. The reasons underlying this choice are that a significant percentage of patients are treated with once-weekly semaglutide in our area and we wanted to evaluate its efficacy in isolation, without the interference of other GLP-1ra. Therefore, our findings cannot be extrapolated to other GLP-1ra.
ConclusionsOnce-weekly 1.00mg semaglutide was associated with an improvement in the HF health status and body weight reduction in patients with HFpEF, obesity, and T2D. There were also reductions in the HF events (emergency department visits for HF decompensation, HF hospitalizations, unplanned outpatient visits), and all-cause hospitalizations. The tolerability and safety profile of semaglutide were good. Further research on GLP-1ra in HFpEF is needed and results from randomized clinical trials are required to provide more evidence on their efficacy and safety.
CRediT authorship contribution statementThe authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Miguel A. Pérez-Velasco: analysis and interpretation of data and preparation of manuscript. María-Rosa Bernal-López: analysis and interpretation of data and preparation of manuscript. Alicia Trenas: analysis and interpretation of data and preparation of manuscript. Michele Ricci: acquisition of subjects and data, analysis and interpretation of data and preparation of manuscript. María-Dolores López-Carmona: analysis and interpretation of data and preparation of manuscript. María D. García de Lucas: analysis and interpretation of data and preparation of manuscript. Ricardo Gómez-Huelgas: concept and design, analysis and interpretation of data and preparation of manuscript. Luis M. Pérez-Belmonte: concept and design, acquisition of subjects and data, analysis and interpretation of data and preparation of manuscript. All authors have participated to drafting the manuscript, read and approved the final version of the manuscript.
Ethics approval and consent to participateThe study was approved by the Institutional Research Ethics Committee of Málaga (Code: REDIME-2019) and written informed consent for the consultation of medical records was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki. Data confidentiality and patient anonymity were maintained at all times.
FundingThis research was funded by grants from Internal Medicine Department, cofounded by the Fondo Europeo de Desarrollo Regional-FEDER (“Centros de Investigación En Red” (CIBER, CB06/03/0018)). The authors have not received any fees related to the development of the manuscript.
Conflict of interestThe authors have no conflicts.