Epidermodysplasia verruciformis (EV) is an autosomal recessive disease of the skin commonly associated with EVER1 and EVER 2 mutations, and is characterized by high susceptibility to infections associated with certain types of human papillomavirus called EV-PVH. Patients have warty lesions on the skin of varying characteristics and are often associated with skin cancer, with a strong association being found with EVER1 and EVER2 mutation gene. The case presented below concerns an absolute CD4+ lymphopenia, and establishes the hypothesis of a possible mutation of the RHOH gene as its origin.
La epidermodisplasia verruciforme (EV) es una enfermedad de la piel autosómica recesiva, relacionada con la mutación EVER1 y EVER2, caracterizada por alta susceptibilidad a infecciones asociadas a ciertos tipos de papillomavirus humano llamadas EV-PVH. Los pacientes presentan lesiones verrucosas en la piel de características variadas y muchas veces asociadas a cáncer de piel no melanocítico, encontrándose una fuerte asociación con la mutación del gen EVER1 y EVER2. El caso que se presenta a continuación documenta linfopenia absoluta de CD4+ por lo que se plantea la hipótesis de una posible mutación del gen RHOH como etiología.
Epidermodysplasia verruciformis (EV) is an autosomal recessive disease of the skin with a prevalence lower than one per million inhabitants; it was initially described by Lewandowsky and Lutz in 1922. It is characterized by a high susceptibility to infections associated with certain types of human papillomavirus (HPV) called EV-HPV, which may not occur in the general population, since they do not integrate to the genome due to the absence of the E5 gene.1,2
