Although vaccination has considerably reduced the risk of hospitalization and death from COVID19, the impact of vaccination and anti-SARS-CoV-2 antibody status on the outcome of patients who required hospitalization has been poorly investigated.
Material and methodsA prospective observational study in 232 patients hospitalized for COVID19 was carried out from October 2021 to January 2022 to evaluate the role on patient outcome of their vaccination and anti-SARS-CoV-2 antibody status and titer, comorbidities, analytical determinations, clinical presentation at admission, treatments and requirements for respiratory support. Cox regression and survival analyzes were performed. The SPSS and “R” programs were used.
ResultsPatients with complete vaccination schedule had higher S-protein antibody titers (log10 3.73 [2.83–4.6]UI/ml vs 1.6 [2.99–2.61]UI/ml; p<0.001), lower probability of radiographic worsening (21.6% vs. 35.4%; p=0.005), less likely required high doses of dexamethasone (28.4% vs. 45.4%; p=0.012), high-flow oxygen (20.6% vs. 35.4%; p=0.02), ventilation (13.7% vs, 33.8%; p=0.001) and intensive care admissions (10.8% vs. 32.6%; p<0.001). Remdesivir (HR=0.38; p<0.001) and complete vaccination schedule (HR=0.34; p=0.008) were protective factors. No differences in antibody status were detected between groups (HR=0.58; p=0.219).
ConclusionsSARS-CoV-2 vaccination was associated with higher S-protein antibody titers and lower probability of radiological progression, immunomodulators requirement and respiratory support or death. However, vaccination but not antibody titters protected from adverse events pointing a role of immune-protective mechanisms in addition to humoral response.
Aunque la vacunación ha reducido considerablemente el riesgo de hospitalización y muerte por COVID-19, se ha investigado poco el impacto de la vacunación y el estado de los anticuerpos anti-SARS-CoV-2 en la evolución de los pacientes que requieren hospitalización.
Material y métodosSe realizó un estudio observacional prospectivo en 232 pacientes hospitalizados por COVID-19 desde octubre del 2021 hasta enero del 2022 para evaluar el impacto en la evolución clínica del estado vacunal, el título de anticuerpos anti-SARS-CoV-2, la presencia de comorbilidades, analítica, la clínica al ingreso, tratamientos y soporte respiratorio. Se realizaron análisis de supervivencia y regresión de Cox. Se utilizaron los programas SPSS y «R».
ResultadosLos pacientes con esquema de vacunación completo presentaron títulos de anticuerpos contra la proteína S más elevados (log10 3,73 [2,83-4,6] UI/mL vs. 1,6 [2,99-2,61] UI/mL; p < 0,001), menor probabilidad de empeoramiento radiográfico (21,6 vs. 35,4%; p = 0,005), requirieron con menor probabilidad dosis elevadas de dexametasona (28,4 vs. 45,4%; p = 0,012), oxígeno de alto flujo (20,6 vs. 35,4%; p = 0,02), ventilación (13,7 vs. 33,8%; p = 0,001) e ingresos en cuidados intensivos (10,8 vs. 32,6%; p < 0,001). El remdesivir (HR = 0,38; p < 0,001) y el esquema completo de vacunación (HR = 0,34; p = 0,008) fueron factores protectores de mala evolución. No se detectaron diferencias en el estado de los anticuerpos entre los grupos (HR = 0,58; p = 0,219).
ConclusionesLa vacunación contra el SARS-CoV-2 se asoció con mayores títulos de anticuerpos contra la proteína S y menor probabilidad de progresión radiológica, requerimiento de inmunomoduladores y soporte respiratorio o muerte. Sin embargo, la vacunación, pero no los títulos de anticuerpos, protegió de los eventos adversos, lo que indica un papel de los mecanismos de protección inmunológica además de la respuesta humoral.
