To assess the utility of the desmopressin (DDAVP) test in the diagnosis and follow-up of a cyclical Cushing's disease (CCS) case.
Material and methodsLaboratory tests included morning and midnight serum cortisol levels, 24h urine free cortisol excretion, midnight salivary cortisol levels, serum cortisol levels after low (1mg) and high (8mg) dexamethasone, plasma ACTH and serum cortisol levels after DDAVP. Magnetic resonance imaging (MRI) was used to assess the presence of a pituitary adenoma. The resected tumor specimen was studied by histological, immunohistochemical and cell biology techniques.
ResultsA patient was referred to our unit with a diagnosis of Cushing's syndrome (CS) for further evaluation and treatment. However, no biochemical evidence of hypercortisolism was observed in the follow-up evaluations. Furthermore, the typical features of CS fluctuated throughout this period. A consistent positive response to the DDAVP stimulation test was observed during the diagnostic work-up, even when overt clinical features of CS were not apparent, raising suspicion for CCS. After two years of follow-up a definitive diagnosis of hypercortisolism was established. An MRI scan revealed a pituitary adenoma, as the source of ACTH production. After transphenoidal surgery, clinical signs of CS resolved and the response to DDAVP became negative. DDAVP induced a significant increase in ACTH levels in cultured pituitary adenoma cells, consistent with the in vivo DDAVP test results.
ConclusionsOur case illustrates the utility of the DDAVP test in the evaluation of patients with suspected CCS. The DDAVP test could facilitate the management of CCS by shortening the time of diagnosis.
Evaluar la utilidad de la prueba de la desmopresina (DDAVP) en el diagnóstico y seguimiento de un caso de enfermedad de Cushing cíclica (ECC).
Material y métodoSe realizaron mediciones de cortisol sérico diurno y nocturno, cortisol libre en orina de 24h, cortisol en saliva nocturno, cortisol sérico tras dosis elevadas y bajas de dexametasona, ACTH plasmática y cortisol tras la DDAVP, y resonancia magnética (RM) para valorar la presencia de un adenoma hipofisario. El tumor extirpado fue analizado mediante técnicas histológicas, inmunohistoquímicas y de biología celular.
ResultadosSe presenta una paciente enviada a nuestra unidad con el diagnóstico de síndrome de Cushing (SC) para evaluación más completa y tratamiento. No obstante durante un periodo de seguimiento de 2 años no se encontró evidencia alguna de hipercortisolismo en los análisis realizados en nuestro laboratorio. Durante este tiempo, la paciente mostró fluctuaciones de los síntomas y signos típicos del SC. De manera interesante, la prueba de DDAVP mostró hiperrespuesta de cortisol y ACTH en todas las evaluaciones. La exploración por RM mostró un adenoma hipofisario que tras extirpación resultó ser positivo para ACTH. El SC se resolvió tras la cirugía y la respuesta a la prueba de DDAVP desapareció en las evaluaciones de seguimiento posquirúrgico. Se incubaron muestras del tumor mostrando este un aumento en la secreción in vitro de ACTH.
ConclusionesEste caso ilustra la utilidad de la prueba de DDAVP en la evaluación de pacientes con sospecha de SCC. Esta prueba podría facilitar el manejo del SCC acortando el tiempo de diagnóstico.
Cyclical Cushing's syndrome (CCS) is a rare form of hypercortisolism characterized by repeated episodes of cortisol excess and normal cortisol secretion. These episodes or cycles of hypercortisolism and the associated clinical signs and symptoms can fluctuate over days, months or even years. Due to this cyclical pattern, hypercortisolism might be difficult to detect in CCS patients through biochemical tests commonly used to assess endogenous hypercortisolism in Cushing's syndrome (CS), such as 24-h urinary free cortisol (UFC) and midnight serum or salivary cortisol.1 In addition, these biochemical tests have intrinsic methodological difficulties and concerns about their precision and accuracy have been recently raised.2 New procedures, such as the measurement of hair cortisol levels, are now becoming available for the diagnosis of CS.3 However, evidence about the utility of this test to assess CCS is still limited. The combination of all these factors makes the diagnosis of CCS a significant challenge for endocrinologists.
