Colon cancer in elderly patients is an increasing problem due to its prevalence and progressive aging population. Prehabilitation has experienced a great grown in this field. Whether it is the best standard of care for these patients has not been elucidated yet.
MethodsA retrospective comparative cohort study of three different standards of care for elderly colon cancer patients (>65 years) was conducted. A four-weeks trimodal prehabilitation program (PP), enhanced recovery program (ERP) and conventional care (CC) were compared. Global complications, major complications (Clavien-Dindo ≥ 3), reinterventions, mortality, readmission and length of stay were measured. Optimal recovery, defined as postoperative course without major complications, no mortality, hospital discharge before the fifth postoperative day and without readmission, was the primary outcome measure. The influence of standard of care in optimal recovery and postoperative outcomes was assessed with univariate and multivariate logistic regression models.
ResultsA total of 153 patients were included, 51 in each group. Mean age was 77.9 years. ASA Score distribution was different between groups (ASA III-IV: CC 56.9%, ERP 25.5%, PP 58.9%; p = 0.014). Optimal recovery rate was 55.6% (PP 54.9%, ERP 66.7%, CC 45.1%; p = 0.09). No differences were found in major complications (p = 0.2) nor reinterventions (p = 0.7). Uneventful recovery favors ERP and PP groups (p = 0.046 and p = 0.049 respectively).
ConclusionsPP and ERP are safe and effective for older colon cancer patients. Fewer overall complications and readmissions happened in ERP and PP patients. Major complications were independent of the standard of care used.
El cáncer de colon (CC) en pacientes de edad avanzada es un problema creciente por su prevalencia y envejecimiento progresivo de la población. La prehabilitación ha experimentado un gran crecimiento en este campo sin haberse dilucidado si es el mejor estándar de cuidados para estos pacientes.
MétodosEstudio retrospectivo comparativo de cohortes de tres estándares diferentes de cuidados para pacientes mayores de 65 años con CC. Se compararon un programa de prehabilitación (PP) trimodal de cuatro semanas, uno de recuperación intensificada (RI) y cuidados convencionales (CC). Se midieron complicaciones globales, complicaciones mayores (Clavien-Dindo ≥ 3), reintervenciones, mortalidad, reingresos y estancia hospitalaria. La recuperación óptima fue la medida de resultado primaria. La influencia del estándar de atención en la recuperación óptima y los resultados postoperatorios se evaluó con modelos de regresión logística univariante y multivariante.
ResultadosSe incluyeron 153 pacientes, 51 por grupo. La edad media fue 77,9 años. La distribución del ASA fue diferente entre los grupos (ASA III–IV: CC 56,9%, RI 25,5%, PP 58,9%; p = 0,014). La tasa de recuperación óptima fue del 55,6% (PP 54,9%, RI 66,7%, CC 45,1%; p = 0,09). No se encontraron diferencias en complicaciones mayores (p = 0,2) ni reintervenciones (p = 0,7). La recuperación sin incidencias favorece a los grupos RI y PP (p = 0,046 y p = 0,049 respectivamente).
ConclusionesPP y RI son seguros y efectivos para pacientes mayores con CC. Las complicaciones generales y reingresos en pacientes con RI y PP fueron menores. Las complicaciones mayores resultaron independientes del estándar de cuidados utilizado.
Colon cancer in elderly population is a current relevant worldwide problem due to its increasing prevalence, progressive aging and frailty of this population group1.
The best standard of care for elderly colon cancer patients has been extensively discussed and it is still arguable: laparoscopy vs. open approach2–4, the feasibility of enhanced recovery programs (ERP)5–7, and potential advantages of prehabilitation programs (PP)8,9.
Some encouraging results have been published concerning PP in elderly patients after colon cancer surgery8,10,11, and therefore, an increasing interest is evident in the scientific community in the last few years12,13.
Despite these results, a recently published randomized controlled trial challenges previous evidence, and therefore, the role of PP could be questioned, mainly when compared to ERP14.
In this context, we designed a study to compare the postoperative outcome after colon cancer surgery in elderly patients following three different standards of perioperative care: conventional care (CC), ERP and PP.
