Traumatic brain injury (TBI) is the most common cause of cognitive and behavioral deficits, disability, and mortality worldwide. Trehalose is a natural non-reducing disaccharide of glucose which its anti-inflammatory and anti-oxidative stress effects have been proven in past in vivo and in vitro studies. This study aims to evaluate the effect of oral trehalose on inflammatory biomarkers, oxidative stress indices and clinical outcomes in hospitalized traumatic brain injury patients.
MethodsTwenty participants with traumatic brain injury were randomly assigned to the intervention or control group in order to evaluate the effect of oral trehalose (30g instead as a part of the daily carbohydrate of enteral feeding) or placebo groups (standard isocaloric hospital enteral feeding) using 12-day parallel study. Inflammatory and oxidative stress biomarkers such as interleukin-6 (IL-6), C-reactive protein (CRP), pro-oxidant-antioxidant balance (PAB), superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA), clinical outcomes, including APACHE II, SOFA scores, NUTRIC score, GCS, ICU discharge time, mechanical ventilator duration, 28-day and 60-day mortality rate were measured at the beginning and end of the study.
ResultsThe trehalose intake could significantly improve CRP (mean change: −30±25mg/l, P˂0.001) and NUTRIC score (mean change: 0(−2,0), P=0.04). In addition, a marginally significant decrease in APACHE II score (mean change: −3±5, P=0.07) and 28-day mortality (0 vs. 30%; P=0.06, {NNT}=3) was observed. Moreover, among anthropometric indicators, only a marginally decrease in MAC (mean change: −0.5(−1,0) was shown. No considerable effect was observed on other biomarkers, anthropometric indices and clinical outcomes in the intervention group as well as the control group.
ConclusionThis trial provided some evidences that trehalose administration improved CRP and marginally improved some clinical outcomes, such as NUTRIC score and 28-day mortality in TBI patients in ICU.
La lesión cerebral traumática (LCT) es la causa más común de déficits cognitivos y conductuales, discapacidad y mortalidad en todo el mundo. La trehalosa es un disacárido natural no reductor de glucosa cuyos efectos antiinflamatorios y antiestrés oxidativo han sido comprobados en estudios in vivo e in vitro previos. Este estudio tiene como objetivo evaluar el efecto de la trehalosa oral en biomarcadores inflamatorios, índices de estrés oxidativo y resultados clínicos en pacientes hospitalizados con LCT.
MétodosFueron asignados al azar 20 participantes con LCT al grupo de intervención o al grupo de control para evaluar el efecto de la trehalosa oral (30g en lugar de una parte de los carbohidratos diarios de la alimentación enteral) o grupos de placebo (alimentación enteral isocalórica estándar del hospital) mediante un estudio paralelo de 12 días. Los biomarcadores de inflamación y estrés oxidativo, como la interleucina-6 (IL-6), la proteína C reactiva (PCR), el equilibrio prooxidante-antioxidante (PAB), la superóxido dismutasa (SOD), el glutatión (GSH) y el malondialdehído (MDA), los resultados clínicos, incluyendo los puntajes APACHE II, SOFA, el puntaje NUTRIC, la escala de coma de Glasgow (GCS), el tiempo de alta de la Unidad de Cuidados Intensivos (UCI), la duración de la ventilación mecánica y las tasas de mortalidad a los 28 y 60 días, se midieron al inicio y al final del estudio.
ResultadosLa ingesta de trehalosa pudo mejorar significativamente la PCR (cambio medio: −30±25mg/l, p˂0,001) y el puntaje NUTRIC (cambio medio: 0(−2,0), p=0,04). Además, se observó una disminución marginalmente significativa en el puntaje APACHE II (cambio medio: −3±5, p=0,07) y la mortalidad a los 28 días (0 vs. 30%; p=0,06, [NNT]=3). Además, entre los indicadores antropométricos, solo se mostró una disminución marginal en la circunferencia de la mitad del brazo (MAC) (cambio medio: −0,5 [−1,0]). No se observó un efecto considerable en otros biomarcadores, índices antropométricos y resultados clínicos en el grupo de intervención ni en el grupo de control.
ConclusiónEste ensayo proporcionó algunas pruebas de que la administración de trehalosa mejoró la PCR y mejoró marginalmente algunos resultados clínicos, como el puntaje NUTRIC y la mortalidad a los 28 días en pacientes con LCT en la UCI.
Traumatic brain injury (TBI) is considered as a growing major global health problem.1 Worldwide, more than 69 million TBIs occur each year.2 TBI affects the health and productivity of individuals and imposes a heavy burden on the individual, their family and society.3 Mechanical tissue deformation, decreasing cerebral blood flow (CBF), and metabolic abnormalities occur at the moment of the injury. As a result, ischemia, cerebral edema, a rise in intracranial pressure (ICP), mitochondrial dysfunction, inflammation, a buildup of reactive oxygen (ROS), and cell death will ensue.4–6
Inflammation is one of the dominant mechanisms of secondary damage after head trauma.7 Dysfunction and altered permeability in BBB allows the circulating of monocytes, neutrophils, and lymphocytes recruited to the lesion.8 The diffuse brain inflammation intensifies neurodegeneration and will result in acute and chronic injury during a lifetime.9 In addition, inflammation has a pivotal role in the pathogenesis and recovery of TBI.10
Microglia is one of the first-line protection barriers between the injured and healthy domains in the brain. Whenever microglia are inappropriately activated, oxidative metabolites will be released.11 In addition, oxidative stress and neuroinflammation will result in secondary injury after TBI.12 Given this background, efforts to reduce oxidative stress and inflammatory markers in TBI patients are crucial.
In general, decreased immune processes will result in nutrition-related complications in the intensive care unit.13,14 This will increase mortality and ventilator dependence.15,16
Trehalose is a natural non-reducing disaccharide of glucose synthesized in all organisms, such as plants, bacteria, yeast, fungi, insects, and shrimp, except vertebrates.17 Several in vitro and in vivo studies have been undertaken to highlight the pharmacological activities of trehalose including induction of autophagy as well as reduction of inflammation and oxidative stress in various conditions.18–24 Many lines of evidence indicate that using trehalose in inflammatory conditions effectively suppresses inflammatory factors such as IL-6, IL-8, IL-1β, TNF-α, and MDA.25–28 Some evidence shows that trehalose can increase the level of antioxidant factors such as GSH in situations resulting in oxidative stress.28,29 Interestingly, there is major interest in the role of trehalose in neuroprotection in animal models of diverse neurodegenerative diseases, such as Huntington's and Parkinson's diseases.21,22,30,31 The dominant hypothesis that trehalose may exert neuroprotective effects is that trehalose induces autophagy, and another one is the influence of trehalose on the gut microbiome.19,32
In an animal study that investigated the effects of trehalose on the inflammatory sequelae associated with endotoxin shock in male rats, the results suggested that trehalose supplementation can be a safe approach for alleviating inflammation in critically ill patients.27 However, data on the clinical efficacy of trehalose administration have been scant.33–36 The aim of this pilot study was thus to explore in humans, for the first time, the potential effect of oral trehalose on inflammatory factors and oxidative stress in patients with head trauma in the intensive care unit. Therefore, a pilot RCT was designed to determine the feasibility of a full-scale trial.