The desmopressin (DDAVP) stimulation test has been proposed as a useful diagnostic tool for Cushing's disease (CD). DDAVP, a long-acting vasopressin analog, stimulates ACTH and cortisol production in the majority of patients with CD, but generally not in healthy subjects, pseudo-Cushing's, adrenal and ectopic Cushing's patients.4–7 The positive ACTH response to DDAVP after surgery is associated with increased risk of CD recurrence8,9 and thus, the DDAVP test might also be useful for the follow-up of CD patients. Here we report a case of CCS in which the positive response to the DDAVP test provided a strong suspicion for the presence of Cushing's disease, warranting further evaluation until a firm diagnosis of hypercortisolism was made. The DDAVP test was also useful to confirm the pituitary origin of excess ACTH as well as to monitor the postsurgical follow-up of CCS. In addition, a cellular and molecular characterization of the pituitary adenoma was performed revealing molecular features similar to those found in most human corticotroph tumors.
Material and methodsHormone assaysACTH levels were determined by electrochemiluminescence immunoassays in E170 Modular Analytics (Roche Diagnostic, Manheimn Germany), with intra-assay and inter-assay coefficients of variability (CVs) of 3.1–9.6% and 5.1–9.2%, respectively (for values between 2.3 and 1121pg/mL). The normal range value was 7.2–63.3pg/mL and the lower detection limit was 1pg/ml. Serum cortisol and UFC levels were determined by electrochemiluminescence immunoassays in E170 Modular Analytics (Roche Diagnostic, Mannheim, Germany), with 1.1–1.7% within-run precision and 1.4–2.8% total precision (cortisol levels from 4.69 to 31.40μg/l). The normal range values for serum cortisol and UFC were 137.5–495nmol/L and 137–412nmol/24h, respectively, and the lower detection limit was 0.5nmol/L.
The DDAVP test was performed after overnight fasting by injecting 10μg of desmopressin (Minirin/DDAVP; Ferring S.A.U., Madrid, Spain) as a slow i.v. bolus (t0). An indwelling catheter was inserted in the patient's forearm vein at 08:30 AM. Blood samples were collected 15min before the test (t-15), at t0 and 15, 30, 60, 90 and 120min after the i.v. injection. An ACTH increment of >50% and cortisol >20% above baseline levels (mean of the two baseline values t-15 and t0) was considered positive. Formula for computation area under the curve (AUC) for plasma ACTH and serum cortisol responses to DDAVP has been previously described.1
After pituitary surgery the patient required physiologic hydrocortisone replacement therapy that was withheld for 48h before each post-surgery evaluation.
