MPM stands as a rare malignancy necessitating improved therapeutic strategies due to its limited treatment choices and unfavorable prognosis. The advent of immune checkpoint inhibitors has heralded a paradigm shift in the therapeutic landscape of MPM, offering promising avenues across diverse clinical scenarios. In the context of advanced stages of the disease, Immune check-point inhibitors targeting programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-as-sociated protein 4 (CTLA-4), have exhibited encouraging potential in clinical trials, particularly manifesting efficacy among patients exhibiting disease progression following chemotherapy regimens. Innovative combination regimens, exemplified by the concurrent administration of nivolumab and ipilimumab, have demonstrated marked improvement in survival and patient's benefits. A deeper comprehension of the intricate genetic underpinnings of MPM, encompassing key mutations such as cyclin-dependent kinase inhibitor 2A (CDKN2A), neurofibromin 2 (NF2), and BRCA1-associated protein 1 (BAP1) mutations, has elucidated novel avenues for targeted therapeutic interventions. This review accentuates the transformative capacity of immunotherapy in revolutionizing the therapeutic outlook for MPM, thereby potentially translating into augmented survival rates and offering glimpses of new approaches on the horizon. Despite the persisting challenges, the synergistic crossroads of interdisciplinary research and collaborative clinical endeavors portend a hopeful landscape for MPM treatment.
El mesotelioma pleural maligno (MPM) es una neoplasia poco frecuente que requiere una mejora de las estrategias terapéuticas debido a sus limitadas opciones de tratamiento y a su pronóstico desfavorable. La llegada de los inhibidores de los puntos de control inmunitario ha supuesto un cambio de paradigma en el panorama terapéutico del MPM, ofreciendo vías prometedoras en diversos escenarios clínicos. En el contexto de los estadios avanzados de la enfermedad, los inhibidores de puntos de control inmunitario dirigidos contra la proteína de muerte celular programada 1 (PD-1) y la proteína4 asociada a los linfocitos T citotóxicos (CTLA-4) han mostrado un potencial alentador en los ensayos clínicos, sobre todo por su eficacia en los pacientes con progresión de la enfermedad tras los regímenes de quimioterapia. Los regímenes combinados innovadores, ejemplificados por la administración concurrente de nivolumab e ipilimumab, han demostrado una mejora significativa de la supervivencia y de los beneficios para los pacientes. Una comprensión más profunda de los complejos fundamentos genéticos del MPM, que abarca mutaciones clave como el inhibidor de la cinasa dependiente de ciclina 2A (CDKN2A), la neurofibromina 2 (NF2) y las mutaciones de la proteína 1 asociada a BRCA1 (BAP1), ha dilucidado nuevas vías para el desarrollo de intervenciones terapéuticas dirigidas. Esta revisión acentúa la capacidad transformadora de la inmunoterapia para revolucionar las perspectivas terapéuticas en el MPM, lo que podría traducirse en un aumento de las tasas de supervivencia y ofrecer nuevos enfoques terapéuticos en el horizonte próximo. A pesar de los retos persistentes, el cruce sinérgico de la investigación interdisciplinar y los esfuerzos clínicos de colaboración auguran un panorama esperanzador en el tratamiento de los MPM.
Malignant pleural mesothelioma (MPM) stands as an aggressive neoplasm arising from mesothelial cells that constitute the serous lining of the pleural cavity. Presently, it is regarded as a rare malignancy, with an approximate incidence of 1.83 cases per 100,000 individuals per year.1 Classification of MPM can be done into three distinct subtypes based on histological morphology: epithelioid, sarcomatoid, or biphasic.
In the contemporary scenario, all forms of asbestos are classified as Class 1 carcinogens by the International Agency for Research on Cancer. A well-established causal relationship between asbestos exposure and MPM development exists, albeit with an average latency period of around 40 years.2 While 85% of mesotheliomas can be attributed to occupational asbestos exposure, merely 10% of those exposed individuals will eventually develop MPM. Moreover, asbestos exposure is also linked to lung cancer, and the combination of smoking and exposure escalates the risk of lung cancer development by 10–100 times compared to non-exposed individuals.3 Despite asbestos being banned in many countries, its extraction and commercialization persist as latent issues, particularly in emerging economies, sustaining the global incidence of exposure.1 This, in turn, portends an exponential surge in mesothelioma cases in these regions due to its unregulated utilization.4
The median age at MPM diagnosis hovers around 70–75 years, displaying a male-to-female ratio of 3:1.5 According to Surveillance, Epidemiology, and End Results (SEER) data, the median reported survival rates for these patients range between 8 and 12 months in recent decades,6 and between 12 and 18 months in the context of the latest clinical trials.7 Estimated global survival rates at 1 and 3 years stand at 40% and 10%, respectively.8 As per the American Cancer Society, around 40,000 individuals succumb annually worldwide due to this pathology.9 Factors predicting outcomes include tumor stage at presentation, functional status, and response to cytostatic treatment.10 This data underscores the critical significance of early detection and optimized therapeutic interventions in enhancing patient prognosis.
