Artificial Intelligence (AI) has become one of the most important and impactful technological tools in different aspects of health, including clinical performance and medical or surgical treatment, with the subsequent increase in patient quality of life.1 It is software comprising complex algorithms designed to learn from a vast amount of data and also, at the same time, be updated automatically. Its purpose is to help physicians to interpret images, improve workflow and reduce medical errors.2
Machine learning (ML) is a computer learning system which, following training to perform a specific task, focuses on the capacity to infer and learn from these computing algorithms in order to make predictions from a new dataset. It has the capacity to automatically learn and improve from each experience and without explicit programming.1,3
Deep learning is an advanced and complex form of ML structured with different levels of specific algorithms known as convolutional neural networks (CNN/ConvNet) that make a powerful prediction with the data provided.4 These networks can learn the characteristics of images based on accumulated images which, when processed automatically and swiftly, can be particularly valuable in clinical medicine for medical image analysis, as well as in imaging-based diagnosis.3,5,6
Endoscopic applications of AI in GastroenterologyOesophagogastroduodenoscopy, together with colonoscopy, are the most commonly-performed procedures by gastroenterologists and are extremely operator-dependent.7 This means that a high-quality endoscopy will depend on certain variables, such as the time taken to complete the procedure, as well as the endoscopist's training and technique for recognising certain conditions. These variables in endoscopic practice are likely to hinder the discovery of disease.3,7 In recent years, an extensive variety of applications have been proposed and developed for AI algorithms in gastrointestinal endoscopy in order to help guarantee high-quality procedures.
The two areas of endoscopy in which AI has been most extensively studied and developed are: computer-aided detection (CADe) and computer-aided diagnosis (CADx). The former is used to develop algorithms to detect conditions, whereas in the latter the algorithms are intended mainly to classify conditions correctly by means of optical biopsy and lesion characterisation.3
The use of CADx has attracted a great deal of attention on account of its usefulness in colonoscopy. It has been shown to facilitate the histological classification of colonic polyps without the need for biopsies. The idea is to perform an optical biopsy based on the amount of surface microstructures that reflect a lesion's histological characteristics. This procedure helps the endoscopist to "resect and reject" polyps in each individual case, without the need to take a sample and perform a histological analysis. Table 1 describes examples of available AI models.8
Examples of artificial intelligence systems.
| Artificial intelligence system | Company | Type | Function |
|---|---|---|---|
| ENDO-AID | Olympus | CADe | Detection of possible lesions such as colonic polyps, malignant neoplasms and adenomas |
| The GI Genius™ | Medtronic | CADe | |
| Wision AI | Shanghai Wision AI Co. | CADe | |
| Discovery | Pentax Medical | CADe | |
| ME-APDS™ | Magentiq Eye LTD | CADe | |
| Ultivision | Docbot, Inc. | CADe | |
| EndoBRAIN-EYE | Cybernet | CADe | |
| EndoAngel | Wuhan EndoAngel Medical Technology Company | CADe | |
| CAD EYE | Fujifilm | CADe-CADx | Real-time detection and diagnosis of the histology of gastrointestinal lesions |
| CADDIE | OdinVision | CADe-CADx |
Oesophageal adenocarcinoma is normally diagnosed at an advanced stage, when the prognosis is already poor. For this reason, appropriate monitoring of Barrett's oesophagus and the eradication of early associated dysplastic and neoplastic lesions are the key to preventing the transformation to adenocarcinoma, since minimally invasive treatments with a high healing rate are currently available.9
Screening currently consists of direct observation by endoscopy together with guided or random biopsies. Dysplasia in Barrett's oesophagus may be difficult to identify, resulting in a lower sensitivity of biopsy samples, despite standardised protocols. As such, it is regarded as relatively inefficient as it is very time-consuming and delivers a low rate of correct diagnoses.