Primary cultures and in vitro analysis of ACTH release in response to desmopressinTo examine the direct effect of desmopressin on ACTH release, the tumor sample were dispersed into single cells by enzymatic and mechanical disruption and cultured as previously described.10,11 After a 24–36h of incubation (37°C/5% CO2), the medium was replaced with fresh, warm, serum-free medium, and the cells were pre-incubated for 1h to stabilize basal hormone secretion. After this preincubation step, the medium was replaced with serum-free medium containing DDAVP [100nM, dose selected according to previous studies12; Ferring SAU; Madrid, Spain] or serum-free medium alone (vehicle). After 4h incubation, medium was recovered and frozen for subsequent analysis of ACTH concentrations using a commercial immunoassay (DRG International, NJ, USA) as previously reported.10
RNA isolation, reverse-transcription and quantitative real-time RT-PCR of human transcripts from the pituitary adenoma sampleDetails of RNA extraction, quantification, reverse-transcription as well as of the development, validation and application of quantitative real-time reverse transcriptase polymerase chain reaction (qrtRT-PCR) to measure expression levels (copy number) of different human transcripts have been previously reported.13 To control for variations in the amount of RNA used in the reverse-transcription reaction and the efficiency of the RT reaction, the expression level (copy-number) of each transcript was adjusted by a normalization factor calculated from the expression level of three housekeeping genes, hypoxanthine-guanine phosphoribosyltransferase (HPRT), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin using the GeNorm 3.3 visual basic application software as previously described.13
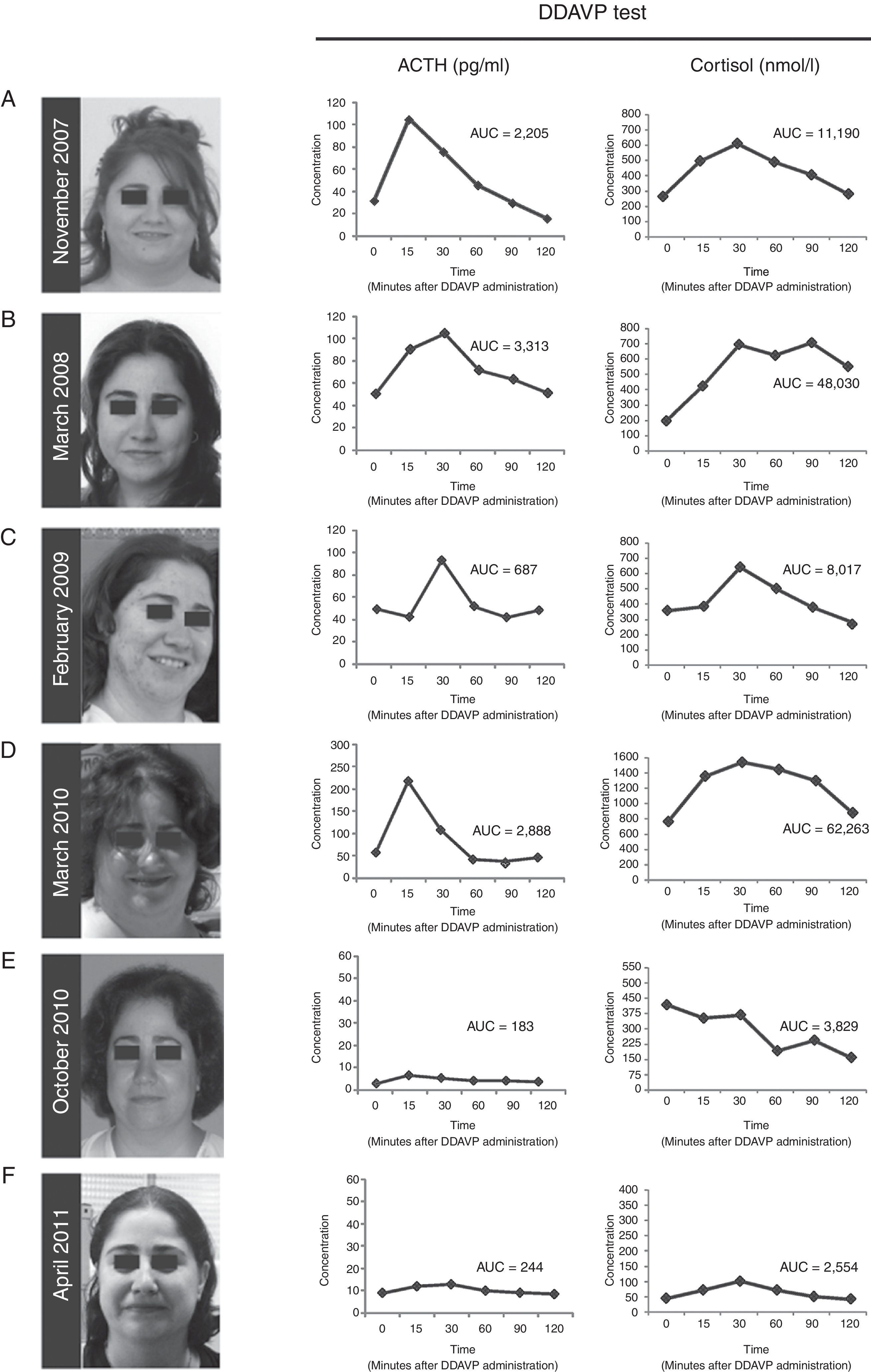
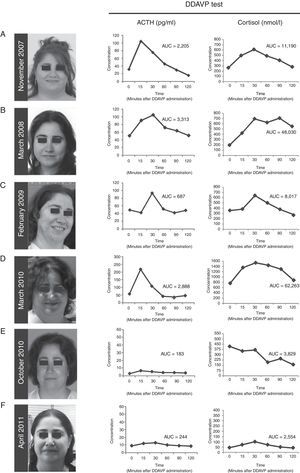
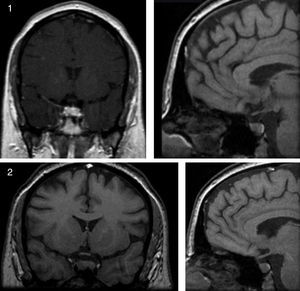
