Dientamoeba (D.) fragilis is a common intestinal protozoan with an unresolved clinical significance. The association between D. fragilis and the etiology of gastrointestinal symptoms in children is unclear. Metronidazole is often used for treatment. The aims of this study are to clarify the clinical relevance of D. fragilis in children with gastrointestinal symptoms, and to determine the clinical and microbiological efficacy of metronidazole in D. fragilis-infected children with gastrointestinal complaints.
MethodsA prospective case–control study was performed from October 2017 to February 2019. A total of 106 individuals aged 1–17 were included. Out of the 106; 59 showed gastrointestinal symptoms (case group), and 47 were without gastrointestinal symptoms (control group). We excluded 2 patients from the case group. D. fragilis was diagnosed by real-time PCR in stool samples. A 10-day course of oral Metronidazole was prescribed in D. fragilis positive children with GI symptoms. Clinical data before and after the treatment as well as peripheral eosinophilia in previous blood samples, were recorded.
ResultsOf the 104 participants, D. fragilis was found in 17 (29.8%) children from the case group, whereas in the control group the parasite was detected in 11 patients (23.4%) with an odds ratio (OR) of 1.39 (IC 95% 0.53–3.75, p=0.46). The most prevalent clinical manifestation was abdominal pain (46/57, 80.7%). Seventeen cases with a positive PCR received anti-parasitic treatment according to the established protocol, although during the collection period we received only 11 stool samples to perform the post-treatment follow-up. The PCR of the D. fragilis remained positive in 3 patients (3/11, 27.27%). Despite achieving the eradication of the parasite, 4/8 patients (50%) continued with digestive symptoms.
ConclusionsAccording to our study there were no differences between the D. fragilis infection in children with or without gastrointestinal symptoms. No relation was found between the clinical and microbiological responses after said D. fragilis treatment. Therefore, we conclude that it is not justified to look specifically for D fragilis in pediatric patients with abdominal symptoms.
Dientamoeba (D.)fragilis es un protozoo intestinal muy común pero con una relevancia clínica incierta. El papel etiopatogénico de la D. fragilis en sintomatología gastro-intestinal no está claramente establecido. El metronidazol es con frecuencia el fármaco de elección. El objectivo de nuestro estudio fue determinar la relación entre D. fragilis y la presencia de clínica digestiva en población pediátrica así como valorar la eficacia del metronidazol en la resolución de los síntomas.
Material y métodosEstudio prospectivo caso-control realizado entre octubre 2017 y febrero 2019. Se incluyeron un total de 106 pacientes entre 1-17 años. 59 pacientes presentaban síntomas digestivos (grupo caso) y 47 pacientes asintomáticos (grupo control). Se excluyeron 2 pacientes del grupo caso. La presencia de D. fragilis se determinó por técnica de PCR real-time(PCR-RT) en muestra de heces. En los pacientes del grupo caso con PCR-RT positiva se realizó tratamiento con metronidazol durante 10 dias. Se recogieron datos clínicos antes y después del tratamiento asi como presencia de eosinofilia periférica.
ResultadosDe los 104 participantes, se detectó D. fragilis en el 17/59 (29,8%) en el grupo caso y en 11/47 pacientes del grupo control (23,4%) con una odds ratio (OR) de 1.39 (IC95% 0.53-3.75, p=0.46). El síntoma más frecuente fue el dolor abdominal (46/57, 80,7%). Los 17 pacientes del grupo caso recibieron metronidazol según protocolo y se realizó PCR-TR D. fragilis post-tratamiento en 11/17 pacientes. La PCR-RT persistió positiva en 3 pacientes (3/11, 27,27%). De los 8 pacientes que negativizaron PCR-RT, 4 (50%) continuaron con dolor abdominal.
ConclusiónSegún nuestro estudio no se encontró relación significativa entre la presencia de infección por D.fragillis y la existencia de sintomatología digestiva. Tampoco se observó mejoría clínica significativa en aquellos pacientes infectados que recibieron tratamiento.