The COVID-19 pandemic caused by the new coronavirus SARS-CoV-2 has led to a global public health and economic crisis. Thanks to both messenger RNA (mRNA) and adenovirus vaccines, the risk of hospitalization and death has been considerably reduced.1,2 However, there are vaccine breakthroughs that lead to the appearance of infections, admissions and deaths.3 Once hospitalized, COVID19 patients can progress to more severe illness, including respiratory failure, acute respiratory distress syndrome, and death.3
SARS-CoV2 vaccines elicit several protective mechanisms including neutralizing antibodies (NAbs) and other immune effectors mechanisms including non-NAbs, memory T cells, and innate immunity.4 SARS-COV-2 infection in vaccinated individuals is expected to recall these specific mechanisms that should blunt disease progression preventing the onset of respiratory failure, the need for mechanical ventilation, and death.4 Currently, there are numerous studies that demonstrate the beneficial effect of vaccination in terms of reducing the risk of infection, hospitalization, and death.1,5–8 However, there are few studies that assess the impact of vaccination on the progression of the disease in hospitalized patients.3,9,10 Tenforde et al.3 found a reduction in the risk of ventilation and death of 67% after 28 days in vaccinated subjects, but they only evaluated patients vaccinated with mRNA. In another study performed in 448 hospitalized patients carried out in the USA by Naleway et al.,9 they observed that unvaccinated patients had a worse clinical evolution (ICU admission, need for ventilation and intubation) but they did not found differences in mortality. In contrast, in a study conducted by Kalligeros et al.,10 in 915 hospitalized patients, between January 2021 and April 2021, they found that vaccination was associated with a 76% reduction in the risk of death. In a CDC report including 706 hospitalized patients with vaccine failure, in the same period (January–April 2021), they described 132 patients that died (18.6%).11 Unfortunately, in this report there was not an unvaccinated control group.
This study aims to assess the impact that vaccination and anti-SARS-CoV-2 antibody status may have on the clinical course (need for ventilation or death) of patients hospitalized for COVID-19 during the sixth wave of the pandemic in Spain.
MethodsStudy design, participants, setting and eligibilityThis prospective observational study developed in Murcia (Spain) between October 2021 and January 2022 included 232 consecutive adult patients admitted to the Reina Sofia Hospital for a clinical syndrome consistent with acute COVID-19 and a positive SARS-CoV-2 PCR test within 10 days after symptom onset. Patients were distributed in two groups: unvaccinated (n=130) and vaccinated (n=102). Vaccinated patients included those with ‘complete vaccination schedule’ that met the following criteria: (1) for Pfizer vaccine, a minimum period between the two doses was ≥19 days and ≥7 days after the second dose; (2) for Moderna vaccine, a minimum period between doses of 25 days and 14 days after the second dose; (3) for AstraZeneca vaccine, a minimum period between doses of 21 days and 14 days after the second dose; and (4) for Janssen vaccine, a minimum period of 14 days before infection. Details of COVID-19 vaccination, including dates and location, vaccine product, and lot number, were ascertained through a systematic process including patient or proxy interview and source verification. Sources of documentation included vaccination cards, hospital records, vaccine state registries, and vaccine records requested from clinics and pharmacies. Vaccine doses were classified as administered if source documentation was identified or if the patient or proxy reported a vaccine dose with a plausible date and location of vaccination.
Demographic, clinical and laboratory data were collected by trained personnel through standardized participant (or proxy) interviews and medical record reviews. The study conformed principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines and was approved by the local Ethics Committee (‘Comité Ético de Investigación Clínica del Hospital General Universitario Reina Sofía de Murcia’).
Laboratory analysisUpper respiratory specimens were collected from enrolled patients, frozen, and shipped to a central laboratory at Reina Sofia Hospital (Murcia, Spain). Reverse transcriptase-polymerase chain reaction (RT-RCP) has routinely been used to confirm diagnosis. Detection of IgG antibodies to SARS-CoV-2 testing in human serum was made with the SARS-CoV-2 IgG assay, a chemiluminescent microparticle immunoassay (CMIA). The Alinity i system calculates the calibrator mean chemiluminescent signal from 3 calibrator replicates, and stores the result. Results are reported by dividing the sample result by the stored calibrator result. The default result unit for the SARS-CoV-2 IgG assay is Index (S/C). Determination of Ferritin and CRP were analyzed with an ADVIA® system and a Dimension Xpand Plus® system respectively (Siemens Healthcare Diagnostics Inc.,Tarrytown, NY, USA). D-dimer was analyzed with the ACL-TOP 500 CTS® system (Werfen, Barcelona, Spain)
COVID-19 severity classificationWe collected data on severity for patients hospitalized with COVID-19. Outcome data were collected until hospital discharge or 28 days after hospital admission. The primary classification of disease severity was a binary measure that divided patients into those who experienced death or invasive or non-invasive mechanical ventilation (progression to high disease severity) and those who did not (no progression to high disease severity).
