With the onset of the COVID-19 (SARS-CoV2) pandemic in Wuhan-China at the end of 2019,1 multiple clinical trials have been conducted seeking medical solutions to mitigate the impact of the virus. Vaccination has been the most important tool to combat infectious agents and their complications.2 In the case of COVID-19, vaccines from different manufacturers have been developed with an action-focused on preventing serious cases and mortality. The Sinovac-life-science inactivated virus vaccine is one of them, with effectiveness studies in different age groups and use in different countries of the world within their vaccination plans.3
Vaccines can have rare, varied adverse effects including neurological entities. Guillain-Barre syndrome (GBS) is associated with multiple infectious agents including COVID-19, 4 it has also been reported after vaccination of polio, hepatitis B, rabies, and influenza in periods from 2 days to 3 weeks5; recently one case of GBS has been reported with the Pfizer vaccine for COVID-19 6 and two cases in the Johnson & Johnson vaccine studies.7 Although this does not generate a causal relationship, it creates the need to follow up all patients vaccinated for COVID-19 who develop neurological pathologies, to increase the evidence and favor a precise medical approach with timely treatments in complex neurological conditions.
We report the case of a 73-year-old man, from Cali-Colombia, who consults a national reference university hospital in March 2021, with four-day symptoms consisting of upper limb (UL) paresis, posterior lower limb involvement (LL) associated with an inability to walk, and dyspnea. Four days before the onset of symptoms, the patient had received the first dose of vaccination for COVID-19-Sinovac. Upon admission to the emergency department, he presented flaccid symmetric quadriparesis with muscle strength in UL of 1/5 and bilateral LL 2/5, generalized hyporeflexia, without sensory alteration or sphincter involvement, without upper motor neuron signs; they suspected motor poly-neuro-radiculopathy with a descending pattern and a high risk of ventilatory failure, for which he was admitted to the intensive care unit with oxygen support, 24 h later with deterioration of the respiratory pattern and a requirement for orotracheal intubation. The patient had a history of GBS 20 years ago, without information on medical history or management, does not report other relevant personal or family history, nor febrile, gastrointestinal, or respiratory episodes in the last month.
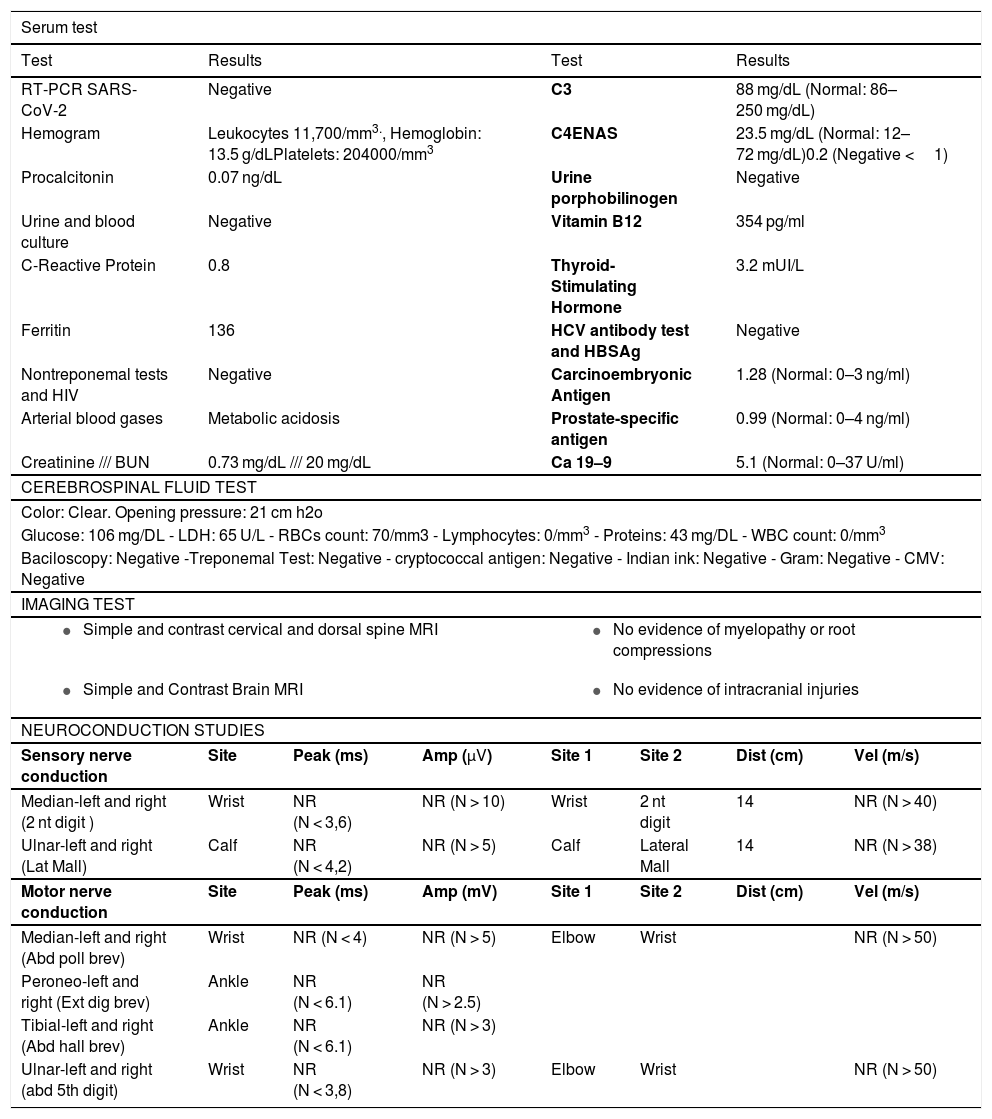