MethodsStudy designThis study was a pilot randomized, prospective, and double-blind controlled clinical trial conducted at adult wards at the trauma referral ICU of Shahid Kamyab Hospital (Mashhad, Iran) from May 2022 to August 2022. As planned, 20 TBI patients were recruited. All participants fulfilled the inclusion criteria such as being aged 18–65 years old, having stable hemodynamic and metabolic conditions in the first 24–48h, Glasgow Coma Scale (GCS) ≥7, having enteral feeding, lack of intolerance to food sources of trehalose such as mushroom, and had signed written informed consent form by themself or legal guardian. Non-entry criteria included being “nil by mouth” for more than 48h, patients who are transferred from other ICUs after one week, having a history of cancer, autoimmune or congenital metabolic diseases, pregnancy and lactation. Exclusion criteria included requests to stop the study by patients’ legal guardians and having enteral nutrition for less than three days. Patients who met enrollment criteria were randomized (in quadruple blocks based on a blinded randomization list generated by an online tool for clinical trials: https://www.sealedenvelope.com) in 1:1 ratio to either oral trehalose (30g instead as a part of the daily carbohydrate of enteral feeding) or placebo groups (standard isocaloric hospital enteral feeding) for 12 days. The whole trehalose was not given in a single meal for safety assessment. Instead, we assessed nutritional and clinical conditions daily to recognize and manage adverse effects such as gastrointestinal discomfort. Primary and secondary endpoints were evaluated at the baseline and the end of the study. The institutional ethics committee approved the study (Ethics Code: IR.MUMS.MEDICAL.REC.1400.113).37
Sample size estimationThis is the first study of oral trehalose consumption in a new patient population and was conducted as a pilot study. Therefore, the sample size was computed to be 10 for each group (a total of 20 patients), and an approximate attrition rate was considered 10%.
OutcomesThe prespecified primary endpoints were the changes in inflammation and oxidative stress levels as measured by serum IL-6, CRP, PAB, SOD, GSH, and MDA. Secondary outcomes were a comparison of changes in GCS, APACHE II, NUTRIC and SOFA scores, blood glucose level, blood pressure, and 28-day and 60-day mortality.