As a secondary assessment, we classified COVID-19 severity using a modified version of the World Health Organization COVID-19 Clinical Progression Scale, a commonly used ordinal scale for assessing COVID-19 severity that ranges from uninfected (level 0) and infected but asymptomatic (level 1) to death (level 6). We classified severity according to the highest ordinal level that the patient experienced during the first 35 days of hospitalization. In this analysis of hospitalized patients, the highest severity level experienced could range from level 2 to 6, including hospitalized without supplemental oxygen (level 2), with standard supplemental oxygen (level 3), with high-flow nasal cannula or noninvasive ventilation (level 4), with invasive mechanical ventilation (level 5), and in-hospital death (level 6).
We also evaluated in-hospital receipt of treatments used for severe COVID-19 (corticosteroids, high-dose dexamethasone (20mg bolus), remdesivir, tocilizumab or baricitinib).
Statistical analysisA descriptive analysis for categorical variables of patients’ characteristics was carried out using frequency tables. Mean and standard deviation (SD) were used for continuous variables. Differences in categorical variables between patients unvaccinated and vaccinated were assessed through the chi-squared test or Fisher test, and t-student tests for continuous variables.
Among patients hospitalized with COVID-19, the association between progression to death or need of mechanical ventilation was calculated with multivariable Cox regression adjusted for the variables that had p less than 0.1 in the univariate analysis: age, obesity, cardiovascular disease, treatment with remdesivir, vaccination status, SARS-CoV-2 S-protein and N-protein antibodies positivity and titer.
To assess the impact of antibody levels on clinical evolution, patients were analyzed differentiating whether or not they were vaccinated. A logarithmic transformation of the antibody titer was made.
The association between death alone and vaccination status was calculated with multivariable logistic regression evaluating the odds of vaccination among patients with COVID-19 who died vs survived.
Kaplan–Meier curves were built to compare time to disease progression between patients vaccinated versus unvaccinated. Nonparametric (log-rank) tests were used to compare event-free survival functions in the 2 study groups.
p<0.05 was considered statistically significant. The statistical analysis was conducted using IBM SPSS Statistics software, version 23.0 (IBM Corp., Armonk, New York) and “r” free software.
ResultsClinical and biological characteristics of COVID-19 patientsA total of 232 COVID-19 patients mostly infected with the SARS-CoV-2 delta (B.1.617.2) variant were included in the study. Patients who were not infected with delta were infected with omicron (B.1.1.529). The data on the type of variant are estimates considering epidemiological data since sequencing has not been performed in all patients. When 90% of patients were admitted, at that time, the dominant variant was delta.
The basal characteristics of patients are shown in Table 1. Patients’ mean age was 58 years, 52.6% men, 81% Spaniards and 7% with an immunocompromising condition. Vaccine breakthrough patients compared to unvaccinated patients tended to be older (mean age 64.2 vs. 54.1 years, p<0.001), and showed higher rates of hypertensive condition (59.8% vs. 30%, p<0.001), cardiovascular disease (27.5% vs. 10.8%, p=0.02) and chronic kidney disease (12.7% vs. 2.3%, p=0.004). Among 102 fully vaccinated patients, 68 (68%) received the mRNA vaccines BNT162b2 or the mRNA-1273 vaccine, 9 (9%) Ad26.COV2-S vaccine and 23 (23%) ChAdOx1-S vaccine.
Patients’ characteristics and clinical evolution according to the vaccination status.