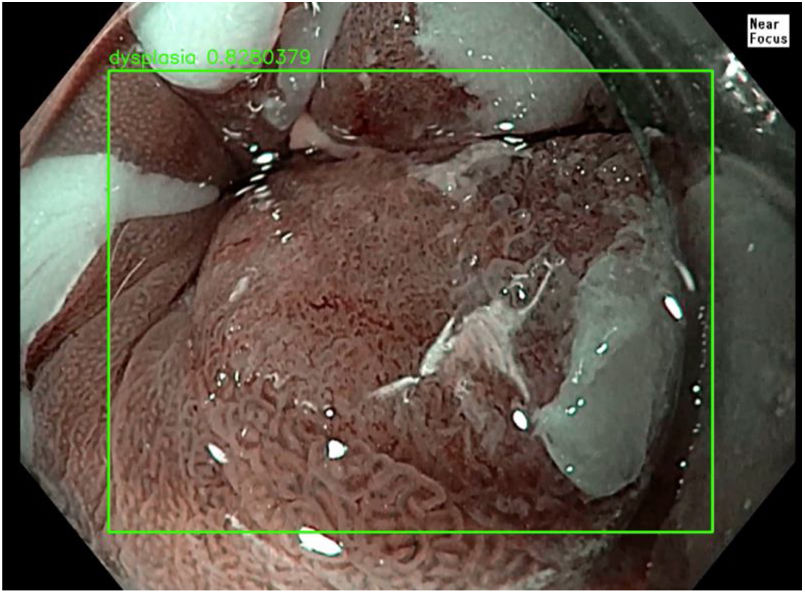
The role of AI in evaluating Barrett's oesophagus focuses on improving oesophageal adenocarcinoma screening. The joint use of AI and advanced imaging techniques, such as volumetric laser endomicroscopy (VLE), white light endoscopy and confocal laser endomicroscopy, have delivered high-performance metrics compared to expert endoscopists, thus improving the procedure's sensitivity and speed. This helps endoscopists to perform targeted biopsies with greater precision, and eliminates the need to perform random biopsies, which have a relatively low sensitivity of around 64% for the detection of dysplasia.3,10 AI has been proven to have sensitivities greater than 90% and specificities above 80% in the early diagnosis of oesophageal adenocarcinoma, with a very subtle endoscopic appearance.3 AI systems are capable of detecting pre-cancerous lesions and early forms of oesophageal squamous cell carcinoma, including those smaller than 10 mm, with a sensitivity of 98.04% and a specificity of 95.03%.3,11Fig. 1 shows severe dysplasia in a segment of Barrett's oesophagus.
Gastric cancerGastric cancer is primarily detected by upper gastrointestinal endoscopy; a precise prediction based on endoscopic images is important in order to create a better treatment strategy for the patient.
For this reason, it is essential to determine the depth of invasion in order to establish the best treatment strategy; however, the general precision of conventional endoscopy is insufficient to define invasion (69%–79%).12
At this moment in time, AI systems have proven to be useful in diagnosing gastric cancer with great precision, detecting blind points and lesions of less than 5 mm; in this way, it distinguishes between malignant and non-cancerous areas in the stomach, thereby heralding a substantial improvement in gastric cancer screening quality. Similarly, AI is useful in assessing the depth of invasion of gastric cancer, distinguishing between lesions that invade more than 500 μm of the submucosa and more superficial lesions.3,12,13
In a recent study performed by Mori et al.,14 which evaluated 790 images from different patients with gastric cancer, AI had a sensitivity of 76% and a specificity of 96% in the identification of deeper cancers compared to visual inspection performed by endoscopists. Similar results were reported in parallel in the study by Zhu et al.12
Identification of Helicobacter pylori infectionHelicobacter pylori has been proven to be associated with gastric cancer by inducing atrophy of the gastric mucosa, as well as intestinal metaplasia. Gastroscopy is useful for improving the diagnostic precision of H. pylori gastritis, although it has a sensitivity of 62% and a specificity of 89%.15 AI is useful to support decision-making related to the diagnosis of H. pylori. Shichijo et al.16 developed a 22-layer-deep CNN algorithm to predict H. pylori during gastroscopy and compared its diagnostic accuracy to that of endoscopists. The CNN's sensitivity, specificity and accuracy were 81.9%, 83.4% and 83.1%, respectively. Similarly, Nakashima et al.10 created an AI system to diagnose H. pylori using blue laser imaging-bright and linked colour imaging, finding sensitivity figures of 96.7% and 96.7%, respectively, for this model, sufficient for introduction into clinical practice.