MeasurementsDemographic information and anthropometric assessments, including height (by using ulna length) at baseline, weight (by using a bed scale Seca Medical 984), and mid-arm circumference (MAC), were measured at the beginning and the end of the study. One investigator conducted all assessments to reduce individual error. At the baseline and the end of the study, 10ml of the venous blood sample was collected and serum inflammatory and oxidative factors such as IL-6, CRP, SOD, GSH, MDA and PAB were analyzed by using ELISA kits (Kiazist, Iran). PAB was evaluated on a previously described method in a single assay.38 Furthermore, the Physiology and Chronic Health Evaluation II (APACHE II) score and Nutrition Risk in the Critically ill Score (NUTRIC score) at baseline and the end of the study, Sequential Organ Failure Assessment (SOFA) score and Glasgow Coma Scale (GCS) at every day will be filled out. Moreover, 28-day and 60-day mortality were evaluated using a telephone call or the patient hospital electronic file.
Statistical analysisStatistical analyses were performed using the SPSS Software (Version 22.0; Inc., Chicago, IL, USA). All analyses were assessed based on the intention-to-treat approach. Data were expressed as mean±SD and number (percentage) or median (25–75th interquartile range). Moreover, we used the chi-square test, Fisher's exact test, Mann–Whitney U test, and independent-sample t-test to compare the baseline variables between the two groups. A paired sample t-test or the Wilcoxon rank-sum test was applied to compare the changes in variables within a group. Also, the independent-sample t-test and Mann–Whitney U test for between-group comparisons was used. A P-value <0.05 was considered significant.
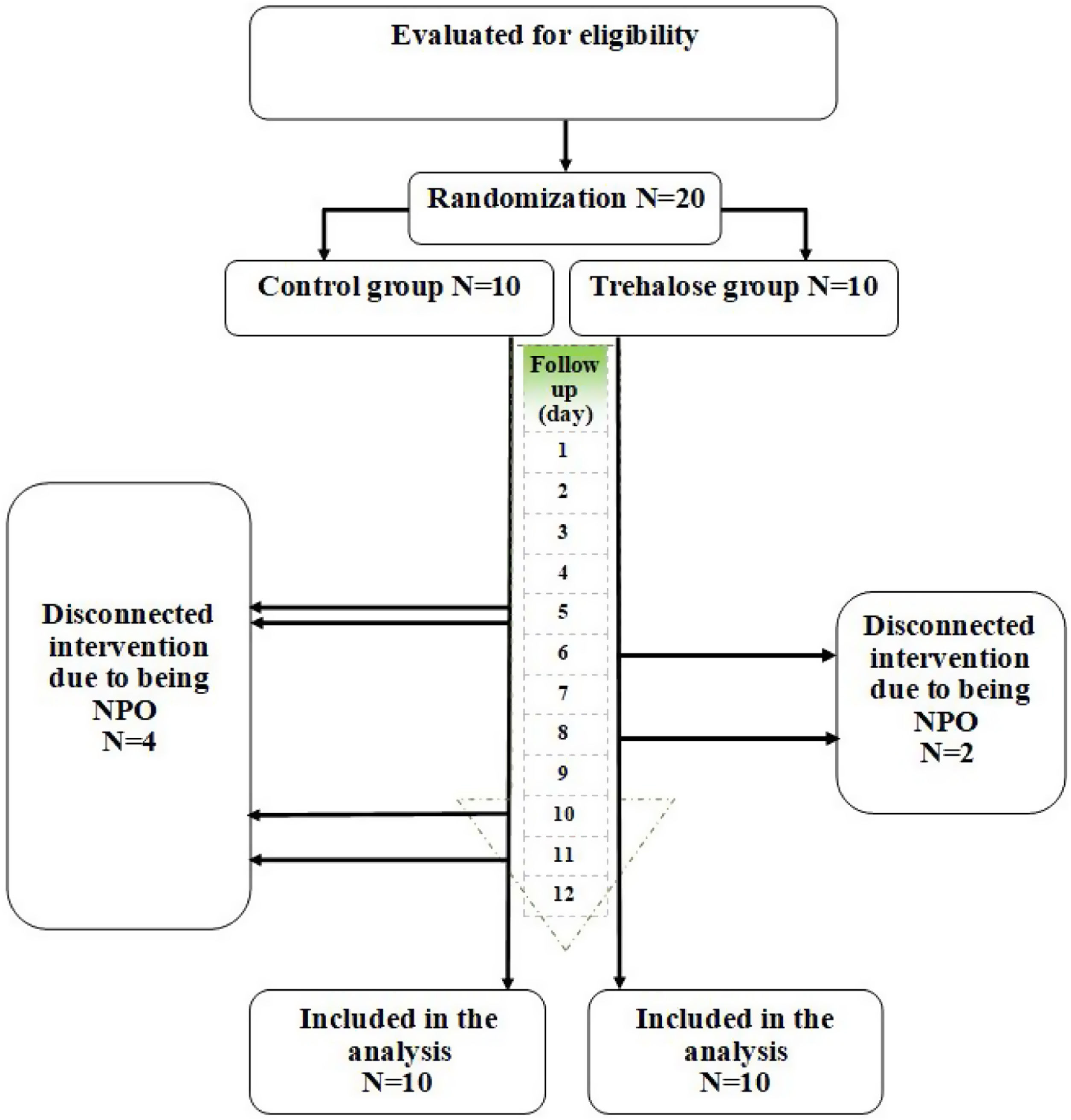
ResultsAs shown in Fig. 1, 20 patients with TBI were recruited in the current study, and all completed the study. Among the study participants in the intervention group, two people were excluded from the study on days 6 and 8 due to becoming NPO, and a total of 4 people from the control group were excluded from the study due to becoming NPO on days 5, 10 and 11. Therefore, data analysis was performed using the intention-to-treat method on 20 patients.
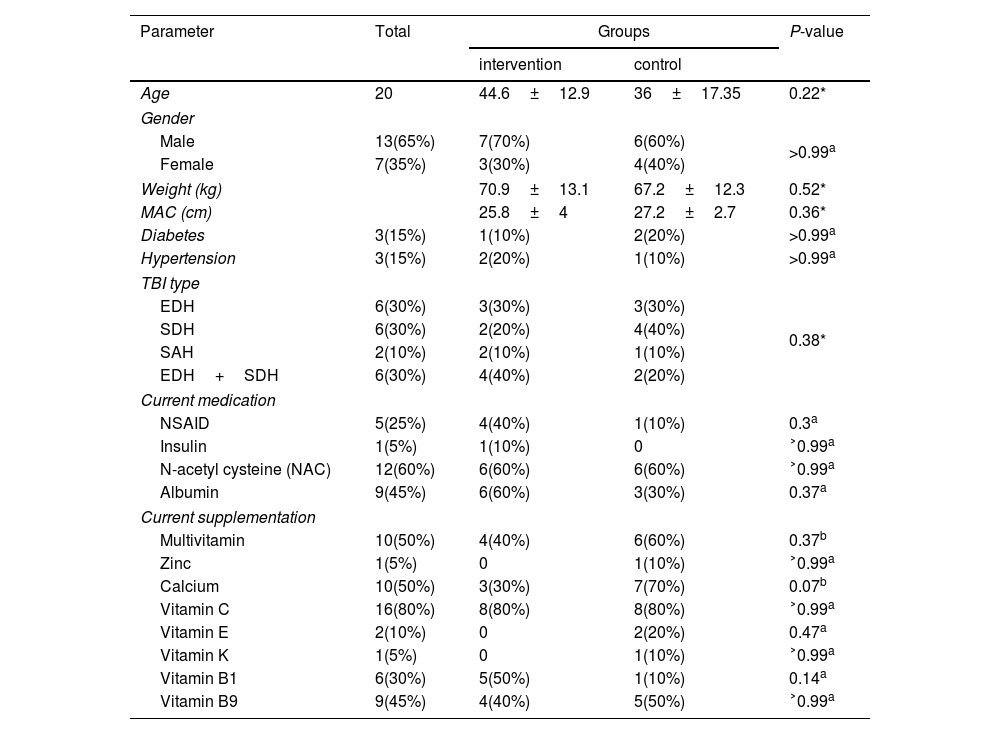
The baseline demographic and clinical characteristics of both groups are presented in Table 1. Notably, no adverse effects were reported following trehalose consumption throughout the study. In addition, there were no significant differences between the intervention and control groups at baseline regarding age, sex, weight, MAC, diabetes and hypertension, TBI type, current medication and supplementation except calcium.
Baseline characteristics of study patients.