Although its pathogenesis is not completely defined, it is known that the combination of genetic factors that lead to a selective immunodeficiency, mainly cellular, are responsible for the disease.3,4
The groups of individuals in whom this condition is most frequently described are patients with infection with the human immunodeficiency virus (HIV) or transplant recipients.5,6 There are also primary familial forms due to autosomal recessive mutations, much less frequent.3,7,8
Patients with EV-HPV have an increased risk for developing non-melanoma skin cancer, especially in areas exposed to the sun, during the second and third decades of life, mainly those associated with serotypes 5 and 8 of HPV.9,10
In the group of patients with EV-HPV in its primary form, are described mutations of the TMC (EVER 1 and 2) genes and more recently, homozygous mutations of the RHOH gene, which condition alterations in the signaling of the T cell receptors that predispose to other opportunistic infectious diseases such as Mycobacteria, Cryptococcus, Pneumocystis jirovecii (P. jirovecii), cytomegalovirus, among others. Their presentation varies according to the CD4 counts.7,8,11
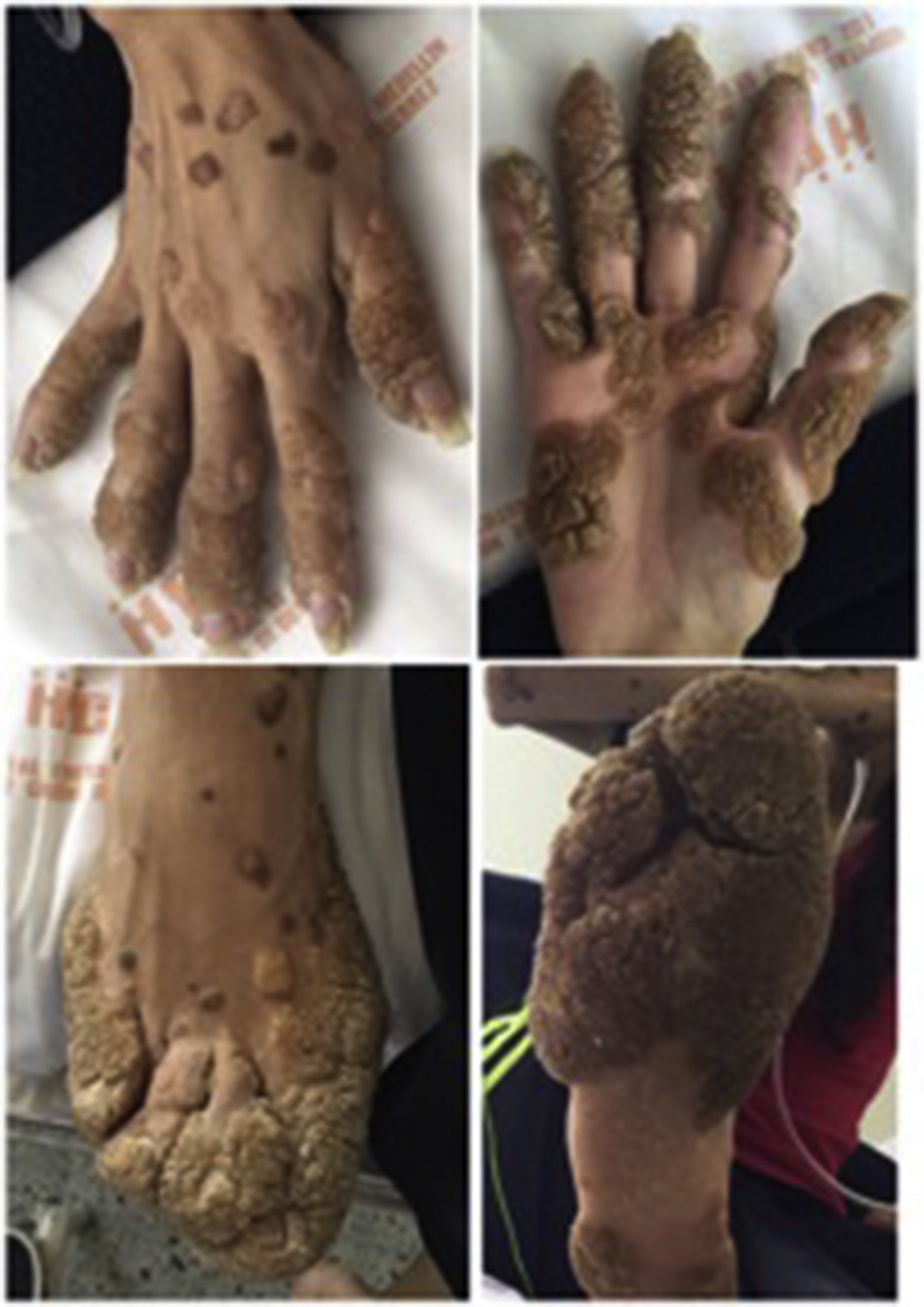
Clinical caseA 27-year-old woman, with a history of vulgar warts on the fingers and toes, lung disease defined as asthma in childhood, who had consulted to emergencies since the age of 12 years due to recurrent infectious respiratory clinical pictures (Fig. 1). As an important family antecedent, is recorded the death of her older brother due to undiagnosed lung disease at the age of 20 years.