Material and methodsStudy designA comparative Cohort study of consecutive patients included in a prehabilitation program was done. Comparison groups were two historical cohorts of patients with same inclusion and exclusion criteria who had been consecutively attended at our colorectal surgery unit of a tertiary referral center following ERP and CC. All the patients were included in the prehabilitation program. The same number of consecutive patients in the ERP and CC cohorts were included from a prospectively maintained data base. The study period was October 2016–May 2019.
Ethical permission for this analysis was provided by the local hospital ethics committee (22/21-4658).
Study participantsInclusion criteria were colon cancer patients, 15 cm or above from anal verge, age of 65 or older, and operated on an elective surgery. The main selection criteria for patients to undergo PP, was a score ≤14 in the frailty screening tool G8 scale15. Exclusion criteria were stage IV disease, inability to understand ERP or PP preoperative and postoperative instructions or disabled patients who were unable to do any physical activity.
Prehabilitation programA trimodal design was used, embracing physical exercising, nutritional supplementation and psychological assessment. Apart from this, during operation and postoperative management, these patients were managed following ERP criteria. A multidisciplinary team of psycho-oncologists, dietitians and endocrinologists, physiatrists, anesthesiologists, internal medicine doctors, oncology nurses and colorectal surgeons was set up.
Nutritional counseling and supplementationPatients were evaluated following the Malnutrition Universal Screening Tool (MUST)16. Advice of appropriate nutrition and counseling for prehabilitation phase were given. Every patient was orally supplemented with immune-nutrient based shakes (Atempero, Vegenat®), 200 mL twice a day during the preoperative week. Those patients at risk of malnutrition were derived to an exhaustive malnutrition diagnosis protocol and individualized assessment.
Physical exerciseThe physiatrist’s visit always took place after nutritional screening. Anemia screening and diabetic profile, when indicated, were done. When severe malnutrition or poor cardiorespiratory conditions were diagnosed, physical exercise was considered contraindicated. Those cases were further discussed in order to establish if they were suitable candidates for surgery or if they were considered appropriate to be discarded.
Patients with normal screening tests were designated an individualized multi-task exercise program during a personal interview in an outpatient visit. Exercise routine consisted of strengthening of respiratory muscles, muscle stretching exercises, moderate aerobic activity counseling with 3–5 weekly sessions and strengthening limb exercises.
Psychological assessmentDuring surgical interview with the colorectal surgeon the hospital anxiety and depression scale (HADS)17 was undertaken. Those with an altered score were referred to personal evaluation with the psycho-oncologist; patients with normal punctuation were forwarded to a group meeting where meditation and stress managing tools were explained and put into practice.
ERPERP has been implemented within our hospital since 2015. Very strict inclusion criteria were considered at the early beginning of the program, when elderly colorectal cancer patients were considered, just fit or healthy patients (e.g. ASA II, non-diabetic) were included in the program.
A 70% adherence was required to be included in the ERP group according to the last ERAS Guidelines for colorectal surgery18,19.
Conventional careConventional care was considered for all those patients that were not included in the ERP or PP preoperatively.
Outcome and measuresThe recorded clinical variables included standard of care, demographic variables, comorbidities, past surgery, substance abuse, chronic medications, ASA score, tumor location, surgical technique, surgical approach, intraoperative complications, and postoperative outcome variables such as anastomotic leak, postoperative ileus, surgical site infection, reintervention and readmission. Postoperative complications were classified according to Clavien-Dindo20. Mayor complications were defined as Clavien-Dindo ≥ III.
Our primary outcome was optimal recovery, which had been previously defined as the postoperative recovery with hospital discharge prior to postoperative day 5 and absence of major complication, nor mortality nor readmission at 30-days21. Secondary outcomes were global rate of complications, length of stay, overall mortality, failure to rescue rate and readmission rate. Failure to rescue had been previously defined as the death of a patient after one or more potentially treatable complications.
Statistical methodContinuous variables with a normal distribution are shown as mean and standard deviation (SD); those with non-normal distribution are shown in median and range. Categorical variables are shown in number and percentage.
The relation among continuous variables was studied with the Mann–Whitney test. The relation among categorical variables was studied with χ2-Test (or Fisher exact test when necessary).
Univariate analysis with χ2-Test for categorical variables was done. Clinically relevant variables and those with a p value <0.1 were introduced in a multivariate logistic regression model with calculation of odds ratio [(OR) (95% CI)]. In multivariate model variables were introduced with a full-model strategy and automatic step selection.