| Unvaccinated (n=130) | Vaccinateda (n=102) | p | |
|---|---|---|---|
| Demographics and clinical background | |||
| Male sex, n (%) | 69 (53.1) | 53 (52.0) | 0.971 |
| Age (mean±SD) | 54 (16) | 64 (17) | <0.001 |
| Age, years range, n (%) | |||
| <40 | 29 (22.3) | 9 (8.8) | <0.001 |
| 40–59 | 49 (37.7) | 23 (22.5) | |
| 60–79 | 45 (34.6) | 47 (46.1) | |
| ≥80 | 7 (5.4) | 23 (22.5) | |
| Origin other than Spain (%) | 32 (25.2) | 13 (12.7) | 0.029 |
| Chronic medical conditionsb, median (IQR) | 1 (0–2) | 2 (1–3) | <0.001 |
| Hypertension, n (%) | 39 (30.0) | 61 (59.8) | <0.001 |
| Type 2 Diabetes, n (%) | 24 (18.5) | 31 (30.4) | 0.049 |
| Obesity (body mass index ≥30), n (%) | 32 (24.6) | 29 (28.4) | 0.613 |
| Current tobacco use, n (%) | 16 (12.3) | 9 (8.8) | 0.302 |
| Cardiovascular disease, n (%) | 14 (10.8) | 28 (27.5) | 0.002 |
| Chronic kidney disease, n (%) | 3 (2.3) | 13 (12.7) | 0.004 |
| Pulmonary disease, n (%) | 19 (14.6) | 26 (25.5) | 0.056 |
| Liver disease, n (%) | 6 (4.6) | 5 (4.9) | 1.000 |
| Dementia, n (%) | 2 (1.5) | 7 (6.9) | 0.082 |
| Immunocompromising conditionc, n (%) | 3 (2.3) | 4 (3.9) | 0.744 |
| COVID-19 status at admission | |||
| Days from symptom onset to admission (mean±SD) | 6.57±3.62 | 5.57±3.38 | 0.032 |
| PaO2/FiO2at admission mmHg (mean±SD) | 410.9±91.9 | 419.7±67.18 | 0.298 |
| CURB-65, n (%) | |||
| 0 | 29 (22.3) | 11 (10.8) | 0.045 |
| 1 | 59 (45.4) | 43 (42.2) | |
| 2 | 30 (23.1) | 38 (37.3) | |
| 3 | 12 (9.2) | 9 (8.8) | |
| 4 | 0 (0.0) | 1 (1.0) | |
| WHO ordinal scale adapted, n (%) | |||
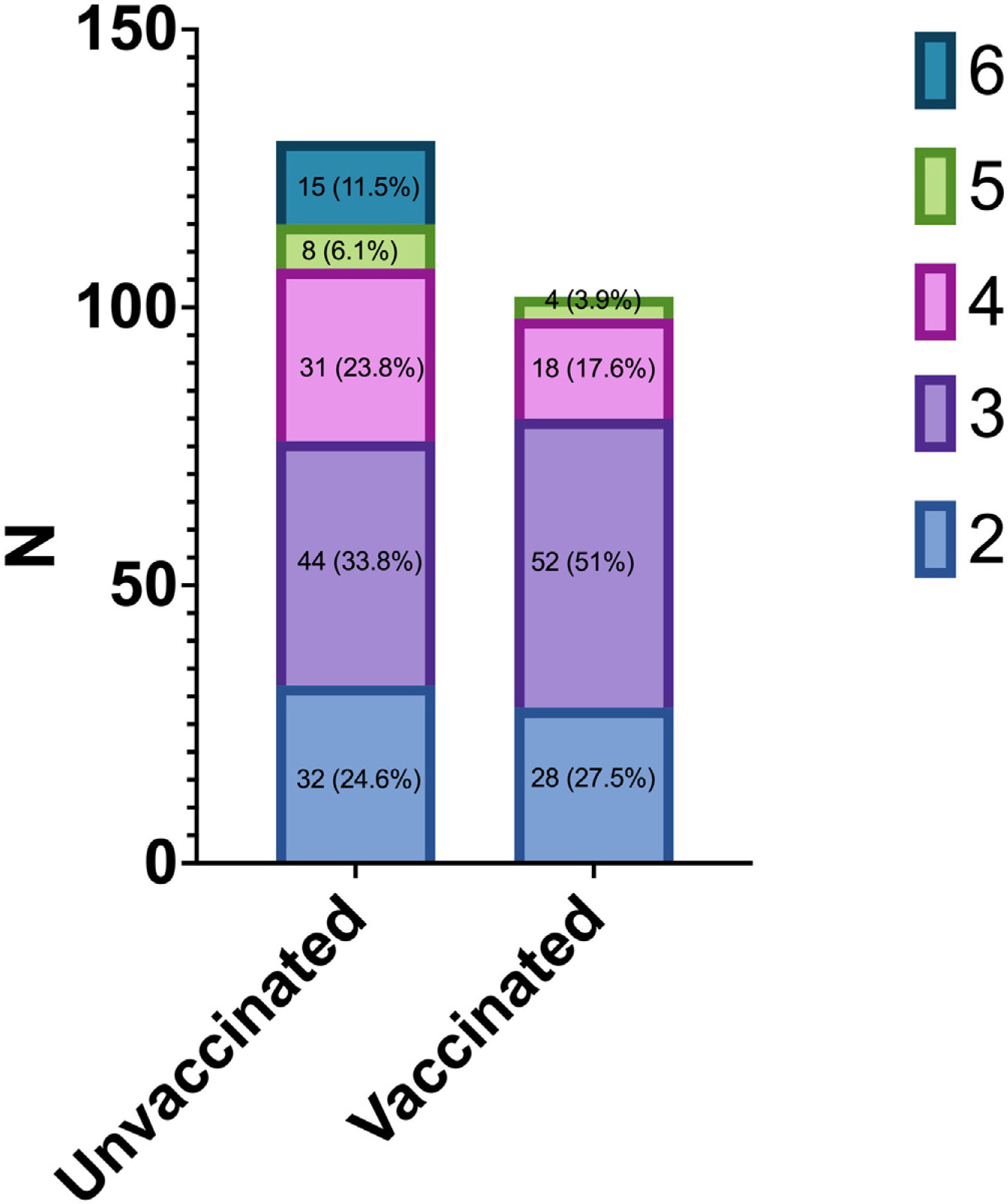
| 2 | 32 (24.6) | 28 (27.5) | 0.002 |
| 3 | 44 (33.8) | 52 (51.0) | |
| 4 | 31 (23.8) | 18 (17.6) | |
| 5 | 8 (6.1) | 4 (3.9) | |
| 6 | 15 (11.5) | 0 (0.0) | |
| SOFA score (mean±SD) | 1.22±1.48 | 1.09±1.46 | 0.514 |
| SOFA>2, n (%) | 39 (30.5) | 31 (31) | 1.000 |
| Chest radiology, n (%) | |||
| No infiltrates | 8 (6.2) | 16 (15.7) | 0.005 |
| Unilateral infiltrates | 8 (6.2) | 14 (13.7) | |