In this ever-evolving landscape of mesothelioma, where meticulous clinical evaluation meets cutting-edge research, novel approaches are being harnessed to ameliorate patient outcomes and provide hope for those battling this complex ailment. Continued investigation, collaboration, and interdisciplinary efforts will undoubtedly illuminate new avenues for tackling the challenges posed by malignant pleural mesothelioma.
Immune checkpoint inhibitors (CPIs)The scrutiny of mutational landscapes within MPM has assumed pronounced significance, serving as a pivotal tool to unearth genetic aberrations or mutational changes. Specifically, the integration of Immune Checkpoint Inhibitors (CPIs) has entrenched itself as a promising therapeutic strategy for these patients.1
Recent years have witnessed a revolution in the management of diverse tumor types through the utilization of CPIs. The fundamental premise underlying immunotherapy involves harnessing the host's immune system to wage a concerted assault against malignant cells. Current investigations in the realm of CPIs encompass not only those targeting T-cell responses or activating T-cell pathways but also the utilization of cytokines such as IL-12 and IL-15, therapeutic vaccines, ablation of immunosuppressive cells, and modulation of other constituents of the immune cascade.11
In the specific context of MPM, a range of promising CPIs have surfaced. On one front stands CTLA-4 (Cytotoxic T-lymphocyte-associated protein 4), a receptor located on the surface of T-cells. Its pivotal role hinges on immune response regulation, achieved by curbing T-cell activation. CTLA-4 interacts with specific ligands on other immune cells, such as antigen-presenting cells, transmitting inhibitory signals that curtail T-cell activity. This finely orchestrated equilibrium sustains immune response balance and averts autoimmunity.12 Empirical evidence underscores augmented suppression of tumor growth upon administration of monoclonal anti-CTLA4 antibodies in murine MPM models.13
On another axis emerges PD-1 (programmed cell death protein 1), expressed on the surface of T-cells as well as other immune constituents like B-cells and dendritic cells. During immune response activation, PD-1 forms interactions with its ligands PD-L1 (programmed death-ligand 1) and PD-L2 (programmed death-ligand 2), orchestrating a cascade that transmits inhibitory signals back to the T-cell itself.14 Notably, heightened PD-L1 expression has been documented in MPM,15 with this expression being subject to variations contingent upon histological subtypes. Notably, this elevated expression extends to 21% of epithelial subtypes, 94% of sarcomatoid subtypes, and 57% of biphasic subtypes.16
The elucidation of these multifaceted interactions between CPIs, immune microenvironment, and the unique cellular terrain of MPM shines a light on the promising prospects of leveraging these mechanisms in the quest to refine therapeutic approaches and ultimately augment patient outcomes.
Immunogenicity of MPMThe comprehension of cytogenetic and molecular facets in mesothelioma carries a profound complexity, primarily attributed to the infrequent occurrence of genetic alterations and the substantial heterogeneity across patients.17–19 Nonetheless, high-throughput analyses have revolutionized molecular characterization, unveiling a deeper understanding of its biological intricacies and the potential therapeutic targets within MPM.
While mutations in specific genes remain relatively rare, noteworthy changes have been identified in pivotal genetic pathways, including those encoding the cyclin-dependent kinase inhibitor 2A (CDKN2A), neurofibromin 2 (NF2), and the BRCA1-associated protein 1 (BAP1).20–22 Unlike the majority of other cancers, TP53 mutations are infrequent in mesothelioma, occurring in less than 10% of cases17,23; however, when isolated, these mutations correspond to a poorer survival rate.24
In approximately 45% of mesothelioma cases, CDKN2A is eradicated, encoding two cell cycle regulators: p16INK4a, which inhibits the cyclin-dependent kinase 4 and 6 phosphorylation of the retinoblastoma protein (RB); and p14ARF, preventing p53 degradation by Mdm2.
Notably, germline mutations in BAP1 predispose individuals to malignant pleural mesothelioma.25 BAP1 plays a pivotal role as a regulator of gene-environment interactions.26 The loss of BAP1 amplifies the susceptibility of fibroblasts and mesothelial cells to external agents like asbestos, thereby fostering mesothelioma development. Intriguingly, patients with mesothelioma harboring germline mutations in BAP1 exhibit significantly improved prognosis, although the underlying mechanisms remain enigmatic.27
NF2 encodes Merlin, a protein that exerts negative regulation over receptor-dependent mitogenic signaling and the activity of phosphatidylinositol 3-kinase (PI3K)-AKT, while also activating the Hippo pathway.28 Intriguingly, NF-κB has been unveiled as a survival factor in mutated human mesothelial cells and human mesothelioma cells, concurrently serving as a factor of resistance to chemotherapeutic treatment.29,30
This mosaic of genetic and molecular underpinnings underscores the intricate tapestry of mesothelioma and unveils novel avenues for therapeutic intervention, transforming the landscape of mesothelioma treatment strategies and propelling us toward more targeted and efficacious therapies.