Lumbar puncture was performed with opening pressure of 21 cm/H2O, cytochemical without alterations, chest X-ray without signs of pneumonia or pleural effusions. The estudies of the patient show a normal biochemical, metabolic, and infectious profile (Table 1), simple and contrasted magnetic resonance imaging (MRI) of the brain and total spine without findings of clinical relevance, and an electrophysiological study of four extremities that reported acute demyelinating motor and sensory polyneuropathy. (Table 1).
Patient studies.
| Serum test | |||||||
|---|---|---|---|---|---|---|---|
| Test | Results | Test | Results | ||||
| RT-PCR SARS-CoV-2 | Negative | C3 | 88 mg/dL (Normal: 86–250 mg/dL) | ||||
| Hemogram | Leukocytes 11,700/mm3., Hemoglobin: 13.5 g/dLPlatelets: 204000/mm3 | C4ENAS | 23.5 mg/dL (Normal: 12–72 mg/dL)0.2 (Negative <1) | ||||
| Procalcitonin | 0.07 ng/dL | Urine porphobilinogen | Negative | ||||
| Urine and blood culture | Negative | Vitamin B12 | 354 pg/ml | ||||
| C-Reactive Protein | 0.8 | Thyroid-Stimulating Hormone | 3.2 mUI/L | ||||
| Ferritin | 136 | HCV antibody test and HBSAg | Negative | ||||
| Nontreponemal tests and HIV | Negative | Carcinoembryonic Antigen | 1.28 (Normal: 0–3 ng/ml) | ||||
| Arterial blood gases | Metabolic acidosis | Prostate-specific antigen | 0.99 (Normal: 0–4 ng/ml) | ||||
| Creatinine /// BUN | 0.73 mg/dL /// 20 mg/dL | Ca 19–9 | 5.1 (Normal: 0–37 U/ml) | ||||
| CEREBROSPINAL FLUID TEST | |||||||
| Color: Clear. Opening pressure: 21 cm h2o | |||||||
| Glucose: 106 mg/DL - LDH: 65 U/L - RBCs count: 70/mm3 - Lymphocytes: 0/mm3 - Proteins: 43 mg/DL - WBC count: 0/mm3 | |||||||
| Baciloscopy: Negative -Treponemal Test: Negative - cryptococcal antigen: Negative - Indian ink: Negative - Gram: Negative - CMV: Negative | |||||||
| IMAGING TEST | |||||||
|
| ||||||
|
| ||||||
| NEUROCONDUCTION STUDIES | |||||||
| Sensory nerve conduction | Site | Peak (ms) | Amp (μV) | Site 1 | Site 2 | Dist (cm) | Vel (m/s) |
| Median-left and right (2 nt digit ) | Wrist | NR (N < 3,6) | NR (N > 10) | Wrist | 2 nt digit | 14 | NR (N > 40) |
| Ulnar-left and right (Lat Mall) | Calf | NR (N < 4,2) | NR (N > 5) | Calf | Lateral Mall | 14 | NR (N > 38) |
| Motor nerve conduction | Site | Peak (ms) | Amp (mV) | Site 1 | Site 2 | Dist (cm) | Vel (m/s) |
| Median-left and right (Abd poll brev) | Wrist | NR (N < 4) | NR (N > 5) | Elbow | Wrist | NR (N > 50) | |
| Peroneo-left and right (Ext dig brev) | Ankle | NR (N < 6.1) | NR (N > 2.5) | ||||
| Tibial-left and right (Abd hall brev) | Ankle | NR (N < 6.1) | NR (N > 3) | ||||
| Ulnar-left and right (abd 5th digit) | Wrist | NR (N < 3,8) | NR (N > 3) | Elbow | Wrist | NR (N > 50) | |