The statistical package used for the analysis was Stata 13.1 (StataCorp, Texas, USA).
ResultsA total of 153 patients were analyzed, 51 in each standard of care group: PP, ERP and CC. Mean age was 77.9 (SD 6.8), with 87 male and 66 female patients (56.9% and 43.1% respectively).
Demographic variables, comorbidities and ASA score classification in the whole sample and in each standard of care group is represented in Table 1. Patients in ERP had a lower age and a significantly higher rate of ASA III patients, compared to the other two groups (p < 0.01). The distribution of the rest of comorbidities among the three groups is equivalent, with the only exception of liver disease that is significantly higher in the CC group (0.03).
Demographic variables and distribution of comorbidities in the global sample, and in each group of care regimen.
| Total (n = 153) | Conventional care (n = 51) | ERP (n = 51) | Prehabilitation (n = 51) | p | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 87 (56.9) | 27 (52.9) | 28 (54.9) | 32 (62.7) | 0.57 |
| Female | 66 (43.1) | 24 (47.1) | 23 (45.1) | 19 (37.3) | |
| Age* | 77.9 (+/−6.8) | 79.2 (+/−6.3) | 75.8 (+/−7.1) | 78.6 (+/−6.5) | 0.028 |
| ASA | |||||
| I | 9 (5.9) | 2 (3.9) | 4 (7.8) | 3 (5.9) | 0.014 |
| II | 72 (47.1) | 20 (39.2) | 34 (66.7) | 18 (35.3) | |
| III | 70 (45.8) | 28 (54.9) | 13 (25.5) | 29 (56.9) | |
| IV | 2 (1.3) | 1 (2) | 0 (0) | 1 (2) | |
| Obesity | 52 (34) | 14 (27.5) | 17 (33.3) | 21 (41.2) | 0.34 |
| Heart disease | 44 (28.8) | 17 (33.3) | 13 (25.5) | 14 (27.5) | 0.66 |
| COPD | 31 (20.3) | 9 (17.6) | 11 (21.6) | 11 (21.6) | 0.85 |
| Diabetes | 55 (35.9) | 20 (39.2) | 15 (29.4) | 20 (39.2) | 0.49 |
| Liver disease | 3 (2) | 3 (2) | 0 (0) | 0 (0) | 0.035 |
| Kidney disease | 25 (16.3) | 7 (13.7) | 9 (17.6) | 9 (17.6) | 0.83 |
| Alcohol use | 13 (8.5) | 3 (5.9) | 4 (7.8) | 6 (11.8) | 0.55 |
| Tobacco abuse | |||||
| Never | 121 (79.1) | 45 (88.2) | 39 (76.5) | 37 (72.5) | 0.3 |
| Past smoker | 28 (18.3) | 5 (9.8) | 10 (19.6) | 13 (25.5) | |
| Current smoker | 4 (2.6) | 1 (2) | 2 (3.9) | 1 (2) | |
Surgical details, such as previous interventions, surgical technique and surgical approach are summarized in Table 2. The highest rate of laparoscopic surgery was found in ERP (88.2%), followed by PP (82.4%) and CC (75.8%) in third place. The lower conversion rate was also seen in the ERP group (7.8%), being PP (13.7%) and CC (11.8%) quite similar.
Operative data in the global sample and in each standard of care group.