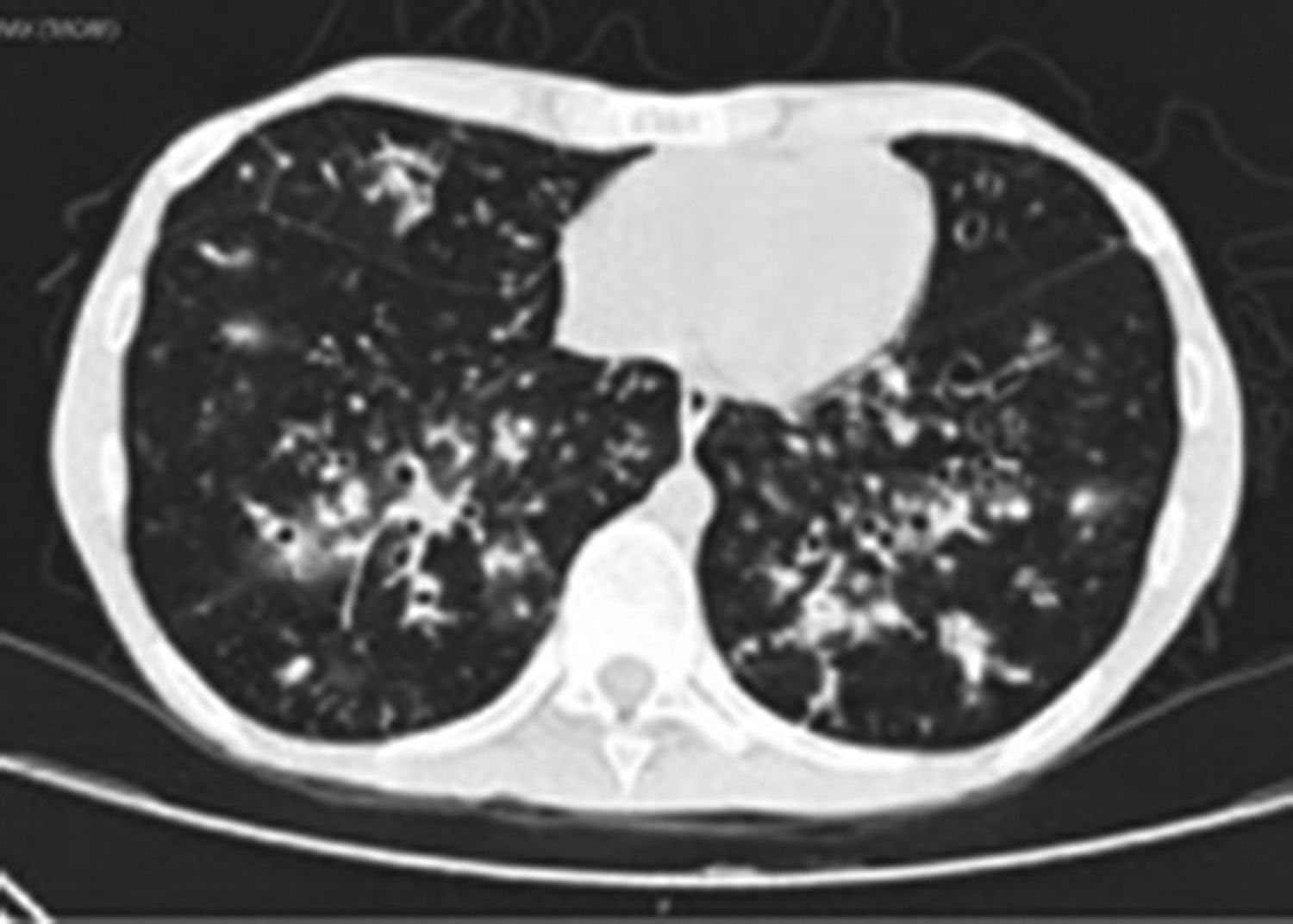
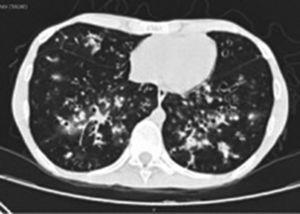
In the last hospitalization, the patient was admitted again to the general hospital of Medellin with a clinical picture suggestive of community-acquired pneumonia, with a high resolution tomographic image (Fig. 2) showing infiltrates of reticulonodular predominance.
Antibiotic management with cefepime and fibrobronchoscopy with alveolar lavage were carried out, but without microbiological isolation. The patient evolved satisfactorily after taking antibiotics for 7 days; however, she had persistent hypoxemia for which supplementary oxygen and outpatient pulmonary function testing were ordered. A spirometry documented a severe obstructive pattern, lung volumes that ruled out restrictive alterations, with a severe alveolar damage by a carbon monoxide diffusion test; but with an acceptable functional class, with a 6min walk test greater than 300m.
The diagnosis of Epidermodysplasia verruciformis was confirmed in the dermatology board by finding on the skin biopsy hyperkeratosis of basket-like tissue, mild acanthosis and vacuolated cells in the upper epidermis, management with topical retinoids was indicated given the advanced stage of her disease and the morbidities that other therapies such as cryotherapy could cause.
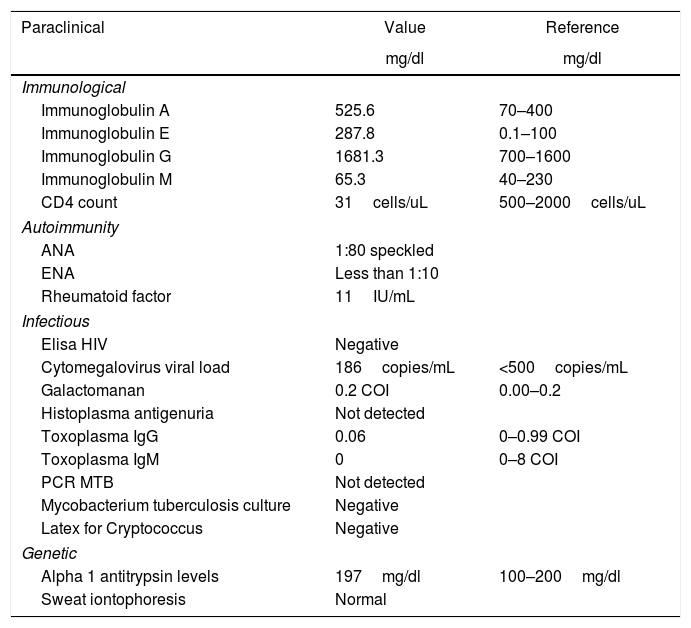
Autoimmunity studies that would explain the lung damage were performed, being all negative. With a negative Elisa test for HIV and normal levels of alpha 1 antitrypsin and sweat iontophoresis (Table 1).