Rationale for the use of CPIs in MPMThe aggressiveness of malignant pleural mesothelioma (MPM), the high percentage of irresectability upon diagnosis, and the lack of highly effective treatments contribute to long-term survival outcomes remaining close to 10% at the 5-year mark post-diagnosis.31
Until October 2020, platinum-based agents in combination with folate antimetabolites such as pemetrexed have stood as the sole approved first-line treatment regimens for MPM since 2004.32–34
The pivotal Phase III CheckMate 743 trial in 2021 ushered in a new era for first-line therapy by introducing immunotherapy. Following the approval of the combined treatment of nivolumab with ipilimumab, the study showcased a remarkable 50% improvement in 2-year overall survival (41% vs. 27%) compared to previously described chemotherapy regimens. This groundbreaking advancement solidified the use of this therapy for patients diagnosed with unresectable MPM who had not previously undergone treatment.35
Subsequent to this milestone publication, the gates to immunotherapeutic interventions swung open in clinical practice, now reigning as the standard treatment approach for MPM patients, having secured approval from leading regulatory bodies such as the FDA and EMA.36–38 Despite the commendable progress achieved, the endurance of therapeutic response remains limited, and the recurrence rate of the disease remains high. Hence, there persists a need for dedicated research initiatives geared toward the dissection of mechanisms underlying immune therapy response, ultimately aiming to enhance treatment outcomes.
In summary, the advent of immune checkpoint inhibitors has heralded a transformative era in MPM treatment, breaking through the traditional boundaries to provide a promising avenue for patients with previously limited options.
Treatment of MPM with CPIs after failure of other therapies (second line and beyond)Upon progression following first-line platinum-pemetrexed chemotherapy, there lacks a standardized therapy and scientific evidence remains limited. Second-line chemotherapy regimens involving vinorelbine, gemcitabine, or the gemcitabine-docetaxel combination, among others, have been evaluated in Phase II studies and retrospective analyses, yielding modest outcomes.39–44
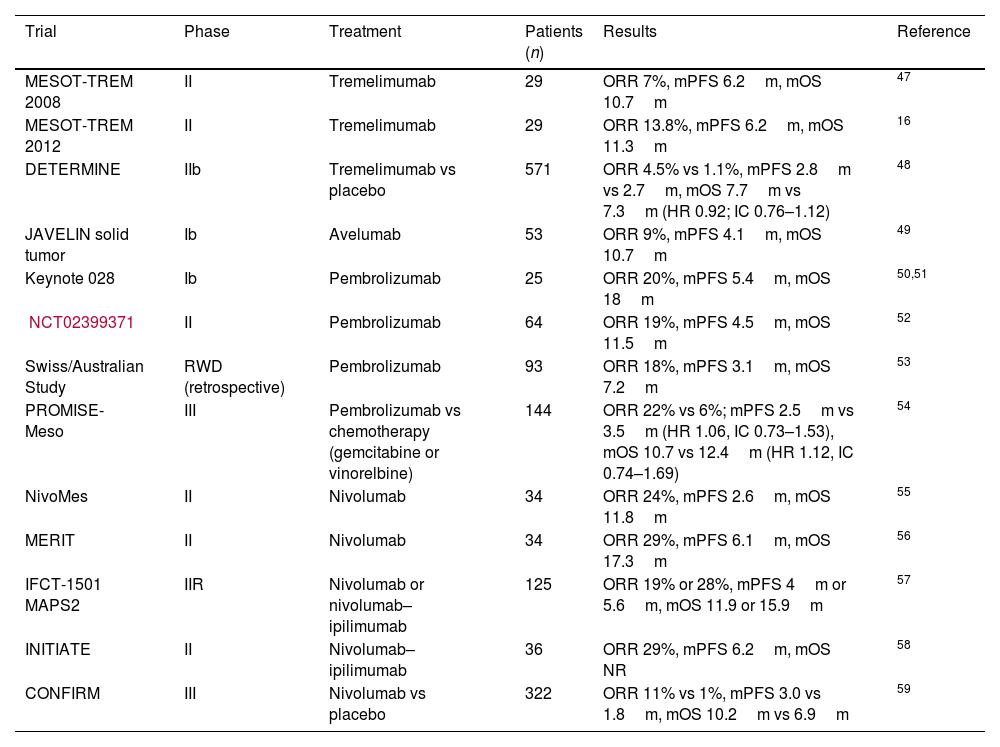
The suboptimal results achieved within this context, coupled with chronic inflammation stemming from asbestos exposure as the main pathogenic factor7 and supported by the heightened expression of PD-L1 (20–60%),45,46 particularly in the non-epithelioid subtype, have spurred systematic investigation into immunotherapy as a second-line approach (Table 1).