Dientamoeba fragilis is a protozoan reported worldwide as a common microorganism present in the human gastrointestinal tract, with an estimated prevalence in Spain of around 17.7%,1 which is equal or exceeds the incidence of giardiasis.2 It remains controversial whether D. fragilis is a commensal parasite or a pathogen. Several studies were published to describe its role, mainly in the adult population,3 but so far, there are few pediatric studies designed with a specific control group.4
The complete life cycle of D. fragilis has not yet been elucidated. It is suggested that the cyst stage is the vehicle that mediates fecal-oral transmission of D. fragilis between hosts.5,6 Although it is unknown how trophozoites can remain outside the body, some authors postulate that they can survive within the eggs of helminths such as Enterobius vermicularis, but, on the other hand, other studies have not shown the correlation between infections caused by D. fragilis and E. vermicularis.7 The classic method to diagnose the parasite is by optic microscopy (permanent stains or a microscopic wet mount examination), but this can be hard to identify due to its morphological similarity to other protozoa. Additionally, the method requires rapid fixation of the stool samples, and even specific stains such as trichrome or another permanent stains which are not routinely used in clinical microbiology laboratories. In any case, highly trained technologist is also required for a correct diagnosis of D. fragilis infection. Another difficulty is the phasic secretion of the parasite and the intermittent elimination of D. fragilis on different days, as in other intestinal parasites, with the risk that in the absence of D. fragilis, a single stool sample conducted a false negative result. To avoid this problem, it is recommended to send 3 samples taken on different days to investigate its presence.8 Introduction of the Polymerase Chain Reaction (PCR) as a diagnostic technique, with a higher sensitivity than a microscopic examination,9–12 makes the diagnosis easier and it has made its prevalence increase in recent years. Another advantage of the PCR is the possibility of using a single sample instead of three.8
Although its real prevalence is not known, the published data presents a high variability with figures ranging between 1% and 70% according to the studies (accurately in developed countries the incidence has been described between 1 and 20%).1,8 The highest incidence rates have been described in patients under 15 years old and in adults with close contact with infected children. The variation in prevalence may be due to the different study designs and specially, the type of diagnostic technique applied. The use of molecular biology techniques would probably increase the prevalence.8,10,13,14 Results based on PCR analysis should be regarded with caution because of the variability from different platforms and the potential for low specificity. These data could be false positive results so there is a need for a standardization of detection assays across all nations screening for D. fragilis.20
The most frequent symptoms described in individuals with D. fragilis are abdominal pain (frequently lasting longer than two weeks) and diarrhea. Less frequently reported symptoms included weight loss, anorexia, flatulence, vomiting and anal itching.15
In the last few years an increasing interest toward D. fragilis has emerged. More studies have been published4,13,14 in which symptoms were correlated with the presence of the parasite in the gastrointestinal tract (the colon). But on the other hand, other recent studies question the role of this parasite13,16,17 because many individuals with D. fragilis were asymptomatic carriers.15
There is no consensus in clinical guidelines about eradication treatment of D. fragilis. Although there are several treatments options, oral metronidazole in monotherapy seems to be the one with the lowest minimal lethal concentration in the moment of treatment.8 Another treatment options are paromomycin, an intraluminal antiparasitic and clioquinol.15 The aim of this study was to assess the prevalence and, to understand the role of D. fragilis in children with gastrointestinal symptoms excluding other causes (infectious or otherwise like celiac disease) in our settings. We designed a pilot case control study to compare the presence of the parasite in children who did and didn’t refer gastrointestinal symptoms.
Materials and methodsPatient populationThis is a prospective case–control study performed at the Hospital Germans Trias i Pujol in Badalona (HGTiP), Spain, between October 2017 and February 2019. The chosen case group comprises of individuals aged between 1 and 17 that attended the hospital or the associated primary care centers from when they have had any kind of gastrointestinal (GI) disorder (abdominal pain, diarrhea, meteorism, nausea, weight loss, abdominal bloating and/or hyporexia) when other organic/infectious causes have been excluded. The existence of a previously diagnosed digestive pathology or a systemic disease, with a possible involvement of the gastrointestinal tract, is considered as an exclusion criterion. The control group was formed by healthy children aged between 1 and 17 years old recruited at the HGTiP or the associated primary care centers for any other reason apart from a GI disorder.