| Total (n = 153) | Conventional care (n = 51) | ERP (n = 51) | Prehabilitation (n = 51) | p | |
|---|---|---|---|---|---|
| Previous Surgery | |||||
| Yes | 59 (38.6) | 27 (52.9) | 20 (39.2) | 12 (23.5) | 0.09 |
| No | 94 (61.4) | 24 (47.1) | 31 (60.8) | 39 (76.5) | |
| Surgical Approach | |||||
| Laparoscopy | 116 (75.8) | 29 (56.9) | 45 (88.2) | 42 (82.4) | 0.0001 |
| Open | 20 (13.1) | 16 (31.4) | 2 (3.9) | 2 (3.9) | |
| Conversion | 17 (11.1) | 6 (11.8) | 4 (7.8) | 7 (13.7) | |
| Surgical Technique | |||||
| Right Hemicolectomy | 89 (58.2) | 28 (54.9) | 31 (60.8) | 30 (58.8) | 0.37 |
| Extended Right Hemicolectomy | 9 (5.9) | 3 (5.9) | 4 (7.8) | 2 (3.9) | |
| Left Hemicolectomy | 14 (9.2) | 3 (5.9) | 2 (3.9) | 9 (17.6) | |
| Sigmoidectomy | 33 (21.6) | 13 (25.5) | 12 (23.5) | 8 (15.7) | |
| Subtotal Colectomy | 2 (1.3) | 0 (0) | 1 (2) | 1 (2) | |
| Total Colectomy | 1 (0.7) | 0 (0) | 0 (0) | 1 (2) | |
| Segmental Colectomy | 3 (2) | 2 (3.9) | 1 (2) | 0 (0) | |
| Hartmann Procedure | 1 (0.7) | 1 (2) | 0 (0) | 0 (0) | |
| Right Hemicolectomy + Sigmoidectomy | 1 (0.7) | 1 (2) | 0 (0) | 0 (0) | |
All the patients that were included in the ERP had an over 70% accomplishment of ERAS items as described in the methods section. PP were managed also following ERAS guidelines, although it was not considered a necessary criterion for inclusion in PP group. Table 3 represents each ERAS items criterion considered and its accomplishment in each ERP and PP groups. There only existed statistically significant differences in the preoperative items: preoperative information, optimization, nutrition and anemia screening (p < 0.01), favoring PP patients.
ERAS items accomplishment in the ERAS (ERP) and Prehabilitation (PP) Cohorts. ERAS items categorization exactly reproduces last version of ERAS Colorectal Surgery Guidelines20.
| ERAS items | ERP | PP | p | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Preadmission Items | Preoperative information | 44 (87) | 51 (100) | 0.01 |
| Optimization | 34 (67) | 51 (100) | <0.01 | |
| Prehabilitation | 0 (0) | 51 (100) | n.a. | |
| Nutrition | 39 (76) | 51 (100) | <0.01 | |
| Anemia screening | 39 (76) | 51 (100) | <0.01 | |
| Preoperative Items | Prevention of nausea and vomiting | 49 (96) | 47 (92) | 0.67 |
| Selective premedication | 29 (57) | 34 (67) | 0.41 | |
| Prophylactic antibiotics | 51 (100) | 51 (100) | n.a. | |
| No bowel preparation | 35 (68) | 32 (63) | 0.83 | |
| Maintaining euvolemia | 49 (96) | 51 (100) | 0.49 | |
| Avoid fasting and carbohydrate drink | 47 (92) | 49 (96) | 0.67 | |
| Intraoperative Items | Standard anesthetic protocol | 51 (100) | 51 (100) | n.a. |
| Fluid normovolemia | 31 (61) | 36 (70) | 0.4 | |
| Normothermia | 33 (65) | 37 (72) | 0.52 | |
| Minimal invasive surgery | 45 (88) | 42 (82) | 0.57 | |
| No drainage | 30 (59) | 33 (65) | 0.68 | |
| Postoperative Items | No gastric drainage | 51 (100) | 51 (100) | n.a. |
| Multimodal analgesia | 33 (65) | 35 (69) | 0.67 | |
| Thromboprophylaxis | 47 (92) | 49 (96) | 0.67 | |
| Fluid normovolemia | 31 (61) | 36 (70) | 0.4 | |
| Urinary catheter early removal | 45 (88) | 47 (92) | 0.74 | |
| Prevent Hyperglycemia | 24 (47) | 29 (57) | 0.32 | |
| Postoperative nutrition | 45 (88) | 44 (86) | 0.98 | |
| Early mobilization | 42 (82) | 39 (76) | 0.62 | |
Data shown represents total number of patients and percentages.
n.a.: Not applicable.