| Parameter | Total | Groups | P-value | |
|---|---|---|---|---|
| intervention | control | |||
| Age | 20 | 44.6±12.9 | 36±17.35 | 0.22* |
| Gender | ||||
| Male | 13(65%) | 7(70%) | 6(60%) | >0.99a |
| Female | 7(35%) | 3(30%) | 4(40%) | |
| Weight (kg) | 70.9±13.1 | 67.2±12.3 | 0.52* | |
| MAC (cm) | 25.8±4 | 27.2±2.7 | 0.36* | |
| Diabetes | 3(15%) | 1(10%) | 2(20%) | >0.99a |
| Hypertension | 3(15%) | 2(20%) | 1(10%) | >0.99a |
| TBI type | ||||
| EDH | 6(30%) | 3(30%) | 3(30%) | 0.38* |
| SDH | 6(30%) | 2(20%) | 4(40%) | |
| SAH | 2(10%) | 2(10%) | 1(10%) | |
| EDH+SDH | 6(30%) | 4(40%) | 2(20%) | |
| Current medication | ||||
| NSAID | 5(25%) | 4(40%) | 1(10%) | 0.3a |
| Insulin | 1(5%) | 1(10%) | 0 | ˃0.99a |
| N-acetyl cysteine (NAC) | 12(60%) | 6(60%) | 6(60%) | ˃0.99a |
| Albumin | 9(45%) | 6(60%) | 3(30%) | 0.37a |
| Current supplementation | ||||
| Multivitamin | 10(50%) | 4(40%) | 6(60%) | 0.37b |
| Zinc | 1(5%) | 0 | 1(10%) | ˃0.99a |
| Calcium | 10(50%) | 3(30%) | 7(70%) | 0.07b |
| Vitamin C | 16(80%) | 8(80%) | 8(80%) | ˃0.99a |
| Vitamin E | 2(10%) | 0 | 2(20%) | 0.47a |
| Vitamin K | 1(5%) | 0 | 1(10%) | ˃0.99a |
| Vitamin B1 | 6(30%) | 5(50%) | 1(10%) | 0.14a |
| Vitamin B9 | 9(45%) | 4(40%) | 5(50%) | ˃0.99a |
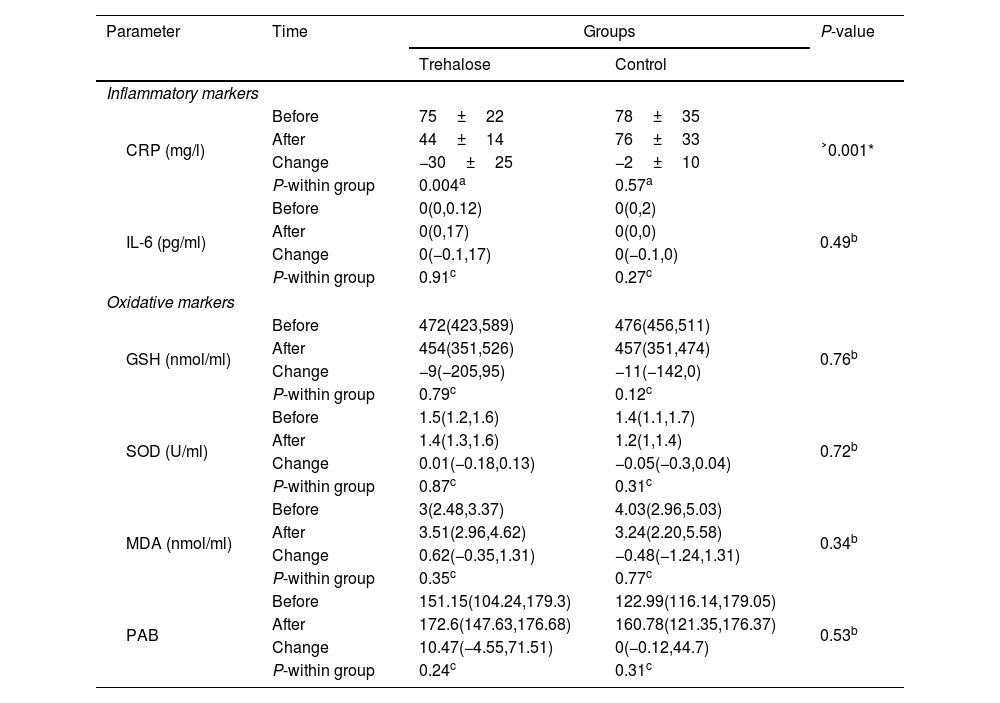
As summarized in Table 2, a significant reduction in CRP was found in the trehalose group (−30±25; P=0.004). We could not demonstrate any significant difference between the trehalose and control groups in changes of IL-6 [0(−0.1,17) vs. 0(−0.1,0); P=0.49]. No meaningful within- and between-group differences were found regarding GSH, SOD, MDA and PAB.
Changes in inflammatory and oxidative markers.
| Parameter | Time | Groups | P-value | |
|---|---|---|---|---|
| Trehalose | Control | |||
| Inflammatory markers | ||||
| CRP (mg/l) | Before | 75±22 | 78±35 | ˃0.001* |
| After | 44±14 | 76±33 | ||
| Change | −30±25 | −2±10 | ||
| P-within group | 0.004a | 0.57a | ||
| IL-6 (pg/ml) | Before | 0(0,0.12) | 0(0,2) | 0.49b |
| After | 0(0,17) | 0(0,0) | ||
| Change | 0(−0.1,17) | 0(−0.1,0) | ||
| P-within group | 0.91c | 0.27c | ||
| Oxidative markers | ||||
| GSH (nmol/ml) | Before | 472(423,589) | 476(456,511) | 0.76b |
| After | 454(351,526) | 457(351,474) | ||
| Change | −9(−205,95) | −11(−142,0) | ||
| P-within group | 0.79c | 0.12c | ||
| SOD (U/ml) | Before | 1.5(1.2,1.6) | 1.4(1.1,1.7) | 0.72b |
| After | 1.4(1.3,1.6) | 1.2(1,1.4) | ||
| Change | 0.01(−0.18,0.13) | −0.05(−0.3,0.04) | ||
| P-within group | 0.87c | 0.31c | ||
| MDA (nmol/ml) | Before | 3(2.48,3.37) | 4.03(2.96,5.03) | 0.34b |
| After | 3.51(2.96,4.62) | 3.24(2.20,5.58) | ||
| Change | 0.62(−0.35,1.31) | −0.48(−1.24,1.31) | ||
| P-within group | 0.35c | 0.77c | ||
| PAB | Before | 151.15(104.24,179.3) | 122.99(116.14,179.05) | 0.53b |
| After | 172.6(147.63,176.68) | 160.78(121.35,176.37) | ||
| Change | 10.47(−4.55,71.51) | 0(−0.12,44.7) | ||
| P-within group | 0.24c | 0.31c | ||
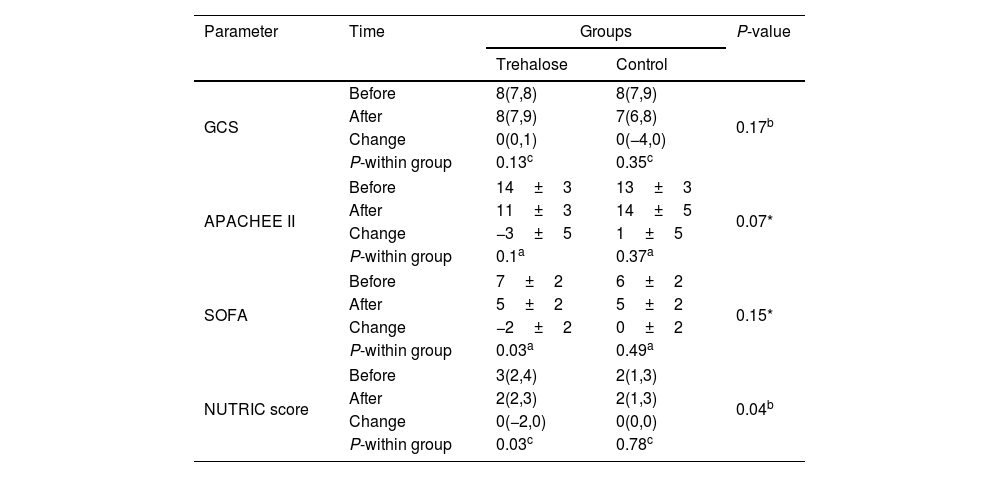
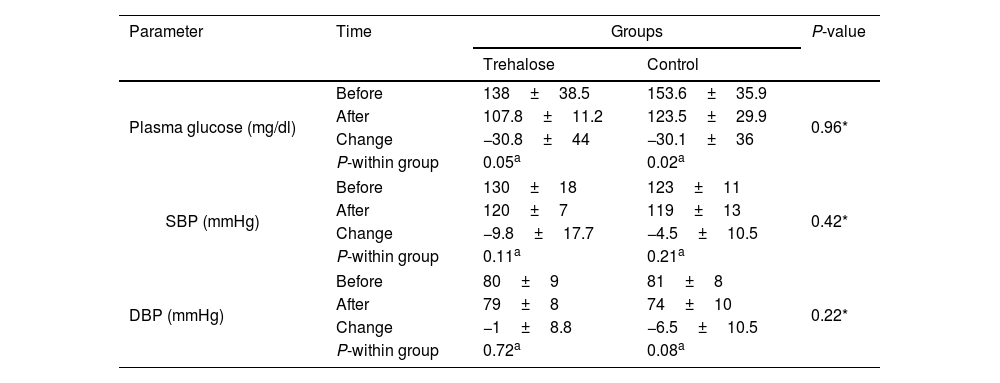