Microbiological analysisWe collected one fresh stool sample for each participant (57 samples of the case group and 47 samples of the control group). Subsequently for the Ova and Parasite (O&P) wet mount examination, samples were fixed in a formalin-free 60 fixative, AlcorFix®, and Mini Parasep® SF (solvent free) collection tube (Parasep®; Apacor; Berkshire, England, UK) for every individual from both the case and the control groups that were treated as routine samples according to the protocol in our settings. Stool cultures for enteropathogens were performed using standard microbiological techniques to exclude the following infections: Salmonella spp., Shigella spp., Campylobacter spp., Arcobacter spp., Yersinia enterocolitica, Vibrio spp., Plesiomonas spp. and Aeromonas spp. Virology testing was performed by an Immunochromatographic screening test (Adeno/Rota STAT-PAK; Chembio Diagnostic Systems Inc., Sydney) for detection of Adenovirus serotypes 40–41 and Rotavirus antigens in stool samples, according to the manufacturer's recommendations.
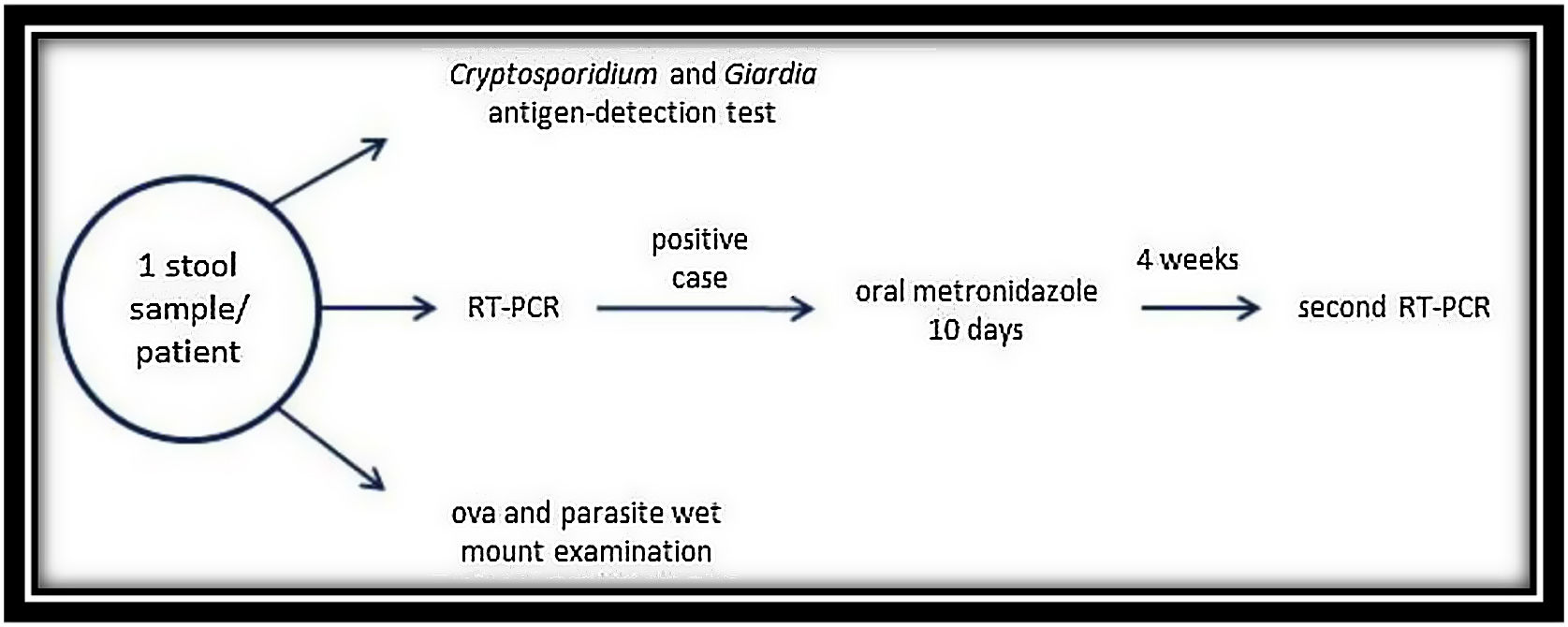
This parasitological study (Fig. 1) consisted in a Cryptosporidium spp. and Giardialamblia antigen-detection test (RIDA®QUICK Cryptosporidium/Giardia Combi, Darmstadt, Germany) followed by an O&P wet mount examination. For the antigen detection test, not-fixed stool sample was used but for the O&P examination, we used fixed samples. Furthermore, we studied the presence of D. fragilis in all the samples by RT-PCR (VIASURE Dientamoebafragilis Real Time PCR Detection Kit, Zaragoza, Spain) following the manufacturer's instructions. DNA extraction was performed by using the Maxwell® 16 Tissue DNA Kit (Promega Corporation, Madison, WI, USA) with the fixed samples used in the O&P wet mount examination. Among the case group, individuals with a positive result for D. fragilis (PCR and/or wet mount microscopy) were treated with oral metronidazole (15/mg/kg/day) for 10 days. A second PCR was performed 4 weeks after the end of the treatment to verify the eradication of the parasite in stools, and the evolution of the symptoms was also recorded (Fig. 1).
Data collectionClinical data was obtained from medical records in the pediatric facilities. We collected the following data on abdominal pain, diarrhea, meteorism, nausea, loss of weight, hyporexia and eosinophilia. Eosinophilia was defined as ≥0.5×109/L in peripheral blood. Once a patient received anti-parasitic treatment according to the protocol, information about the new clinical status was also compiled. For all individuals, epidemiological data such as age and gender was registered.