Clinical trials in MPM with ICIs in 2nd line therapy and beyond.
| Trial | Phase | Treatment | Patients (n) | Results | Reference |
|---|---|---|---|---|---|
| MESOT-TREM 2008 | II | Tremelimumab | 29 | ORR 7%, mPFS 6.2m, mOS 10.7m | 47 |
| MESOT-TREM 2012 | II | Tremelimumab | 29 | ORR 13.8%, mPFS 6.2m, mOS 11.3m | 16 |
| DETERMINE | IIb | Tremelimumab vs placebo | 571 | ORR 4.5% vs 1.1%, mPFS 2.8m vs 2.7m, mOS 7.7m vs 7.3m (HR 0.92; IC 0.76–1.12) | 48 |
| JAVELIN solid tumor | Ib | Avelumab | 53 | ORR 9%, mPFS 4.1m, mOS 10.7m | 49 |
| Keynote 028 | Ib | Pembrolizumab | 25 | ORR 20%, mPFS 5.4m, mOS 18m | 50,51 |
| NCT02399371 | II | Pembrolizumab | 64 | ORR 19%, mPFS 4.5m, mOS 11.5m | 52 |
| Swiss/Australian Study | RWD (retrospective) | Pembrolizumab | 93 | ORR 18%, mPFS 3.1m, mOS 7.2m | 53 |
| PROMISE-Meso | III | Pembrolizumab vs chemotherapy (gemcitabine or vinorelbine) | 144 | ORR 22% vs 6%; mPFS 2.5m vs 3.5m (HR 1.06, IC 0.73–1.53), mOS 10.7 vs 12.4m (HR 1.12, IC 0.74–1.69) | 54 |
| NivoMes | II | Nivolumab | 34 | ORR 24%, mPFS 2.6m, mOS 11.8m | 55 |
| MERIT | II | Nivolumab | 34 | ORR 29%, mPFS 6.1m, mOS 17.3m | 56 |
| IFCT-1501 MAPS2 | IIR | Nivolumab or nivolumab–ipilimumab | 125 | ORR 19% or 28%, mPFS 4m or 5.6m, mOS 11.9 or 15.9m | 57 |
| INITIATE | II | Nivolumab–ipilimumab | 36 | ORR 29%, mPFS 6.2m, mOS NR | 58 |
| CONFIRM | III | Nivolumab vs placebo | 322 | ORR 11% vs 1%, mPFS 3.0 vs 1.8m, mOS 10.2m vs 6.9m | 59 |
The CTLA-4 inhibitor, Tremelimumab, was the pioneer immune checkpoint inhibitor studied in refractory MPM through non-randomized Phase II trials: the MESO-TREM 2008 trial47 and the MESO-TREM 2012 trial,16 each exploring different dosing schedules of the drug. Modest objective response rates (3–13%) were observed, but the survival outcomes were encouraging (median progression-free survival of 6.2 months and median overall survival of 11 months). However, Tremelimumab failed to significantly improve overall survival compared to placebo in the DERTERMINE study.48
The anti-PD-L1 antibody, Avelumab, demonstrated sustained antitumor activity in a basket-type Phase Ib study,49 particularly in a cohort of heavily pretreated mesothelioma patients. However, further clinical development in this pathology is still pending.
Nivolumab and pembrolizumab, both anti-PD-1 agents, boast the largest body of efficacy data in pre-treated MPM patients. Early efficacy and safety data with Pembrolizumab were gathered in the MPM cohort of the Phase Ib KEYNOTE-028 trial,50,51 enrolling 25 PD-L1-positive patients (≥1%, 22C3 IHC assay), achieving a 20% objective response rate and a median duration of response of 12 months. These results echoed previous Phase II findings from Desai et al.,52 where no patient selection based on PD-L1 status was performed. Furthermore, real-world data from Switzerland and Australia revealed similar efficacy outcomes for Pembrolizumab-treated patients with MPM progression,53 although with slightly inferior survival outcomes. Favorable outcomes were linked to elevated PD-L1 expression and non-epithelioid histologies.