As summarized in Table 3, no meaningful within- and between-group difference was found in GCS [0(0,1) vs. 0(−4,0); P=0.17]. However, based on a within-group analysis, a meaningful decrease in the SOFA score was observed in the trehalose group at the end of the trial (−2±2; P=0.03). In addition, based on a between-group analysis, a marginal change was found in the APACHE II score (−3±5 vs. 1±5; P=0.07). Also, analyses reveal a significant difference between the trehalose and control groups in changes in NUTRIC score [0(−2,0) vs. 0(0,0); P=0.04]. In addition, a meaningful reduction of the NUTRIC score was observed in the trehalose group (P=0.03). As shown in Table 4, a significant decrease in the blood glucose was observed in both groups (−30.8±44; P=0.05 vs. −30.1±36; P=0.02), but the mean change in the blood glucose level was not significantly different between both groups (P=0.96). No meaningful change between-group differences were found in terms of SBP (−9.8±17.7 vs. −4.5±10.5; P=0.42) and DBP (−1±8.8 vs. −6.5±10.5; P=0.22). However, the within-group analysis revealed a meaningful change in DBP in the control group (P=0.08). There was no significant difference in the intervention group, compared to the placebo group [10(8,12) vs. 12(10,12); P=0.37] regarding the duration of mechanical ventilation.
Changes in clinical parameters.
| Parameter | Time | Groups | P-value | |
|---|---|---|---|---|
| Trehalose | Control | |||
| GCS | Before | 8(7,8) | 8(7,9) | 0.17b |
| After | 8(7,9) | 7(6,8) | ||
| Change | 0(0,1) | 0(−4,0) | ||
| P-within group | 0.13c | 0.35c | ||
| APACHEE II | Before | 14±3 | 13±3 | 0.07* |
| After | 11±3 | 14±5 | ||
| Change | −3±5 | 1±5 | ||
| P-within group | 0.1a | 0.37a | ||
| SOFA | Before | 7±2 | 6±2 | 0.15* |
| After | 5±2 | 5±2 | ||
| Change | −2±2 | 0±2 | ||
| P-within group | 0.03a | 0.49a | ||
| NUTRIC score | Before | 3(2,4) | 2(1,3) | 0.04b |
| After | 2(2,3) | 2(1,3) | ||
| Change | 0(−2,0) | 0(0,0) | ||
| P-within group | 0.03c | 0.78c | ||
Changes in blood glucose and blood pressure.
| Parameter | Time | Groups | P-value | |
|---|---|---|---|---|
| Trehalose | Control | |||
| Plasma glucose (mg/dl) | Before | 138±38.5 | 153.6±35.9 | 0.96* |
| After | 107.8±11.2 | 123.5±29.9 | ||
| Change | −30.8±44 | −30.1±36 | ||
| P-within group | 0.05a | 0.02a | ||
| SBP (mmHg) | Before | 130±18 | 123±11 | 0.42* |
| After | 120±7 | 119±13 | ||
| Change | −9.8±17.7 | −4.5±10.5 | ||
| P-within group | 0.11a | 0.21a | ||
| DBP (mmHg) | Before | 80±9 | 81±8 | 0.22* |
| After | 79±8 | 74±10 | ||
| Change | −1±8.8 | −6.5±10.5 | ||
| P-within group | 0.72a | 0.08a | ||
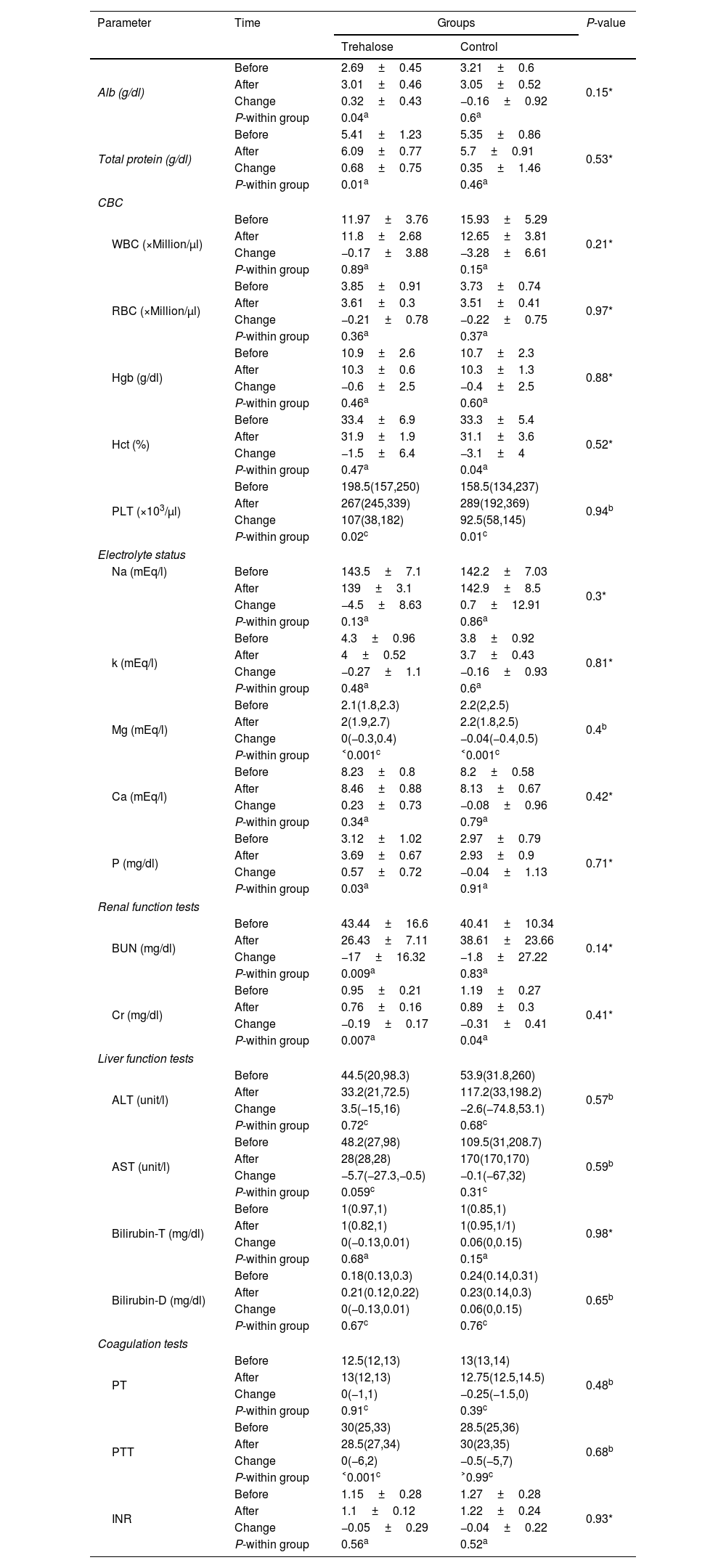
As presented in Table 5, hematological factors of the study patients, including the levels of WBC (−0.17±3.88; P=0.89 vs. −3.28±6.61; P=0.15), Hb (−0.6±2.5; P=0.46 vs. −0.4±2.5; P=0.6), and RBC (−0.21±0.78; P=0.36 vs. −0.22±0.75; P=0.37) did not significantly change neither in the study groups. However, hematocrit (HCT) has a significant reduction in the control group, based on a within-group analysis (−3.1±4; P=0.04). In addition, according to the within-group analysis, we observed a meaningful increase of platelet (PLT) in either group [107(38,182); P=0.02 vs. 92.5(58,145); P=0.01]. Furthermore, the within-group analysis revealed a significant change in