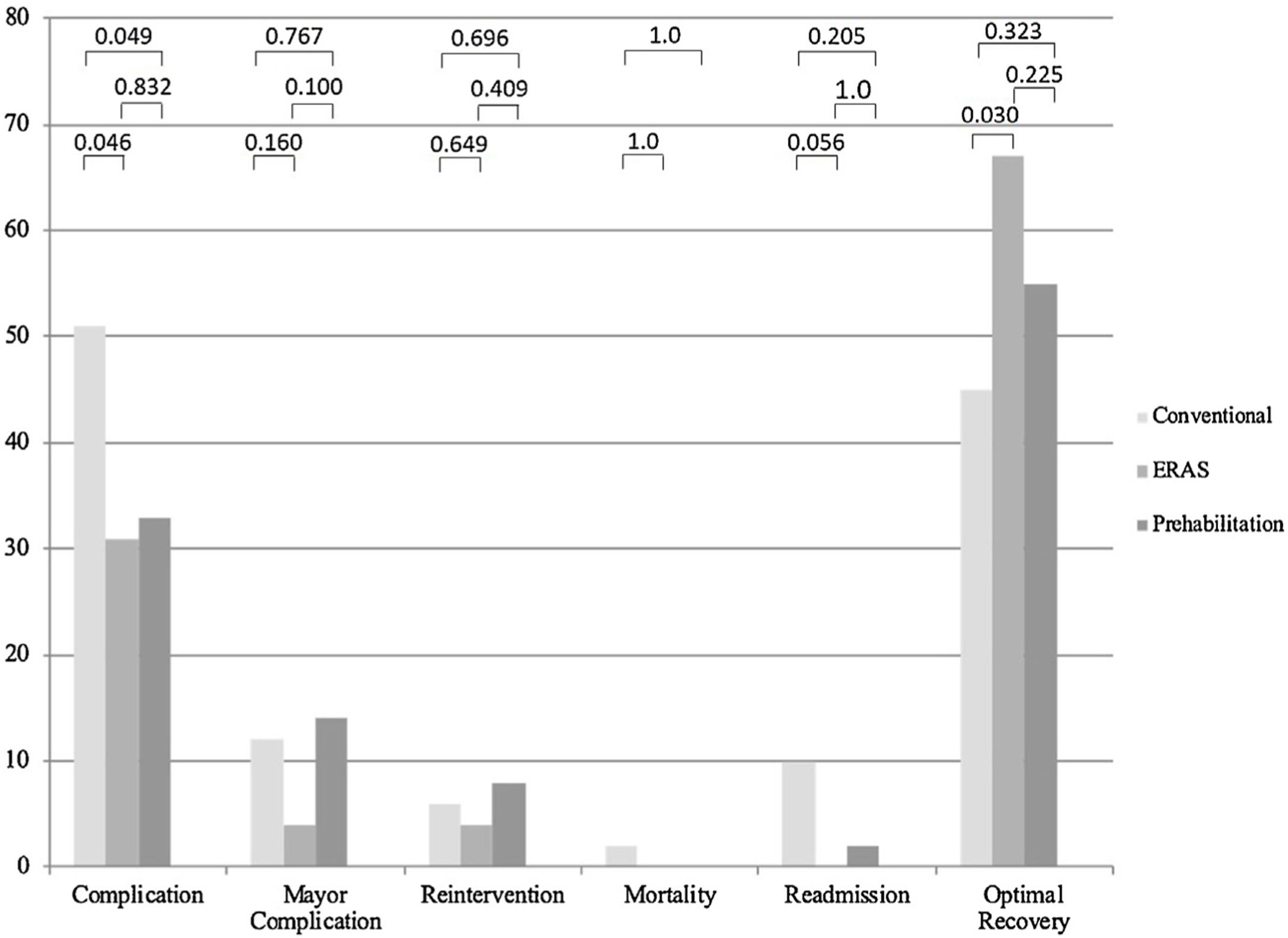
According to the adopted definition, 85 patients, 55.6% of the total sample, experienced an optimal recovery. No differences among the three groups were found (p = 0.09), although when compared by pairs differences existed between the ERP group and the CC one. Uneventful postoperative course, meaning no complications happening, was more frequent in both the ERP (68.6%) and PP (66.7%), compared to the CC group (49%) (p = 0.047). Overall complications classified according to Clavien-Dindo’s were similar in the three groups. Major complications happened in 15 patients, 9.8% of the sample, of whom nine patients (5.9%) required a reoperation and there was just one case of mortality. Length of hospital stay and readmission rates also favors both ERP and PP against CC; differences between ERP and PP programs are not significative. Every postoperative outcome measure and their distribution in every standard of care group are represented in Table 4.