Statistical analysisData was recorded and a univariate non-stratified analysis for the pre-treatment and post-treatment groups was performed to study the Odds Ratio (OR) among the variables, as well as the result of the PCR. Additionally, a univariate and multivariate analysis to assess risk and confusion factors was performed by using the Stata 14 (StataCorp LP, TX, USA) statistical package.
Ethical approvalThis study was approved by the Ethics committee of our hospital (PI-17-166). Parents of the children were informed about the study and written consents were obtained, and when appropriate, consent from the child was also retrieved.
ResultsDuring the period of study, a total of 106 individuals were enrolled. Out of them, 59 children were included in the case group but 2 were excluded because they were diagnosed with Campylobacter spp. and Salmonella spp. infections. Ultimately 57 children met the inclusion criteria for the case group. The control group was composed of 47 participants. There were no significant differences between gender and age in both groups. The average age of the cases was 7.45 years old and 6.23 for the control group. Out of the 57 participants of the case group 27 were females and 30 were males (47.36% and 52.64% respectively).
Among children with symptomatology, D. fragilis was found in 17 patients (29.8%), whereas in the control group the parasite was detected in 11 patients (23.4%). The effect of the parasite on the clinical features is positive with an odds ratio (OR) of 1.39. Despite this positive association, there is still a lack of statistical significance (IC 95% 0.53–3.75, p=0.46). Light microscopy did not allow detecting the presence of D. fragilis in any of the cases.
The most prevalent clinical manifestation was abdominal pain (in 46 cases, 80.7%), followed by diarrhea (28, 49.1%), meteorism (15, 26.3%), abdominal distension (12, 21.1%), hyporexia (5, 8.8%) and weight loss (2, 3.5%). None of these symptoms were significantly associated with the exposure to the parasite. Nevertheless, the p value for meteorism is slightly greater than the significance (p=0.0967). The p values can be seen in Table 1.