ResultsCase presentationA 31-year-old female patient was referred to our Endocrinology Unit in November 2007 with a diagnosis of CS for further evaluation and treatment. For the previous two months she had experienced a weight gain of 17kg and developed reddish stretch marks on abdomen, forearms and breasts during the summer. She also reported frontal headaches and arterial hypertension. The initial diagnosis of CS was based on typical clinical symptoms and laboratory test results. More specifically, morning serum cortisol was 577.5nmol/L [Normal Values (NV) 137.5–495nmol/L (6–23μg/dL)], serum cortisol after overnight low-dose (1mg) dexamethasone suppression test (LDDST, also known as Nugent's test) was 467nmol/L [NV<50nmol/L (1.8μg/dL)], and urinary free cortisol was 1.056μg/24h (2.851nmol/24h) [NV 50–150μg/24h (137–412nmol/24h)]. A gadolinium-enhanced MRI study revealed a microadenoma of 5mm at the right side of the pituitary without displacement of the infundibular stem (Supplemental Figure 1). Physical examination of the patient revealed typical signs of CS such as rounding of the face (moon face), fatty hump between the shoulders (buffalo hump) and abdominal striae. Additional laboratory tests were performed at our Hospital to confirm the hypercortisolism as well as to determine the source of hypercortisolism. These tests included UFCx3, midnight serum and salivary cortisol, and serum cortisol after overnight high-dose dexamethasone suppression test with 8mg. In addition, a DDAVP stimulation test was performed. In our unit the DDAVP test is used in patients with clinical suspicion of CS to determine whether further testing for hypercortisolism is warranted. Unexpectedly UFC, midnight serum and salivary cortisol levels were normal (Table 1). Furthermore, the patient showed a normal response to the overnight high-dose (8mg) dexamethasone test. However, the DDAVP stimulation test was positive, with ACTH and cortisol increases of 331% and 202% above baseline levels, respectively (Fig. 1A). Since no biochemical evidence of clinical hypercortisolism could be found, active CS was excluded and the patient was discharged. A follow-up appointment was scheduled for 4 months later.