Summary of laboratory tests.
| Paraclinical | Value | Reference |
|---|---|---|
| mg/dl | mg/dl | |
| Immunological | ||
| Immunoglobulin A | 525.6 | 70–400 |
| Immunoglobulin E | 287.8 | 0.1–100 |
| Immunoglobulin G | 1681.3 | 700–1600 |
| Immunoglobulin M | 65.3 | 40–230 |
| CD4 count | 31cells/uL | 500–2000cells/uL |
| Autoimmunity | ||
| ANA | 1:80 speckled | |
| ENA | Less than 1:10 | |
| Rheumatoid factor | 11IU/mL | |
| Infectious | ||
| Elisa HIV | Negative | |
| Cytomegalovirus viral load | 186copies/mL | <500copies/mL |
| Galactomanan | 0.2 COI | 0.00–0.2 |
| Histoplasma antigenuria | Not detected | |
| Toxoplasma IgG | 0.06 | 0–0.99 COI |
| Toxoplasma IgM | 0 | 0–8 COI |
| PCR MTB | Not detected | |
| Mycobacterium tuberculosis culture | Negative | |
| Latex for Cryptococcus | Negative | |
| Genetic | ||
| Alpha 1 antitrypsin levels | 197mg/dl | 100–200mg/dl |
| Sweat iontophoresis | Normal | |
ANA: antinuclear antibodies; ENA: antibodies against extractable nuclear antigens; MTB: Mycobacterium tuberculosis; HIV: human immunodeficiency virus.
Two months after discharge the patient re-entered with the same clinical picture. Empiric antibiotic management was started, and a new chest tomography with contrast was taken, evidencing the changes described above. New bronchoalveolar lavage without microbiological isolation. The culture of mycobacteria from the previous hospitalization was reported negative. In this occasion was performed a transbronchial lung biopsy that showed changes of moderate chronic inflammation and areas with foamy exudates and anthracosis without mention of cytopatic changes or inclusion bodies compatible with cytomegalovirus.
It was carried out a new Elisa test for HIV, which was negative, and therefore were performed immunological tests, such as levels of immunoglobulins which were normal and CD4 cell count reported as 31cells/uL. With a new CD4 cell count, once stabilized, of 171cells/uL, a finding that allowed us to define a primary immunodeficiency. Complementary tests for opportunistic infections were performed, which were reported as negative.
Given the clinical and radiological findings, associated with hypoxemia and low CD4, empirical treatment with trimethoprim sulfamethoxazole was started, with clinical improvement. The patient was discharged with prophylaxis for P. jirovecii until reaching CD4 counts higher than 200.
DiscussionEV is a rare clinical condition, whose main manifestation is the involvement of the skin by vulgar warts associated with HPV.3 However, we documented in this patient a quantitative decrease in CD4+ cell counts associated with recurrent lung infections, which have led to the development of a severe structural lung damage with emphysema and cylindrical bronchiectasis associated with indirect signs of infection with P. jirovecii, with good response to empiric treatment.
The most frequent mutations in EV occur in the EVER1 and EVER2 genes, also called TMC6 and TMC8 in the 17q25 region, in up to 75% of cases,12,13 which are closely linked to the cutaneous manifestations of HPV, as is the case of our patient, in addition to the higher incidence of non-melanoma cancer.14,15 However, in this reported case, the infectious pulmonary pathology has determined the clinical evolution and even more important is the finding of absolute decrease of CD4+ in a context not associated with HIV; finding in this group of patients with cutaneous manifestations and recurrent infections a higher frequency of mutations in the RHOH genes, which encode a G protein with a defect in the GTPase activity that is expressed in hematopoietic cells, which implies the alteration in the activation of integrins, differentiation and activation of T cells, favoring the quantitative deficiency of T cells, in this case of CD4+.7,16
Other possible causes of deficiency of CD4+ lymphocytes such as CMV infection by cut-off points lower than 2600copies/mL,17 infection with HIV or chronic infection with hepatotropic viruses were ruled out. Likewise, possible primary autoimmune or hematological etiologies were discarded as an explanation of the absolute deficiency or T cells.
That is how we finally considered that the manifestation of the EV-HPV in this patient could be secondary to a quantitative cell alteration of the CD4s, possibly associated with mutations of the RHOH gene, rather than a predisposition to the growth of the HPV linked to mutations of the EVER1-2 gene, in the presence of cellular immunological competence as it is classically described in the pathophysiology of EV; although its genetic characterization was not possible, this hypothesis is proposed. From the pathophysiological basis, the possible beneficial effect of the allogeneic bone marrow transplantation in symptomatic primary immunodeficiencies of CD4 is proposed as a research topic8–20 and the prophylaxis for major opportunistic germs such as P. jirovecii, among others is proposed as the basic treatment.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Díaz Coronado JC, Soto Osorio MC, Sánchez Jaramillo JS. Epidermodisplasia verruciforme asociada a linfopenia idiopática de CD4+. Rev Colomb Reumatol. 2017;24:254–258.