Data obtained in the case group.
| SymptomsN=57 | PCR+ | PCR− | IC (95%) | |
|---|---|---|---|---|
| Abdominal painn=46 (80%) | 15 | 31 | .37–22.9 | (OR 2.17) |
| Diarrhean=28 (49%) | 6 | 22 | .11–1.65 | (OR .44) |
| MeteorismN=15 (31%) | 7 | 8 | .66–11.38 | (OR 2.8) |
| Abdominal distensionN=12 (21%) | 4 | 8 | .22–5.62 | (OR 1.2) |
| AnorexiaN=5 (8%) | 1 | 4 | .01–6.3 | (OR .56) |
| Weight lossN=2 (3.5%) | 0 | 2 | 0–4.6 | (OR 0) |
Seventeen cases with a positive PCR received anti-parasitic treatment according to the established protocol: although during the collection period we only received 11 stool samples to perform the post-treatment PCR. The Univariate non-stratified analysis in post-treatment cases of the clinical symptoms shows five patients (5/11) who continued with gastrointestinal symptoms (4 had abdominal pain and one had meteorism), but only in one did the PCR of D. fragilis remain positive (1/11). Two other samples (2/11) were positive for the parasite, but those children no longer had gastrointestinal complains.
Eosinophilia in peripheral blood was also recorded but only 9 patients met these criteria, so we excluded it in the final analysis. Despite this fact, the persistence of eosinophilia in the post-treatment group does appear to be significantly associated with the persistence of a positive PCR (p=0.02). But there are very few cases (n=6), so this data must be carefully considered.
The persistence of meteorism does appear to be almost significantly associated with the persistence of a positive PCR in the post-treatment group (p=0.09), but there are also very few registered cases.
DiscussionSeveral authors have tried to determine the role of D. fragilis as an etiology of a gastrointestinal disorder, but it seems that this question has been left unanswered. Some authors suggest that it is a real pathogen. Nevertheless, several publications consider this parasite to be a commensal protozoan since it has been recovered from both symptomatic and asymptomatic individuals.18
This fact also explains the epidemiological variations in the incidence of the D. fragilis infection. The wide range of prevalence levels of Dientamoebiasis established by different authors can be explained by the lack of standardization of a D. fragilis diagnosis.19 Direct comparisons between regions can be misleading due to different study designs: fixed or direct sample, diagnosis using molecular techniques or light microscopy, or the type of PCR, smear, and study population, or the number of samples sent to the laboratory, etc. Furthermore, this information is sometimes not detailed, so results cannot be accurately interpreted, and experiments cannot be replicated.20 It seems that children are one of the groups with the highest prevalence of Dientamoebiasis, and for this reason we decided to focus on this demographic. In our study, D. fragilis was found more often amongst children with symptomatology than in the control group. Despite this tendency, results are not statistically significant. Several authors defend the commensal nature of D. fragilis, through different approaches. Detection of the D. fragilis was higher among healthy children and was not associated with an increase in fecal calprotectin concentration, compared with children with chronic abdominal pain and diarrhea in whom other somatic gastrointestinal disorders were excluded.17 Moreover, in other European countries the estimated prevalence was around 68.3% in children attending day care centers, and no statistical association between a history of infant colic or recent – gastrointestinal symptoms and testing positive for D. fragilis, was observed.13,21
On the other hand, we can also find authors who support the pathogen role of D. fragilis. Wit et al. found that D. fragilis infections were more common in control patients (15–75 years old) than in case patients, except for the 0–14-year age group, where the percentage of positive cases was higher in the case group.22 Similarly, Girginkardeşler et al. described in their group of cases, 35 patients (8.8%) aged between 3 and 60 years old that were positive for D. fragilis. Of all the cases, the infection rates were found to be significantly higher in the 8–15-year age group (14.8%).23 This could correlate with our data, but despite this fact, the low number of patients included is a limitation.