Postoperative results in the global sample and in each standard of care group.
| Total (n = 153) | Conventional care (n = 51) | ERP (n = 51) | Prehabilitation (n = 51) | p | |
|---|---|---|---|---|---|
| Optimal Recovery | |||||
| Yes | 85 (55.6) | 23 (45.1) | 34 (66.7) | 28 (54.9) | 0.09 |
| No | 68 (44.4) | 28 (54.9) | 17 (33.3) | 23 (45.1) | |
| No complications | 94 (61.4) | 25 (49) | 35 (68.6) | 34 (66.7) | 0.047 |
| Clavien-Dindo | |||||
| I | 13 (8.5) | 6 (11.8) | 6 (11.8) | 1 (2) | 0.15 |
| II | 31 (20.3) | 14 (27.5) | 8 (15.7) | 9 (17.6) | |
| IIIa | 3 (2) | 1 (2) | 0 (0) | 2 (3.9) | |
| IIIb | 10 (6.5) | 4 (7.8) | 2 (3.9) | 4 (7.8) | |
| IVa | 1 (0.7) | 0 (0) | 0 (0) | 1 (2) | |
| IVb | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| V | 1 (0.7) | 1 (2) | 0 (0) | 0 (0) | |
| Mayor Complication | 15 (9.8) | 6 (11.8) | 2 (3.9) | 7 (13.7) | 0.2 |
| Reintervention | 9 (5.9) | 3 (5.9) | 2 (3.9) | 4 (7.8) | 0.7 |
| Readmission | 6 (3.9) | 5 (9.8) | 0 (0) | 1 (2) | 0.048 |
| Length of stay* | 7.7 (6.7) | 9.5 (8.6) | 5.9 (3.8) | 7.8 (6.7) | 0.030 |
Likewise, Fig. 1 shows a graphic representation of the most relevant postoperative outcome measures and the p values of χ2-Test of the three groups and when they are compared by pairs (CC vs. ERP, CC vs. PP and ERP vs. PP). A significative higher rate of complications was diagnosed in the CC group vs. both ERP and PP groups (p = 0.046 and 0.049 respectively), with no differences between ERP and PP (p = 0.8).
Table 5 represents the results of univariate and multivariate logistic regression analysis to determine a possible relation between the standard of care and optimal recovery. In univariate analysis the factors related to optimal recovery were age, ASA score and surgical approach; none of them was still significant in multivariate analysis. Standard of care shows a clinical trend in univariate analysis but this relation disappeared in multivariate analysis.
Univariate and multivariate analysis of possible factors influencing postoperative Optimal Recovery.
| Optimal Recovery | |||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| n (%) | p | OR | 95% CI | p | |
| Standard of Care | |||||
| Conventional Care | 23 (45.1) | 0.09 | 1 | ||
| ERP | 34 (66.7) | 1.3 | 0.53–3.3 | 0.545 | |
| Prehabilitation | 28 (54.9) | 1.1 | 0.47–2.70 | 0.786 | |
| Age* | 76.6 (7.0) | 0.007 | 0.96 | 0.91–1.01 | 0.094 |
| ASA | |||||
| I–II | 52 (65) | 0.01 | 1 | 0.113 | |
| III–IV | 33 (45.2) | 0.56 | 0.27–1.14 | ||
| Surgical Approach | |||||
| Laparoscopy | 73 (62.9) | 0.005 | 1 | ||
| Open | 6 (30) | 0.33 | 0.11–1.04 | 0.057 | |
| Conversion | 6 (35.3) | 0.43 | 0.14–1.29 | 0.131 | |
Optimal recovery, considered as postoperative course with no major complications, hospital discharge before the fifth postoperative day, no mortality and no readmission, as it had been previously defined21, was achieved in a 55.6% of elderly colon cancer patients attended at our unit. When comparing among groups, CC clearly obtained the lower rate (45.1%) vs. 54.9% in the PP group and 66.7% among the ERP patients. Although there are not many other references to compare with, as this concept has not been widely used, the authors who originally described it, reported a 49.7% in a mixed cohort of colon and rectal cancer patients of any age. Total complication rates and readmissions favors ERP and PP programs, without differences between them, and being clearly lower and significantly different to the CC group. Patients without any complications have been over 60% in our series, what shows to be higher than other available data in equivalent patients14; specifically, patients managed according to our prehabilitation regimen experienced a difference higher than a 10% of uneventful postoperative stay compared to those of Carli et al.14. Major complications and readmission rates are comparable, although our LOS is higher, probably because of a higher rate or reinterventions in our PP group. In this sense, a possible explanation might be that they included in this group a 27.7% of patients with primary created stomas, some of whom might be protective ileostomies that could prevent from clinically relevant anastomotic leaks and reinterventions, or even definitive colostomies in rectal cancer patients.