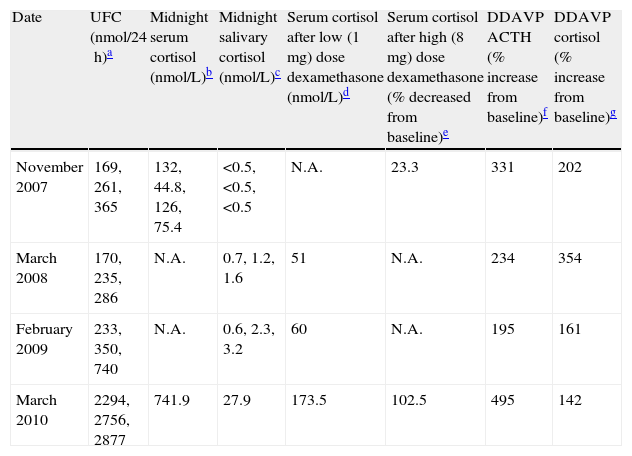
Preoperative biochemical tests.
| Date | UFC (nmol/24h)a | Midnight serum cortisol (nmol/L)b | Midnight salivary cortisol (nmol/L)c | Serum cortisol after low (1mg) dose dexamethasone (nmol/L)d | Serum cortisol after high (8mg) dose dexamethasone (% decreased from baseline)e | DDAVP ACTH (% increase from baseline)f | DDAVP cortisol (% increase from baseline)g |
| November 2007 | 169, 261, 365 | 132, 44.8, 126, 75.4 | <0.5, <0.5, <0.5 | N.A. | 23.3 | 331 | 202 |
| March 2008 | 170, 235, 286 | N.A. | 0.7, 1.2, 1.6 | 51 | N.A. | 234 | 354 |
| February 2009 | 233, 350, 740 | N.A. | 0.6, 2.3, 3.2 | 60 | N.A. | 195 | 161 |
| March 2010 | 2294, 2756, 2877 | 741.9 | 27.9 | 173.5 | 102.5 | 495 | 142 |
UFC: urinary free cortisol; DDAVP: desmopressin test; N.A.: data not available.
(A–D) Photographs of the patient and desmopressin (DDAVP) stimulation test. Both ACTH and cortisol levels increase in response to DDAVP administration during periods of worsening of signs and symptoms of Cushing's syndrome, and during periods of clinical remission. (E and F) After surgical removal of the ACTH-secreting adenoma, DDAVP administration did not induce increase of ACTH and cortisol levels. AUC=area under the curve for plasma ACTH and serum cortisol responses to DDAVP calculated using the trapezoidal method.
The patient was evaluated again in March 2008. The physical phenotype of CS was absent in the patient and UFC, midnight salivary cortisol levels were normal (Table 1). Serum cortisol levels after LDDST were slightly elevated although they cannot be considered as suggestive of CS (Table 1). Nevertheless, the response to the desmopressin stimulation test was again positive, with ACTH and cortisol increases of 234% and 354% above baseline levels, respectively (Fig. 1B). Active CS was again excluded. A follow-up visit was scheduled for a year later and the patient was asked to schedule an appointment earlier if symptoms of hypercortisolism were noticed.
In February 2009 the patient was referred again to our unit because she had suddenly developed hirsutism, acne and significant weight gain. The screening evaluation for hypercortisolism (UFC, midnight salivary cortisol) performed in our hospital revealed normal values except for one of the three samples of UFC (233nmol/24h, 350nmol/24h, 740nmol/24h) and a slightly elevated response to the overnight LDDST. A clear hyperresponsiveness to DDAVP was again observed, with plasma ACTH and serum cortisol increases of 195% and 161% above baseline levels, respectively (Fig. 1C). Again, hypercortisolism could not be confirmed and the patient was discharged. A two-week period of midnight salivary cortisol collections was ordered with the aim to determine a potential cycle of hypercortisolism. The patient was carefully instructed to obtain proper saliva samples and their preservation. All the saliva samples showed normal cortisol levels (mean [range]=1.96 [0.5–4.02]nmol/L [NV<10nmol/L]).2
On March 2010 the patient was again admitted to our unit due to a worsening in the symptoms that she had described in her last visit (Fig. 1D). She displayed a typical CS phenotype and the screening tests revealed elevated cortisol levels (Table 1). Further laboratory tests performed at our hospital, including midnight serum cortisol and serum cortisol after low and high dose of dexamethasone, confirmed ACTH-dependent Cushing's syndrome (Table 1). To identify the source of ACTH hypersecretion, a DDAVP stimulation test and a gadolinium-enhanced MRI scan of the pituitary gland were performed. A clear ACTH and cortisol increase in response to DDAVP was observed (495% and 142% increases above baseline levels, respectively). The MRI scan revealed a lesion of 8mm on the right side of the pituitary without displacement of the infundibular stem or cavernous sinus invasion (Supplemental Figure 2). These results confirmed the diagnosis of pituitary ACTH-dependent hypercortisolism (Cushing's disease).