Despite these encouraging results, the Phase III Pembrolizumab trial (ETOP-PROMISE-meso) did not meet its primary endpoint of progression-free survival. The study included 144 MPM patients progressing after platinum-based chemotherapy, with no PD-L1 selection, who were randomized 1:1 to receive either Pembrolizumab or chemotherapy (gemcitabine or vinorelbine as per investigator's choice). Progression-free survival was numerically inferior in the experimental arm (2.5 months vs. 3.4 months; HR 1.06; 95% CI 0.73 vs. 1.53; p=0.76), and there was no advantage in overall survival (10.7 months vs. 12.4 months; HR 1.12; 95% CI 0.74–1.79; p=0.59). Despite a 63% crossover rate, analysis adjusted for this factor failed to detect differences. However, the objective response rate was higher for the Pembrolizumab arm, aligning with previously discussed Phase II findings (ORR 22% vs. 6%). Exploratory analysis based on PD-L1 status (<1% or ≥1%, determined by E1L3N antibody) also failed to reveal differences in outcomes.54
Nivolumab in MPM progressing after chemotherapy has been evaluated both as monotherapy and in combination with anti-CTLA4. The German Phase II NivoMes trial enrolled 34 unselected PD-L1 population patients to assess efficacy and safety. The primary objective of disease control rate at 12 weeks was 47%, with an objective response rate of 26% and a median duration of response of 7 months. Survival data revealed median progression-free survival of 3.6 months and median overall survival of 11.8 months.55 Another similar study, the MERIT trial conducted in a Japanese population, yielded more favorable results with a disease control rate of 68% and extended survival medians (median progression-free survival of 6.1 months and median overall survival of 17.3 months). The objective response rate remained independent of histological subtype at 29%, while the sarcomatoid subtype demonstrated a remarkable 67% response rate.56
The French Phase II IFCT-1501 MAPS2 trial is a multicenter, randomized, non-comparative study evaluating nivolumab (n=63) or nivolumab–ipilimumab (n=62) as second-line treatment. The primary objective of disease control rate at 12 weeks was achieved at 44% and 50%, respectively. Other efficacy variables were consistent with previously reported nivolumab data and favored the combination arm (objective response rate 19% vs. 28%; median progression-free survival 4 months vs. 5.6 months; median overall survival 11.9 months vs. 15.9 months). Grade 3–4 toxicity was slightly higher in the combination arm.57 Another single-center Phase II study utilizing nivolumab–ipilimumab in second-line settings aligned with previously discussed results.58
The Phase III CONFIRM trial demonstrated the efficacy of Nivolumab compared to placebo. A total of 332 MPM patients who had progressed after first-line platinum-based chemotherapy, without PD-L1 selection, were randomized 2:1 to receive nivolumab or placebo. The study successfully met its co-primary endpoints with an absolute survival benefit of 3.3 months (median overall survival of 10.2 months vs. 6.9 months; HR 0.69) and a slight statistically significant increase in median progression-free survival (3.0 months vs. 1.8 months; HR 0.67). The objective response rate was 11% vs. 1% for placebo.59
An Italian-authored meta-analysis pooled evidence from 13 studies encompassing a total of 888 patients treated with anti-PD-L1/anti-PD-1 therapies. The overall response rate and disease control rate were 18.1% (95% CI: 13.9–22.8%) and 55.4% (95% CI: 48.1–62.5%), respectively. Median progression-free survival and overall survival demonstrated an increase from 2.1 months to 5.9 months and from 6.7 months to 20.9 months, respectively. The authors concluded that anti-PD-(L)1 ICIs can be considered a treatment option in MPM progressing after chemotherapy, even in the absence of identified predictive response factors.60
In conclusion, the landscape of treating MPM following failure of initial therapies has undergone a paradigm shift with the advent of immune checkpoint inhibitors. These agents have unlocked new possibilities for patients facing limited alternatives, ushering in a new era of hope and exploration.
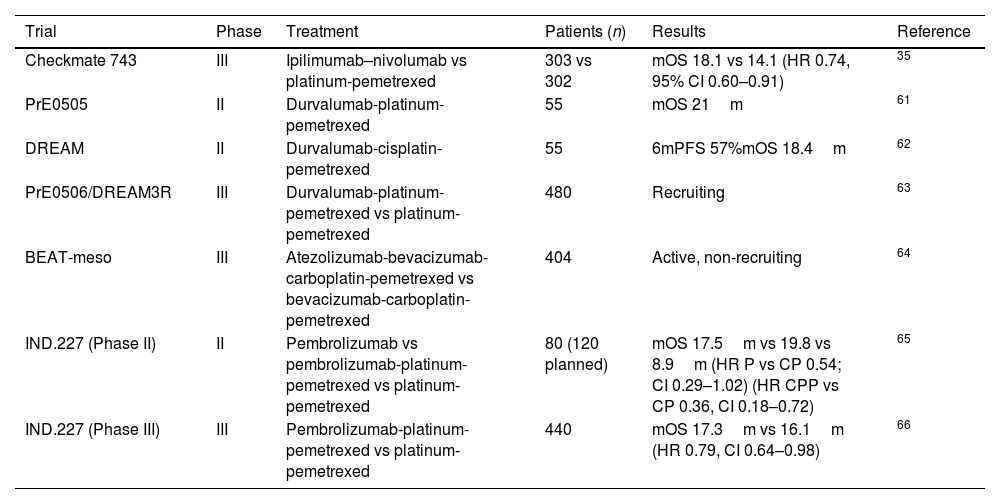
Treatment of malignant pleural mesothelioma with ICIs in first lineThe utilization of immunotherapy in the treatment of previously untreated MPM has been explored in numerous studies, both in monotherapy and in combination (Table 2).