Capsule endoscopyThe capsule endoscopy is a technique developed to obtain endoscopic images of the entire small intestine to detect and diagnose different conditions. Interpreting the images obtained constitutes a challenge for most gastroenterologists in that it requires a high level of concentration and dedication.9,11
Artificial intelligence, specifically computer viewing and automatic learning methodologies, uses algorithms focused essentially on the detection of bleeding and lesions, decreased viewing time, the location of the capsule's position in the small intestine and/or improvements in video quality. All these tools help physicians to read and interpret images, thereby improving efficiency and diagnostic accuracy.3,17
Computer-aided diagnostic algorithms help to increase diagnostic accuracy through the classification of anomalies. The characteristics of endoscopically-obtained images can be classified using automatic learning algorithms, such as a support vector machine, a neural network or a binary classifier.17 The most efficacious tool used in computer-aided diagnosis is the identification of bleeding in the small intestine. This AI system uses colour-based feature extraction, using ratios of the intensity values of the images in the red, green and blue, or hue, saturation and intensity domain, to help distinguish bleeding-containing frames from those without bleeding.9
Leenhardt et al.18 reported the use of convolutional neural networks to improve the detection of gastrointestinal angiodysplasia in the small intestine identified with wireless capsule endoscopy. The sensitivity and specificity of the computer-aided diagnostic algorithm were 100% and 96%, respectively, for the detection of these vascular ectasias. Moreover, Tsuboi et al.19, after training a deep convolutional neural network based on 2,237 capsule endoscopy angiodysplasia images, found that they had a sensitivity and specificity of 98.8% and 98.4%, respectively. With regard to its effectiveness in polyp detection, Saito et al.6 trained a deep convolutional neural network: using 30,584 capsule endoscopy images of protuberant lesions from 292 patients as a training image data set. In total, 17,507 images from 93 patients were used to test the CNN. The sensitivity and specificity of the conventional neural networks were 90.7% (95% CI: 90.0%–91.4%) and 79.8% (95% CI: 79.0%–80.6%), respectively. In a subgroup analysis of the category of protuberant lesions, such as polyps, nodules, epithelial tumours, submucosal tumours and venous structures, the sensitivities were 86.5%, 92.0%, 95.8%, 77.0% and 94.4%, respectively.
Although these systems have the potential to be an excellent method of detection, identifying areas of bleeding and/or vascular and neoplastic lesions, there is still room for improvement and further studies are called for to achieve better diagnostic performance.
Detection of colorectal polypsColorectal cancer is one of the leading causes of death worldwide. Colonoscopy is the technique of choice for preventing colorectal cancer, the incidence of which can be reduced by adenoma detection and resection. However, the lesion detection rate varies between endoscopists, with a percentage loss of up to 27% due to factors related to the characteristics of the polyp and the operator.8 This reduction in lesion detection increases the likelihood that the lesion will subsequently progress to colorectal cancer. For every 1.0% increase in the adenoma detection rate, there is an associated 3.0% reduction in the risk of interval colorectal cancer.1,9
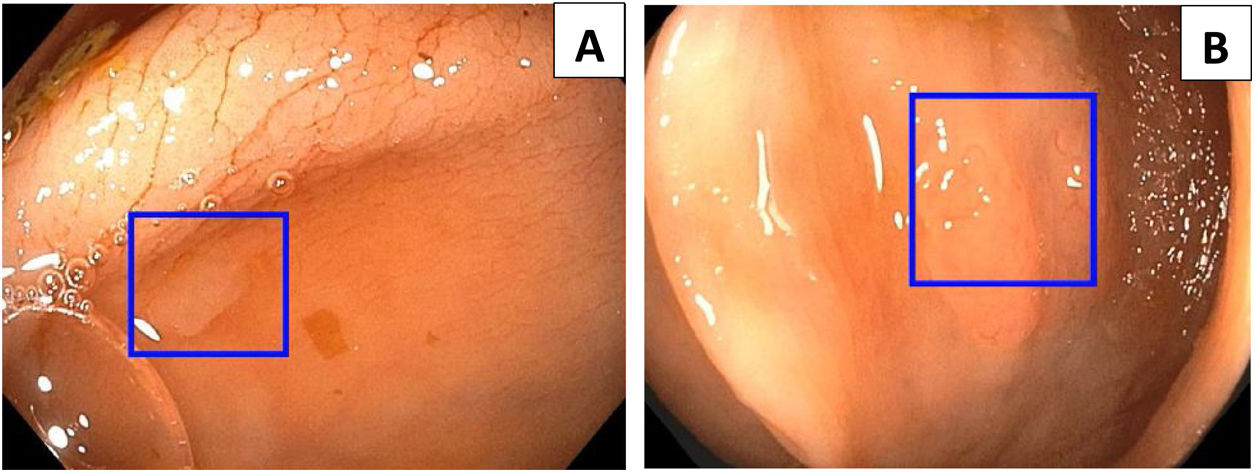
AI and automatic learning systems, particularly in the domain of deep learning, have prompted the development of computer-aided detection programs to help endoscopists detect polyps and adenomas during colonoscopy, essentially focusing on the detection of flat or small lesions.20 The CADe system used in Fig. 2 is Wision AI, which helps the operator to locate polyps that are difficult to detect due to their size or location.
Endoscopic images captured by the automatic polyp detection system (CADe), which shows colonic polyps marked on the screen by a blue square denoting their location. (A) 3-mm polyp, IIA according to the Paris classification. (B) 5-mm polyp, IS according to the Paris classification. Images courtesy of Dr Tyler Berzin, Co-director of the Advanced Endoscopy Department at the Beth Israel Deaconess Medical Center.