Treatment with ketoconazole was initiated at low dose (200mg/day) and progressively increased up to 800mg/day, with weekly control of transaminases, over the first two months. The 800mg/day dose was maintained until surgery. Since adrenal insufficiency is a potential complication of ketoconozale therapy the patient was kept under close surveillance during the treatment period. No side effects of ketoconozale were noted during the treatment and the clinical signs and symptoms of the disease progressively improved. Similarly, the UFC and midnight salivary cortisol values decreased to normal values (236nmol/24h and 3.2nmol/L, respectively). In June 2010 the patient underwent transsphenoidal surgery for pituitary tumor removal. Laboratory tests (plasma ACTH and serum cortisol levels) performed at 4 and 10 months after the surgery indicated remission of the disease (data not shown). Importantly, the response to the DDAVP test was negative (Fig. 1E and F). Since the surgical procedure the patient has required hydrocortisone replacement therapy due to the severe corticotroph deficiency developed. The patient has now been in remission for 24 months.
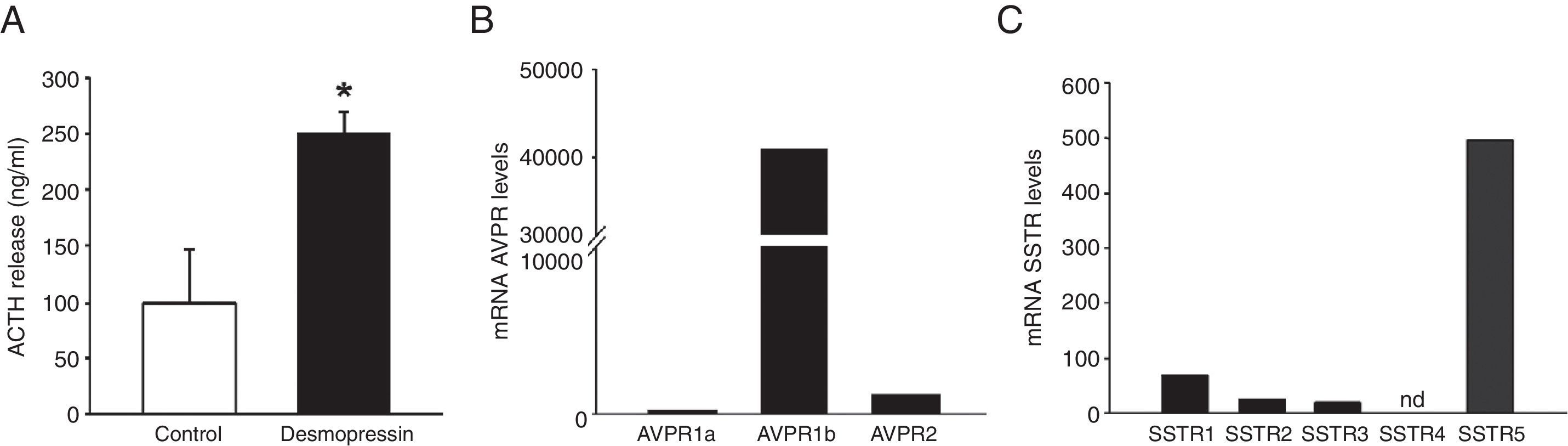
Histological, immunohistochemical and in vitro studiesThe resected tumor specimen was collected for histological, immunohistochemical and in vitro studies. Histological examination of the resected tumor specimen revealed a pituitary adenoma with basophilic neoplastic cells positive for ACTH immunohistochemistry (data not shown). The Ki-67 labeling index was 4–4.5%. Primary cultures from the tumor were established to evaluate the response to DDAVP in vitro. Treatment with DDAVP significantly increased ACTH secretion compared to vehicle-treated control corticotropinoma cells (Fig. 2A). To characterize the molecular features of the pituitary adenoma, the expression levels of arginine-vasopressin and somatostatin receptors were measured by quantitative real-time RT-PCR. The vasopressin receptor AVPR1b was highly expressed in the pituitary adenoma. Expression of AVPR1a and AVPR2 was also observed, albeit at significantly lower levels than AVPR1b (Fig. 2B). All somatostatin receptors but SSTR4 were expressed in the pituitary adenoma. SSTR5 exhibited the highest expression, followed by SSTR1, SSTR2 and SSTR3 (Fig. 2C).