Clinical trials in MPM with ICIs combinations in 1st line therapy.
| Trial | Phase | Treatment | Patients (n) | Results | Reference |
|---|---|---|---|---|---|
| Checkmate 743 | III | Ipilimumab–nivolumab vs platinum-pemetrexed | 303 vs 302 | mOS 18.1 vs 14.1 (HR 0.74, 95% CI 0.60–0.91) | 35 |
| PrE0505 | II | Durvalumab-platinum-pemetrexed | 55 | mOS 21m | 61 |
| DREAM | II | Durvalumab-cisplatin-pemetrexed | 55 | 6mPFS 57%mOS 18.4m | 62 |
| PrE0506/DREAM3R | III | Durvalumab-platinum-pemetrexed vs platinum-pemetrexed | 480 | Recruiting | 63 |
| BEAT-meso | III | Atezolizumab-bevacizumab-carboplatin-pemetrexed vs bevacizumab-carboplatin-pemetrexed | 404 | Active, non-recruiting | 64 |
| IND.227 (Phase II) | II | Pembrolizumab vs pembrolizumab-platinum-pemetrexed vs platinum-pemetrexed | 80 (120 planned) | mOS 17.5m vs 19.8 vs 8.9m (HR P vs CP 0.54; CI 0.29–1.02) (HR CPP vs CP 0.36, CI 0.18–0.72) | 65 |
| IND.227 (Phase III) | III | Pembrolizumab-platinum-pemetrexed vs platinum-pemetrexed | 440 | mOS 17.3m vs 16.1m (HR 0.79, CI 0.64–0.98) | 66 |
One of the earliest pieces of evidence of clinical activity was reported in 2017 with the phase 1b KEYNOTE 028 study involving an anti-PD-1 agent, subsequently leading to investigations in the context of relapsed disease and later in the first-line treatment setting.51
Combination of ICIsCombining two immune checkpoint inhibitors with distinct mechanisms of action has demonstrated the potential for profound and enduring responses in other tumors. Anti-CTLA-4 agents induce proliferation of T cells and de novo antitumor T cell responses, while anti-PD-1 agents restore function in existing antitumor T cells.67
Given the proven efficacy of combination immunotherapy in relapsed disease, as shown in the NIBIT-MESO 1 phase II trial with durvalumab and tremelimumab,68 along with the INITIATE phase II and IFCT-1501 MAPS phase III trials evaluating the nivolumab and ipilimumab combination,57,58 the phase III Checkmate 743 trial was developed.
Checkmate 743 represents the first phase III study demonstrating a survival benefit with immunotherapy versus chemotherapy (pemetrexed combined with cisplatin or carboplatin) as first-line treatment for patients with MPM. This trial achieved its primary endpoint of overall survival with a minimum follow-up of 22.1 months, showing a median OS of 18.1 months with nivolumab plus ipilimumab versus 14.1 months with platinum pemetrexed, yielding a Hazard Ratio (95% confidence interval) of 0.74 (0.60–0.91).35
This benefit was confirmed in an update with extended follow-up published in February 2022, reporting a median follow-up of 43.1 months.69 In this update, the overall survival rate was 23% in the combination arm versus 15% in the chemotherapy arm, progression-free survival rate was 14% versus 1%, and objective response rate was 40% versus 44%, respectively. Moreover, at 3 years, 28% versus 0% of responders maintained a response. These findings align with ipilimumab's biological effect on the immune system by inducing memory T cells, thus yielding durable responses.
Clinical benefit and antitumor immune response were observed with nivolumab plus ipilimumab across all subgroups, irrespective of histology PD-L1 expression.
Although overall survival improved with the combination versus chemotherapy in both epithelioid and non-epithelioid histologies, a greater benefit was seen in epithelioid histology. Malignant pleural mesotheliomas of non-epithelioid histology, particularly the sarcomatoid subtype, are characterized by aggressive natural history and significant chemoresistance. Survival in the Checkmate study for chemotherapy-treated patients was 8.8 months in the non-epithelioid subgroup versus 16.5 months in the epithelioid subgroup (HR 0.47, 95% CI 0.35–0.63). Conversely, results with immunotherapy were similar in both subgroups (18.1 versus 18.7 months; HR 0.93, 95% CI 0.68–1.28), with 3-year survival rates of 24% and 22%, respectively.