MRI: magnetic resonance imaging, HIV: human inmmunodeficiency virus, BUN: blood urea nitrogen, ENAS: extractable nuclear antigen antibodies, HCV: hepatitis c virus, HBSag: hepatitis b Surface antigen, RBC: red blood cell; WBC: White blood cell, LDH: lactate dehydrogenase, CMV: cytomegalovirus, 2 nt:second, Lat: lateral, Mall: Malleolus, 5th: fifth, abd poll brev: abductor pollicis brevis, ext dig brev: extensor digitalis brevis, abd hall brev: abductor hallux brevis, abd: abductor, amp: amplitude, dist: distance, vel: velocity, N:normal, NR: no responce.
Neurology evaluates reporting flaccid quadriparesis, hyporeflexia, and acute ventilatory failure due to GBS, for which plasmapheresis with 3% albumin was started (five sessions), the first being performed 72 h after admission. After the second plasmapheresis, the patient partially regains movement in the LL and UL with limited finger mobility, without cranial nerve involvement and communication through gestures. At the end of the plasmapheresis sessions, it is assessed by physiatry, finding bilateral muscle strength in UL: elbow flexion and extension: 2/5, wrist flexion-extension: 2/5, without complete mobility of the fingers of the hands; LL: adductors 4/5, knee flexion-extension: 3/5, foot dorsiflexion: 1/5, foot plantarflexion: 2/5, toe flexion-extension: 1/5; right patellar reflex + / ++++, which was previously absent. A rehabilitation plan was started with objectives (Table 2), in addition to orthotic management for proper positioning, prevention of contractures, and pain management.
Rehabilitation in Guillain-Barre syndrome (GBS).
| GBS-rehabilitation goals. |
|
The patient presented in this case meets clinical and electrophysiological criteria for GBS8 with a favorable response to medical treatment, in addition to initiating symptoms after COVID-19 vaccination in a period similar to that reported with other infectious agents.5 Vaccination in the COVID-19 pandemic is an appropriate and widely applied intervention, which generates the need to study possible complications to offer the best medical treatment in the shortest time. Although, observational studies are required to define a solid causal relationship, a basis is created for the reporting of future cases and their treatment. to our knowledge, this case presented in March 2021 is the first report in Latin America.
FundingThe authors did not receive funding to carry out this report.
Ethical considerationThe patient gave his consent for the use of clinical and paraclinical information, it was authorized by the ethics committee of the Hospital Universitario del Valle, Cali, Colombia.
To the Hospital Universitario del Valle Evaristo García E.S.E and its department of electro-diagnosis and clinical neurology.