In vitro secretion of ACTH in response to desmopressin (DDAVP) and receptor expression profile by quantitative real-time reverse transcriptase polymerase chain reaction studies. (A) Effect of DDAVP (100nM) vs. vehicle on ACTH secretion in corticotropinoma cell cultures (4h incubation). Values are expressed as percentage of vehicle-treated controls (set at 100%) and represent the mean±SEM (3–4 wells/treatment). *P<0.05, Student's t-test. (B and C) Expression profile of arginine-vasopressin and somatostatin receptor subtypes in the pituitary adenoma. mRNA copy numbers of each transcript were adjusted with the normalization factor calculated from the level of hypoxanthine-guanine phosphoribosyltransferase (HPRT), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin using the GeNorm 3.3 visual basic application for Microsoft Excel.
CCS can be extremely difficult to diagnose due to its heterogeneous clinical presentation. Since no specific clinical features can be used for the diagnosis of CCS, a correct differential diagnosis is critical to establish the presence of cyclic hypercortisolism.1,14,15 CCS should be strongly suspected in: (1) Patients with signs and symptoms consistent with CS but with normal or variable biochemical findings (ACTH and cortisol levels). Several conditions, such as exogenous administration of steroids (factitious) and pseudo-Cushing states, must be ruled out in these patients before establishing a definitive diagnosis of CCS. (2) Patients with typical features of CS and evident or suspected adrenocortical insufficiency (factitious Cushing's must be ruled out in the patients). (3) Patients with biochemical evidence of hypercortisolism but without overt clinical features of CS. In these cases, Cushing's syndrome (adrenal adenoma), pseudo-Cushing state, Cushing by aberrant receptors (macronodular adrenal hyperplasia) and glucocorticoid resistance should be considered in the differential diagnosis.
The patient described here displayed signs and symptoms suggestive of CS on arrival to our unit. In addition, the laboratory tests performed prior to referral revealed hypercortisolism. However, the typical features of CS fluctuated during the follow-up evaluations at our unit (likely paralleling the periods of cortisol excess and normal cortisol production). Furthermore, results of biochemical tests performed during the follow-up in our unit were normal misleading us to exclude the diagnosis of hypercortisolism on three different occasions. The cyclic or intermittent pattern of hypercortisolism poses an additional diagnostic challenge in CCS, particularly in patients with prolonged intervals of normal cortisol production. To overcome this problem, repeated sequential measurements of cortisol (urinary free cortisol and/or midnight salivary cortisol) have been proposed.16 In an attempt to establish a cortisol timeline in our patient, repeated testing of midnight salivary cortisol was performed on 15 consecutive days but no abnormal cortisol levels were found. However, it needs to be acknowledged that this approach might be useful in CCS patients with short cycles of normal/excess cortisol production but might not be adequate for patients with long cycle intervals, as suspected in our patient.