Despite PD-L1 expression being a established biomarker in other advanced solid tumors,70 survival outcomes in the immunotherapy arm were similar for patients with tumor PD-L1 expression≥1% [18.0 months (95% CI 16.8–21.4 months)] and PD-L1<1% [17.3 months (95% CI 10.1–23.9 months)], whereas in the chemotherapy group, survival was shorter for patients with tumor PD-L1 expression>1% [13.3 months (95% CI 11.6–15.4 months)] compared to those with PD-L1 tumor<1% [16.6 months (95% CI 13.4–20.8 months)]. These findings confirm the absence of predictive PD-L1 capacity to mark benefit from combination immunotherapy in MPM, although the trend favors the combination in cases of absent expression of this biomarker.
With these findings, nivolumab plus ipilimumab was approved for first-line treatment of unresectable MPM by the FDA in October 2020 and by the EMA in June 2021, making the combination the preferred choice according to NCCN guidelines.71
Chemotherapy plus CPIsCombining an immune checkpoint inhibitor with chemotherapy has shown a synergistic effect in other tumor types72 and has also been investigated in this scenario. Two phase II single-arm clinical trials with durvalumab and chemotherapy in first-line advanced pleural mesothelioma have been conducted.
In the PrE505 study, patients received platinum-based pemetrexed chemotherapy with durvalumab for up to 6 cycles, followed by durvalumab maintenance for up to 1 year. OS was 21.1 months, and the 1-year OS rate was 70%.61 In the DREAM study, the treatment scheme also combined platinum-based pemetrexed chemotherapy with durvalumab, followed by durvalumab maintenance, with a 6-month progression-free survival rate of 57%.62 Both studies met prespecified safety and activity criteria.
In February 2021, the phase III PrE0506/DREAM3R study was initiated to assess the efficacy of combining chemotherapy (cisplatin or carboplatin and pemetrexed) with durvalumab versus current standard treatment (chemotherapy or choice of immunotherapy combination) as first-line treatment for MPM. Results are awaited, and there are no published data available at the moment.63
In the same vein, the ongoing multicenter randomized phase III BEAT-meso trial compares the combination of atezolizumab plus bevacizumab and standard chemotherapy versus bevacizumab and standard chemotherapy as first-line treatment for MPM. The primary endpoint is OS, with secondary endpoints including PFS, ORR, duration of response, and disease control at 24 weeks.64
CCTG IND.227 is an initial multinational phase II trial conducted at Canadian, Italian, and French centers, evaluating the combination of Pembrolizumab with chemotherapy in previously untreated MPM.65 In the phase 2 study, progression-free survival of treatment with platinum pemetrexed was compared against platinum pemetrexed plus Pembrolizumab and Pembrolizumab monotherapy. Recruitment in the Pembrolizumab arm was halted after an interim analysis (16-week disease control rate). The combination was not only well-tolerated but also demonstrated a superior median survival and overall response rate of 19.8 months [95% confidence interval or CI: 8.4–41.36] versus 8.9 months [95% CI: 5.3–12.8] and 47% [95% CI: 24–71%] versus 19% [95% CI: 5–42%], respectively. Progression-free survival was similar between both arms.
Given the promising results of IND.227, the clinical trial was modified to a randomized phase III design, whose results reported during the annual American Society of Clinical Oncology (ASCO) congress. The primary objective was overall survival (OS). A total of 440 previously untreated MPM patients with an ECOG PS 0–1 were randomized. 218 patients received carboplatin-pemetrexed, and 222 received carboplatin-pemetrexed-pembrolizumab. The primary objective was achieved, with an OS benefit observed in the group receiving chemo-immunotherapy, with a HR of 0.79 (95% CI 0.64–0.98, p=0.0324). However, the numerical difference was modest (17.28 months in the triplet group vs. 16.13 months in the chemotherapy-only group). At 3 years, 25% of patients in the experimental group were still alive compared to 17% in the control group. Subgroup analysis showed a consistent benefit without differences based on sex, age, lineage, or PD-L1 expression.72
All these data suggest that combining immunotherapy with chemotherapy is a potential territory to explore in MPM treatment given its potential benefit in survival.
Adjunvant therapy with ICIsImmunotherapy is also being investigated in the adjuvant setting. AtezoMeso is a phase III trial randomizing patients to receive atezolizumab for 12 months or placebo (2:1) after surgical resection of pleural mesothelioma without macroscopic residual disease and having received at least 4 cycles of perioperative cisplatin/carboplatin and pemetrexed therapy. The primary endpoint is the evaluation of atezolizumab efficacy in terms of disease-free survival (PFS), with secondary objectives including safety, survival, and quality of life.73 The trial is ongoing.