In our cohort, abdominal pain (80.7%) and diarrhea (49.1%) are the most frequent symptoms, and this is also in accordance with other authors research.13,23,24 These two symptoms were found to be significantly more frequent in patients with only Dientamoebiasis, when the cases were compared. But our results show a positive effect of the parasite, with no statistical significance, especially for meteorism. We think that this result should be interpreted carefully due to the low number of cases involved.
Regarding an antiparasitical treatment, the case group patient received metronidazole when D. fragilis was detected. Only 17 patients were checked up upon after the first PCR, and only 11 samples were sent to the laboratory to perform a second PCR. Despite the treatment, 45% of the patients continued to have gastrointestinal symptoms and the PCR remained positive in one of them. Two other samples were positive for the parasite, but children no longer had gastrointestinal complains. One explanation may be because the PCR detected the parasite due to its high sensitivity, but it was not viable anymore. Another explanation may be a failure of the treatment. There is evidence of the wide use of metronidazole to treat dientamoebiasis, although treatment failures are also reported, and more frequently so than with other antiparasitical agents such as secnidazol.14,23,25,26 Paromomycin or clioquinol are antibiotics of choice based on their small spectrum of activity, fewer side effects and better eradication rates than metronidazole.15 Metronidazole is widely used as an antiparasitic treatment despite some authors suggesting that its resistance and failures to comply with the whole treatment are reasons for which some patient cases who are on dientamoebiasis do not respond to the metronidazole treatment.20 Our choice of treatment with metronidazole was due to its high availability and its frequent use for other protozoan infections. Further studies with more participants should be performed and other treatments will be evaluated in case the role of D. fragilis is finally established. Eosinophilia in peripheral blood was also one of the parameters that was associated with a D. fragilis infection,27 but our results show that there are not statistically significant outcomes when comparing case and control groups. Little data was collected so we finally excluded it from the analysis. Curiously, it was one of the clinical data that showed a positive association (p=0.02) with the persistence of a positive PCR. This is a very preliminary conclusion, but it could be considered as a marker for a positive outcome for any antiparasitic treatment.
Other clinical data that has shown a positive association, is meteorism, but no sufficient evidence of this was observed. These kinds of symptoms must be carefully analyzed because some of the side effects of metronidazole are gastrointestinal symptoms, so a confusion factor must be excluded before considering it as reliable data with a sound evolution.
We detected several limitations in our study. First of which being the number of patients enrolled is low so the statistical results must be regarded carefully. Secondly, we have the loss in the follow-up of the patients in the case group. Only 17 patients were enrolled in the second part of the study but in only 11 cases could we obtain a stool sample to confirm the eradication of the D. fragilis by the PCR and O&P examination. Even then, the PCR is not the best tool to assess the eradication due to the high sensitivity of the molecular techniques. And finally, there is the chosen treatment. Other authors have already observed that metronidazole provides no clinical benefit for children with chronic GI complaints who have D. fragilis in their stools, and the microbiological effect of metronidazole is only moderate and transient.26
More studies with more extensive samples are needed in order to confirm our results. In addition, further studies should be performed to investigate household transmission of the D. fragilis. As E. vermicularis has been postulated to have a role in the transmission of the parasite, studies of the presence of the pinworm in D. fragilis infected patients and studies assessing the treatment of both parasites, are also needed.
ConclusionTo our knowledge this is the first prospective case–control study focused on a pediatric demographic, with the control group consisting of asymptomatic children. Since healthy children and children with a GI symptomatology present similar frequency of the D. fragilis infection, we consider that D. fragilis could be considered as a commensal parasite of the gastrointestinal tract. When a treatment is proposed our results show that we do recommend avoiding metronidazole because of its high rates of therapeutic failures and the lack of improvement in gastrointestinal symptoms. We conclude that it is not justified to look specifically for D fragilis in pediatric patients with abdominal symptoms. Further cohort studies with bigger sample, double-blind, randomized and placebo-controlled trials are necessary.
Conflict of interestsThe authors declare that they have no conflict of interest.