The DDAVP test has been proposed as a reliable tool for the diagnosis of CD, both in peripheral ACTH4–8 and in inferior petrosal sinus sampling.9,17 At our unit, the DDAVP test is consistently included in the algorithm for testing patients with suspected CS. We have successfully applied the DDAVP test to exclude pseudo-Cushing's states, to distinguish between central and peripheral source of ACTH in CS, and to predict the recurrence of CD for more than 20 years. In the patient reported here, the DDAVP test yielded consistent positive results (for both ACTH and cortisol values) during the diagnostic period, even when overt clinical features of CS were not apparent. Indeed, the positive response to DDAVP together with the fluctuating symptoms and signs observed in the patient prompted us to continue the monitoring of the patient until a firm diagnosis could be made.
CCS is commonly caused by ACTH-secreting pituitary adenomas but it has also been associated to other non-pituitary diseases including typical and atypical carcinoids, adrenal adenomas, pheochromocytoma and rare forms of pigmented variant of micronodular adrenocortical hyperplasia.18 DDAVP elicits marked ACTH and cortisol responses in the majority of CS patients with pituitary tumors, but generally not in healthy subjects, pseudo-Cushing's, adrenal and ectopic Cushing's patients, thus allowing the diagnosis and localization of the lesion with a sensitivity and specificity similar to that achieved with the CRH test.9,19,20 Here we show that the DDAVP test might be also helpful in the identification of the pituitary source of ACTH overproduction in a case of CCS. In agreement with our observations, DDAVP does not seem to induce ACTH hypersecretion in ectopic ACTH secretion with cyclical behavior (CCS).21,22 DDAVP tests were also performed in the patient after surgery to monitor remission or recurrence. These tests were negative, consistent with the observed biochemical remission. Thus, in the case reported here the DDAVP stimulation test proved to be very useful in both the diagnostic work-up and of postsurgical follow-up (2 years).
The in vitro DDAVP studies performed in primary cultures from the pituitary adenoma of the patient were consistent with the in vivo DDAVP test results. We observed that DDAVP treatment significantly stimulated ACTH secretion in primary pituitary cell cultures suggesting that the pituitary adenoma was the direct mediator of the effect of DDAVP on ACTH secretion observed in the patient. The molecular mechanisms underlying the DDAVP-stimulated ACTH secretion in patients with CD are not completely understood. It has been suggested that it might be related to abnormal overexpression of arginine-vasopressin receptors in pituitary adenomas.12,16,23,24 Interestingly, previous studies have reported that AVPR1b is overexpressed in human pituitary corticotropinomas.23,25,26 In agreement with these observations, our results revealed that AVPR1b was highly expressed compared to the other arginine-vasopressin receptor subtypes in the pituitary tumor of the patient described here. It is tempting to speculate that the response to the DDAVP test observed in patients with Cushing's disease may be due to abnormal expression of AVPR1b in pituitary tumors. Interestingly, the expression pattern of arginine-vasopressin and somatostatin receptors in the case described here are similar to those reported in most pituitary adenomas of CD. Although speculative at this stage, these observations raise the possibility that selective antagonists for AVPR1b might be used for the treatment of CCS.
In conclusion, our case illustrates the utility of the DDAVP test in the evaluation of patients with suspected CCS. The DDAVP test could facilitate the management of cyclical Cushing's disease by shortening the time of diagnosis.
Conflict of interestThe authors have no conflict of interest to declare.
A.L.C. was supported by grants from the Spanish Health Ministry (FIS PS09/01165) and the Andalusian Regional Government (CTS-444). D.A.C. was supported by the “Ramón y Cajal” program from the Spanish Ministry of Science and Innovation (RYC-2006-001071). R.M.L. and J.P.C. are supported by the following grants: RYC-2007-00186, CTS-5051, CIBERobn and BFU2010-19300.