Future in the treatment of malignant pleural mesotheliomaWhile most current studies focus on CTLA-4 and PD-1 checkpoint inhibitors and their combinations, there are numerous other potential checkpoints that could be targeted in the future.
On one hand, mesothelin, a tumor antigen highly expressed in many tumors, including MPM and adenocarcinomas of the pancreas, ovaries, and lungs. It's an appealing therapeutic target due to its limited expression in mesothelial cells, which are dispensable. There are currently various therapeutic agents in clinical evaluation.74 Although most clinical trials with CAR T cells (NCT02414269, NCT04577326, NCT04489862) are in phase I or I/II, the results so far are promising, and all trials show manageable toxicity, although response rates have been low.75
Oncolytic viruses are emerging as targeted therapy for MPM due to their ability to destroy tumor cells without affecting non-tumor cells, releasing antigens that activate T cells through dendritic cells.76 In the case of MPM, therapy with adenoviruses,77 poxviruses,78 reoviruses,79 herpesviruses,80 and measles virus81 is being investigated. Virotherapy is one of the most promising alternatives, with various studies showing that human MPM cells are sensitive to many oncolytic viruses through direct cell death or immunomediated mechanisms. However, extensive research is still needed to define treatment alternatives more clearly.
WT1 (Wilms Tumor-1) is a highly overexpressed transcription factor in MPM that is widely used for diagnostic purposes. However, WT1 peptide antigens can trigger T cell responses in MPM cell lines.82 The adjuvant role of WT1 peptide analog vaccines within a multimodal treatment is being studied within multimodal treatment protocols, showing promising results with increases in both OS and EFS.83
Lastly, the role of microRNAs (miRNAs) must be highlighted. They posttranscriptionally regulate the expression of most protein-coding genes. In MPM, miRNA expression changes are characterized by global downregulation and are associated with the loss of tumor suppressor pathways. Although research on the role of different miRNAs is ongoing, for example, overexpression of miR-16 and miR34a, alone or in combination, slows cell cycle progression, modulates p53 and HMGB1 expression, and increases sensitivity to cisplatin, enhancing the drug's apoptotic effect.84 Furthermore, there are several phase I clinical trials with miR-16-5p,85,86 which have demonstrated safety and early signs of activity against MPM. Therefore, characterizing miRNAs in the future may offer an opportunity to identify different immune biomarkers that improve early diagnosis of this disease and provide novel therapeutic targets.
ConclusionsMalignant pleural mesothelioma remains a challenging neoplasm with a certain bad prognosis. As a rare malignancy, MPM's incidence, despite being relatively low, underscores the importance of understanding its pathogenesis, clinical course, and evolving therapeutic strategies.
The etiology of MPM is intricately linked to asbestos exposure, emphasizing the need for stringent occupational safety measures. Moreover, the nexus between asbestos exposure, lung cancer, and the synergistic impact of smoking mandates comprehensive public health interventions.
The advent of immune checkpoint inhibitors (ICIs) has ushered in a transformative era in MPM treatment. The landscape of ICIs in MPM, particularly focusing on PD-1 and CTLA-4 inhibition, has brought promising results in both monotherapy and combination approaches. Nivolumab, a PD-1 inhibitor, exhibited notable activity in MPM patients progressing after chemotherapy, with response rates varying across trials. The phase III CONFIRM study underscored nivolumab's efficacy by improving overall survival and progression-free survival compared to placebo. Additionally, combination strategies, like nivolumab–ipilimumab, have shown encouraging results, further highlighting the potential of dual immune checkpoint inhibition.
The complexity of MPM's molecular landscape, marked by specific gene alterations and pathways, has been illuminated by advances in high-throughput analyses. Genes like CDKN2A, NF2, and BAP1, along with their respective mutations, offer insights into the disease's biology and potentially serve as therapeutic targets.
In summary, MPM's formidable challenges persist, with limited treatment options and a historically grim prognosis. However, the emergence of immune checkpoint inhibitors has introduced a ray of hope, leading to unprecedented improvements in survival outcomes. While further investigations into predictive biomarkers and resistance mechanisms are essential, the rapidly evolving field of immunotherapy underscores its potential as a cornerstone in the management of MPM.
As we delve deeper into understanding MPM's molecular intricacies and harness the immune system's power, we stand at the cusp of a new era, one marked by personalized therapeutic strategies that promise to reshape the landscape of this formidable malignancy. As we continue to unravel its complexities, interdisciplinary collaboration and continued research efforts will be instrumental in offering a brighter future for MPM patients worldwide.
FundingThis review received no external funding.
Authors’ contributionsAll authors participated in the conception and design of the work. All authors believe that the manuscript represents valid work, have read it, and fully approved it.
Conflicts of interestThe authors declare no conflict of interest related to this review.