Mg level in both groups [0(−0.3,0.4); P˂0.001 vs. −0.04(−0.4,0.5); P˂0.001], a meaningful increase in P level in trehalose group [0.35(3.3,3.9); P=0.04], a significant decrease in AST level in trehalose group [−5.7(−27.3,−0.5); P=0.05], a significant reduction in urea level in trehalose group [−17±16.32; P=0.009], a marginally decrease in Cr level in trehalose group (−0.19±0.17; P=0.007) and a meaningful decrease in the control group (−0.31±0.41; P=0.04), a significant change in Ptt in trehalose group [0(−6,2); P˂0.001], and a meaningful increase in Alb (0.32±0.43; P=0.04) and total protein level (0.68±0.75; P=0.01) in trehalose group. No other significant change was detected after treatment.
Changes in biochemical factors.
| Parameter | Time | Groups | P-value | |
|---|---|---|---|---|
| Trehalose | Control | |||
| Alb (g/dl) | Before | 2.69±0.45 | 3.21±0.6 | 0.15* |
| After | 3.01±0.46 | 3.05±0.52 | ||
| Change | 0.32±0.43 | −0.16±0.92 | ||
| P-within group | 0.04a | 0.6a | ||
| Total protein (g/dl) | Before | 5.41±1.23 | 5.35±0.86 | 0.53* |
| After | 6.09±0.77 | 5.7±0.91 | ||
| Change | 0.68±0.75 | 0.35±1.46 | ||
| P-within group | 0.01a | 0.46a | ||
| CBC | ||||
| WBC (×Million/μl) | Before | 11.97±3.76 | 15.93±5.29 | 0.21* |
| After | 11.8±2.68 | 12.65±3.81 | ||
| Change | −0.17±3.88 | −3.28±6.61 | ||
| P-within group | 0.89a | 0.15a | ||
| RBC (×Million/μl) | Before | 3.85±0.91 | 3.73±0.74 | 0.97* |
| After | 3.61±0.3 | 3.51±0.41 | ||
| Change | −0.21±0.78 | −0.22±0.75 | ||
| P-within group | 0.36a | 0.37a | ||
| Hgb (g/dl) | Before | 10.9±2.6 | 10.7±2.3 | 0.88* |
| After | 10.3±0.6 | 10.3±1.3 | ||
| Change | −0.6±2.5 | −0.4±2.5 | ||
| P-within group | 0.46a | 0.60a | ||
| Hct (%) | Before | 33.4±6.9 | 33.3±5.4 | 0.52* |
| After | 31.9±1.9 | 31.1±3.6 | ||
| Change | −1.5±6.4 | −3.1±4 | ||
| P-within group | 0.47a | 0.04a | ||
| PLT (×103/μl) | Before | 198.5(157,250) | 158.5(134,237) | 0.94b |
| After | 267(245,339) | 289(192,369) | ||
| Change | 107(38,182) | 92.5(58,145) | ||
| P-within group | 0.02c | 0.01c | ||
| Electrolyte status | ||||
| Na (mEq/l) | Before | 143.5±7.1 | 142.2±7.03 | 0.3* |
| After | 139±3.1 | 142.9±8.5 | ||
| Change | −4.5±8.63 | 0.7±12.91 | ||
| P-within group | 0.13a | 0.86a | ||
| k (mEq/l) | Before | 4.3±0.96 | 3.8±0.92 | 0.81* |
| After | 4±0.52 | 3.7±0.43 | ||
| Change | −0.27±1.1 | −0.16±0.93 | ||
| P-within group | 0.48a | 0.6a | ||
| Mg (mEq/l) | Before | 2.1(1.8,2.3) | 2.2(2,2.5) | 0.4b |
| After | 2(1.9,2.7) | 2.2(1.8,2.5) | ||
| Change | 0(−0.3,0.4) | −0.04(−0.4,0.5) | ||
| P-within group | ˂0.001c | ˂0.001c | ||
| Ca (mEq/l) | Before | 8.23±0.8 | 8.2±0.58 | 0.42* |
| After | 8.46±0.88 | 8.13±0.67 | ||
| Change | 0.23±0.73 | −0.08±0.96 | ||
| P-within group | 0.34a | 0.79a | ||
| P (mg/dl) | Before | 3.12±1.02 | 2.97±0.79 | 0.71* |
| After | 3.69±0.67 | 2.93±0.9 | ||
| Change | 0.57±0.72 | −0.04±1.13 | ||
| P-within group | 0.03a | 0.91a | ||
| Renal function tests | ||||
| BUN (mg/dl) | Before | 43.44±16.6 | 40.41±10.34 | 0.14* |
| After | 26.43±7.11 | 38.61±23.66 | ||
| Change | −17±16.32 | −1.8±27.22 | ||
| P-within group | 0.009a | 0.83a | ||
| Cr (mg/dl) | Before | 0.95±0.21 | 1.19±0.27 | 0.41* |