Our results highlight the problem that still exists for proper patient selection for the most advantageous standard of care in each case among this elderly population. As a whole, postoperative outcome in this sample of elderly patients have kept within the quality standards proposed at a national level for colorectal surgery units, considering these standards as global, not only in the elderly population22.
Patients included in both groups of ERP and PP were managed trying to follow scrupulously the ERP Guidelines published at each time18,19. Although CC group might be influenced by some of ERP learnings at some point, it clearly poses a totally different standard of care as it is reflected in the much higher rate of open surgery compared to the two other groups. It is also quite obvious that every preadmission and preoperative items are impossible to be followed without a proper planification, including avoiding fasting, bowel preparation, optimization, nutritional screening and intensification or treating anemia.
Following our PP, reserving it for the frailest patients attended at our unit, we were able to obtain equivalent postoperative results to those obtained with ERP in a quite healthier population. Although these results are not statistically significant to those in CC, a clear clinical difference of 10% rate of optimal recovery was observed. It is probable that these differences did not reach statistical significance, because not enough study population.
It is noticeable that many of the studies carried out on PP are not based on strict inclusion and exclusion criteria, and patients of all ages are admitted, without any selection according to frailty or morbidity20,23–26. Thus, the beneficial results of PP may be questionable, especially with the recently published results obtained by Carli et al.14. Nevertheless, although it is a randomized clinical trial, it is not exempt of certain limitations, like a 40% of the prehabilitation group patients having to be excluded from the per-protocol analysis because of low accomplishment with the prehabilitation program. In this sense, our PP cohort showed quite higher rates, as it is shown in the preadmission items of ERAS recorded in Table 3.
Thus, even taking into account the selection bias present in the present work, we consider PP results as quite positive as long as it has probably opened the ERP door to a group of patients who, otherwise, would have been managed by the CC and could not have benefitted from the virtues of none of ERP or PP.
Major complications, most of the times, are related to technical issues and therefore it is not clear in which manner they can be avoided depending on the standard of care applied in every case, and therefore in most series major complications are the same in every group of comparison8,14,27.
One of the great uncertainties concerning perioperative care in colon cancer surgery at this moment is an adequate selection of patients for just ERP or previous prehabilitation. Conventional care at this moment should be avoided, as results are worse in terms of overall complications or readmission rates27,28, and there should not be any more concerns about the applicability of ERP nor PP in elderly patients.
Further research in this field is still needed. Appropriate patients’ selection for PP has not been elucidated yet. Some issues that have been investigated with contradictory results include duration25,29, possibility of ambulatory performing8,30, or its eventual ability to modify oncological outcome of certain types of tumors10; definitely a very exciting field for research.
This paper has some limitations such as the obvious selection bias of patients to be included in the ERP and CC and its retrospective data collection in both historic cohorts. Nevertheless, it has some important strengths, as being the first paper in which CC, ERP and PP groups are compared in terms of postoperative results in elderly patients having surgery for colon cancer. Selection biases have also been overcome with the rigorous statistical analysis using a multivariate logistic regression model to depict confounders.
ConclusionsThis study demonstrates that older colon cancer patients benefit from new standards of care such as ERP or PP better than conventional care, with good results in terms of applicability and safety, with 66.7% and 54.9% of optimal recovery respectively, with fewer overall complications and readmissions in ERP and PP patients.
Additional benefit of prehabilitation to ERP could not be elucidated from our experience, therefore, further work for appropriate selection of patients is warranted.
FundingNo funding was received for the present study.
Conflicts of interestThe authors of the article do not have any commercial association that might pose a conflict of interest in relation to this article.
Ethics approvalThe study protocol was approved by the Local Ethics Committee.
DisclosuresNone of the authors have any conflicts of interest nor disclosure.
Please cite this article as: Cerdán Santacruz C, Merichal Resina M, Báez Gómez FD, Milla Collado L, Sánchez Rubio MB, Cano Valderrama O, et al. “Recuperación óptima” tras cirugía por cáncer de colon en el paciente añoso. Resultados de un estudio de cohorte: Cuidados convencionales, Recuperación intensificada y programa de prehabilitación. Cir Esp. 2022