The meta-analysis of prospective trials published by Barua et al.20 showed that colonoscopy with AI increased the adenoma detection rate (rate of 29.6% [95% CI: 22.2–37.0]) and polyp detection rate compared to colonoscopy without AI (rate of 19.3% [95% CI: 12.7–25.9]). Another study conducted by Wang et al.17 found that the automatic real-time polyp detection system significantly increases the adenoma detection rate (29.1% versus 20.3%, p < 0.001). Min et al. created a CADx system to predict adenomatous polyps compared to the histology of non-adenomatous polyps using colour images. The system achieved a sensitivity of 83.3%, a specificity of 70.1% and a precision of 78.4%,11 demonstrating that the CADe system can be combined with a CADx system to support the detection and diagnosis strategy of hyperplastic polyps that do not require polypectomies, to thereby improve the workflow and workload of endoscopists and pathologists.
Endoscopic ultrasoundEndoscopic ultrasound (EU) has been established as an important tool for the diagnosis and treatment of gastrointestinal diseases, although it has certain limitations, such as image interpretation.21 The processing and analysis of EU images using AI-related CAD (AI-CAD) can overcome these limitations.22
EU-based CNN data are still limited, although some studies have reported positive results with its use. The study performed by Chang et al., in which they developed a EU-CNN for discriminating gastric subepithelial tumours in EU images, distinguishing between GIST and leiomyomas, obtained an AUC per image of 0.9234, with a corresponding sensitivity of 95.6% and a specificity of 82.1%, and an AUC per patient of 0.9929, with a corresponding sensitivity of 100.0% and specificity of 85.7%.23 These findings could be due to EU-CNN's capacity to analyse images at pixel level, which is difficult for humans to accomplish. In another study, Minoda et al.22 obtained similar findings, concluding that EU-AI delivers high-performance in the prediction of GIST and good prediction for the diagnosis of gastric subepithelial tumours.23 Research is still ongoing into other uses of AI in EU, such as EU elastography, EU with contrast and EU-guided fine-needle aspiration. While the results seem to be positive for evaluating both benign and malignant conditions, further studies are still required.21
Costs to health systemsAI has been implemented effectively in different endoscopic techniques. Although it is expected to be accompanied by a reduction in costs, at this moment in time the evidence is scant. A single study in the literature, published by Mori et al.,24 addresses this topic. The authors calculated the costs of colonoscopy applying an AI-aided diagnosis strategy and not resecting small polyps defined as non-neoplastic compared to the approach of resecting all polyps identified. In 207 patients with 250 small polyps located in the rectum and sigmoid colon, procedure costs fell by 18.9%, 6.9%, 7.6% and 10.9% in Japan, England, Norway and the United States, respectively, using the first strategy compared to the second type of approach.
ConclusionAI algorithms initially emerged for the purpose of limiting intraoperative variability, preventing human error and reducing diagnostic failures. As such, they lead to increased productivity, capacity and diagnostic quality, and facilitate a more efficient way of working that has a positive impact on patient care. In the field of gastrointestinal endoscopy, AI has progressed significantly in recent years, particularly in terms of its potential impact in improving various aspects of endoscopy quality.
The future of AI is promising, with multiple studies showing that it can improve the detection rate of numerous conditions, such as the identification of polypoid lesions, the detection of gastrointestinal cancers and small intestine bleeding areas, and even the endoscopic identification of H. pylori.
Despite all these breakthroughs, there are still challenges in terms of its application in clinical practice. High-quality prospective clinical trials are required to evaluate the true clinical impact and the impact on costs for healthcare systems. Moreover, many more endoscopic images may potentially be required for the database. This will ensure continuous improvement of the models through regular updates and lead to reliable performance in the clinical setting. We believe that this new technology for endoscopy could be implemented on a large scale in clinical practice in the near future.
FundingNon-funded study.
AuthorsAll the authors contributed equally to this article.
Conflicts of interestKenneth Ernest-Suárez is a consultant for Janssen, Pfizer, Astra Zeneca, Ferring and Sandoz.
Tyler M. Berzin has acted as a consultant for Wision AI, Magentiq Eye, Docbot, Endovigilant and Medtronic.
The remaining authors have no conflicts of interest to declare.
Please cite this article as: Aguilera-Chuchuca MJ, Sánchez-Luna SA, González Suárez B, Ernest-Suárez K, Gelrud A, Berzin TM, El papel emergente de la inteligencia artificial en la endoscopia gastrointestinal: una revisión de la literatura, Gastroenterología y Hepatología. 2022;96:492–497.