| After | 0.76±0.16 | 0.89±0.3 | ||
| Change | −0.19±0.17 | −0.31±0.41 | ||
| P-within group | 0.007a | 0.04a | ||
| Liver function tests | ||||
| ALT (unit/l) | Before | 44.5(20,98.3) | 53.9(31.8,260) | 0.57b |
| After | 33.2(21,72.5) | 117.2(33,198.2) | ||
| Change | 3.5(−15,16) | −2.6(−74.8,53.1) | ||
| P-within group | 0.72c | 0.68c | ||
| AST (unit/l) | Before | 48.2(27,98) | 109.5(31,208.7) | 0.59b |
| After | 28(28,28) | 170(170,170) | ||
| Change | −5.7(−27.3,−0.5) | −0.1(−67,32) | ||
| P-within group | 0.059c | 0.31c | ||
| Bilirubin-T (mg/dl) | Before | 1(0.97,1) | 1(0.85,1) | 0.98* |
| After | 1(0.82,1) | 1(0.95,1/1) | ||
| Change | 0(−0.13,0.01) | 0.06(0,0.15) | ||
| P-within group | 0.68a | 0.15a | ||
| Bilirubin-D (mg/dl) | Before | 0.18(0.13,0.3) | 0.24(0.14,0.31) | 0.65b |
| After | 0.21(0.12,0.22) | 0.23(0.14,0.3) | ||
| Change | 0(−0.13,0.01) | 0.06(0,0.15) | ||
| P-within group | 0.67c | 0.76c | ||
| Coagulation tests | ||||
| PT | Before | 12.5(12,13) | 13(13,14) | 0.48b |
| After | 13(12,13) | 12.75(12.5,14.5) | ||
| Change | 0(−1,1) | −0.25(−1.5,0) | ||
| P-within group | 0.91c | 0.39c | ||
| PTT | Before | 30(25,33) | 28.5(25,36) | 0.68b |
| After | 28.5(27,34) | 30(23,35) | ||
| Change | 0(−6,2) | −0.5(−5,7) | ||
| P-within group | ˂0.001c | ˃0.99c | ||
| INR | Before | 1.15±0.28 | 1.27±0.28 | 0.93* |
| After | 1.1±0.12 | 1.22±0.24 | ||
| Change | −0.05±0.29 | −0.04±0.22 | ||
| P-within group | 0.56a | 0.52a | ||
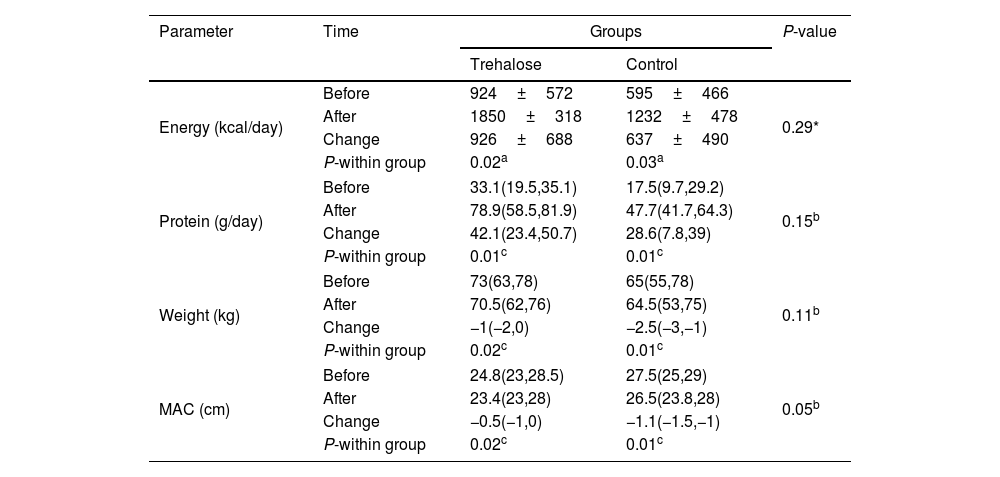
Table 6 shows a meaningful change in energy intake in both groups (926±688; P=0.02 vs. 637±490; P=0.03). Also, based on within-group analysis, a significant increase in protein intake in both groups was observed [42.1(23.4,50.7); P=0.01 vs. 28.6(7.8,39), P=0.01]. Additionally, we could demonstrate a significant weight change [−1(−2,0); P=0.02 vs. −2.5(−3,−1); P=0.01] based on within-group analysis and MAC [−0.5(−1,0) vs. −1.1(−1.5,−1); P=0.05] found on between and within-group analysis.
Changes in nutritional factors.
| Parameter | Time | Groups | P-value | |
|---|---|---|---|---|
| Trehalose | Control | |||
| Energy (kcal/day) | Before | 924±572 | 595±466 | 0.29* |
| After | 1850±318 | 1232±478 | ||
| Change | 926±688 | 637±490 | ||
| P-within group | 0.02a | 0.03a | ||
| Protein (g/day) | Before | 33.1(19.5,35.1) | 17.5(9.7,29.2) | 0.15b |
| After | 78.9(58.5,81.9) | 47.7(41.7,64.3) | ||
| Change | 42.1(23.4,50.7) | 28.6(7.8,39) | ||
| P-within group | 0.01c | 0.01c | ||
| Weight (kg) | Before | 73(63,78) | 65(55,78) | 0.11b |
| After | 70.5(62,76) | 64.5(53,75) | ||
| Change | −1(−2,0) | −2.5(−3,−1) | ||
| P-within group | 0.02c | 0.01c | ||
| MAC (cm) | Before | 24.8(23,28.5) | 27.5(25,29) | 0.05b |
| After | 23.4(23,28) | 26.5(23.8,28) | ||
| Change | −0.5(−1,0) | −1.1(−1.5,−1) | ||
| P-within group | 0.02c | 0.01c | ||
ICU 28-day mortality was marginally different in the intervention group compared to the placebo group (0 vs. 30%; P=0.06 number needed to treat {NNT}=3). In this regard, ICU 60-day mortality was not significantly different in the intervention group compared to the placebo group (10% vs. 40%; P=0.13 number needed to treat {NNT}=3).
SafetyThe safety profile of oral trehalose was evaluated as excellent since no side effects related to its use with the method presented in our study occurred.
DiscussionThis is the first pilot-scale, randomized, double-blinded controlled trial to evaluate the potential effect of oral trehalose administration on inflammatory and oxidative stress biomarkers, clinical outcome, and nutritional factors in TBI patients in the intensive care unit. The current trial indicated that consumption of 30g oral trehalose instead as a part of daily carbohydrate in enteral feeding for 12 days could significantly reduce CRP and blood glucose, AST, urea, and SOFA score in TBI patients at ICU. In addition, a marginally significant decrease in Cr, APACHE II score and 28-day mortality, a significant increase in Alb, total protein, and energy and protein intake, a marginally significant increase in platelet (PLT), and a significant change in MAC, weight, Ptt, Mg, NUTRIC score was observed with oral trehalose administration in TBI patients at ICU. Moreover, no significant differences could be found in the IL-6, GSH, SOD, MDA, PAB, GCS, SBP, DBP, dependence on a ventilator, and 60-day mortality between the two groups after oral trehalose administration in TBI patients at ICU.
Contrary to our results, a pilot clinical trial investigating the effects of intravenous trehalose administration in the change of arterial wall inflammation in 15 patients with a history of myocardial infarction with the weekly received of 15g intravenous trehalose for 12 weeks led to non-significant differences in the serum concentrations of hs-CRP.39 This inconsistency between our finding and the result of mentioned trial may be related to the different routes of administration (oral and intravenous), different dosages of trehalose, dissimilarity of duration, or the difference in the severity of critically ill between the two studies. Moreover, the differences between the biomarkers that evaluated the inflammation in these two studies could be the reason for inconsistent results. In an in vitro study investigating the effects of various concentrations of trehalose in the LPS-stimulated human peripheral blood mononuclear cells from Six healthy donors, it was reported trehalose in 500mM concentration could prevent inflammation by significantly reduce in IL-6 level.33 Furthermore, another in vitro study was conducted to evaluate the effect of different concentrations of trehalose against inflammation in human corneal epithelial cells exposed to hyperosmotic stress. It showed trehalose in 450mOsM concentrations significantly deduced IL-6 levels.40 In an animal model study, male rats received trehalose (1g/kg) before and 2h after the LPS challenge for inducing endotoxic shock. The results show that trehalose inhibited the inflammatory stream by a significant reduction in IL-6 level. Moreover, trehalose administration significantly decreased MDA levels. Furthermore, this study observed a significant reduction in blood alanine aminotransferase (ALT) levels.41 In an in vivo experiment, mice were pretreated with a daily single intraperitoneal injection of trehalose (2g/kg) from 3 days before renal IR surgery. The results showed that trehalose treatment could inhibit inflammation and oxidative stress by markedly reducing MDA, IL-6, and blunted SOD levels decline.28 As well as in a study, the trehalose application has a promising role in treating CHIP-mutation-related ataxia and could increase GSH free radical levels.42 Our results showed the GCS was not significantly different between the two study groups. However, the differences in APACHE II scores between the two intervention groups were marginally significant, indicating that using trehalose can effectively reduce mortality risk in traumatic brain injury patients. Considering the lower nutritional adequacy, the lower NUTRIC score is calculated. Since the NUTRIC score is related to the increase in mortality, it can be concluded the improvement of the NUTRIC score in the intervention group has been related to the reduction of 28-day mortality.43
The present clinical trial showed non-significant weight changes between the two groups. However, the weight reduction in the intervention group was lower than control. Moreover, despite the decrease in MAC in both groups, the control group shows a two-fold reduction compared to the trehalose group. The comparison of these changes between the two groups is marginally significant. Previous studies have shown that frequent episodes of hyperglycemia in patients with head trauma in the intensive care unit have been associated with a higher risk of death and a longer hospital stay.44,45 In this regard, our result demonstrated the non-significant changes between the two study groups, which is not in line with previous studies conducted on animals and human beings. The contradiction between the results of our study and previous studies can be justified by the use of different tools, methods of evaluation, the type of target group, and the difference in the study design. Supplementation with trehalose in this study marginally reduced 28-day mortality. Additionally, it has been shown that one unit reduction in 28-day mortality is expected for every three patients with traumatic brain injury hospitalized in the intensive care unit who receive 30g of oral trehalose supplement. Also, for every three patients with traumatic brain injury hospitalized in the intensive care unit who receive 30g of oral trehalose supplement, one unit reduction in 60-day mortality is expected. Common adverse effects of trehalose administration, such as loose stools and abdominal bloating, were not observed in any of the patients in our two study groups.46 Splitting doses over time has increased the digestive system's ability, as we did in our study.47 In addition, it has been shown that when disaccharides (trehalose) are used in a meal (not as a drink or a separate meal from the main meals) digested for a longer period of time.48 Our results were similar to the results of Bolte et al. in that 60 German people without gastrointestinal disease were given a dose of 50g of trehalose in 400ml of water, and none of the patients showed signs of intolerance (diarrhea and flatulence).49
The strength of our study is that it is the first RCT to evaluate the effect of trehalose in acute conditions and critically ill patients. Moreover, the impact of trehalose on some inflammatory and oxidative stress biomarkers and clinical outcomes was investigated for the first time. However, due to limited funding, we could not assess the effect of trehalose consumption on other inflammatory and oxidative stress biomarkers. Also, some variables, such as the use of medications, supplements and dietary intake, which were not controllable, may affect the outcomes. However, these variables were also adjusted in statistical analysis as covariates.
ConclusionsIn conclusion, our study showed that trehalose administration improved CRP and marginally improved some clinical outcomes, such as NUTRIC score and 28-day mortality in TBI patients in ICU. However, its effect on other favorable biomarkers was not proven. Future well-conducted studies with a longer intervention duration and larger sample sizes provide more precise results.
Conflict of interestThe authors declare that they have no conflict of interest.