| Bilateral infiltrates | 114 (87.7) | 72 (70.6) | |
| SARS-CoV-2 antibody status at admissionf | |||
| SARS-CoV-2 S antibody positive, n (%) | 33 (40.7) | 58 (80.6) | <0.001 |
| SARS-CoV-2 N antibody positive, n (%) | 28 (34.6) | 21 (29.2) | 0.475 |
| Log10 SARS-CoV-2 N antibody UI/ml, median (IQR) | −0.18 (−0.83–0.59) | −0.54 (−1.09–0.41) | 0.243 |
| Log10 SARS-CoV-2 S antibody UI/ml, median (IQR) | 1.6 (2.99–2.61) | 3.73 (2.83–4.6) | <0.001 |
| SARS-CoV2 viral load at admissiong | |||
| CT, mean (IQR) | 20.6 (17.35) | 20 (16.27) | 0.567 |
| CT higher than 30, n (%) | 31 (83.8) | 30 (85.7) | 0.540 |
| Treatments | |||
| Remdesivir, n (%) | 59 (45.4) | 43 (42.2) | 0.720 |
| Dexamethasone, n (%) | 72 (55.4) | 61 (59.8) | 0.726 |
| Dexamethasone boluses (20mg/d), n (%) | 59 (45.4) | 29 (28.4) | 0.012 |
| Tocilizumab, n (%) | 25 (19.2) | 14 (13.7) | 0.349 |
| Baricitinimb, n (%) | 7 (5.4) | 2 (2.0) | 0.318 |
| HBPM prophylaxis (%) | 102 (78.5) | 83 (82.2) | 0.270 |
| Clinical evolution | |||
| Hospital mean stay (mean±SD) | 9.30±6.68 | 8.96±6.99 | 0.707 |
| Complications during hospitalizationd, n (%) | 41 (31.5) | 37 (36.3) | 0.537 |
| Radiological worsening | 46 (35.4) | 22 (21.6) | 0.011 |
| Highest CRP levels, mg/dl median (IQR) | 7.5 [3,13] | 9.3 [4,14] | 0.252 |
| Highest D-dimer levels, ng/mL median (IQR) | 1006.50 [561.00, 1655] | 742.50 [468.75, 1482.50] | 0.066 |
| Higher ferritin levels, ng/mL median (IQR) | 506 [288,1043] | 348 [196,620] | 0.003 |
| Lowest SaO2/FiO2mmHg (mean±SD) | 303.4±142 | 346.4±116 | 0.010 |
| PaO2/FiO2less than 200mmHg, n (%) | 39 (34.5) | 15 (16.7) | 0.007 |
| ICU admission, n(%) | 42 (32.6) | 11 (10.8) | <0.001 |
| Need for high-flow oxygen, n (%) | 46 (35.4) | 21 (20.6) | 0.020 |
| Need for non-invasive mechanical ventilation, n (%) | 44 (33.8) | 14 (13.7) | 0.001 |
| Need for invasive mechanical ventilation, n (%) | 15 (11.5) | 4 (3.9) | 0.063 |
| Need for ventilation, n (%) | 44 (33.8) | 14 (13.7) | 0.001 |
| Death, n (%) | 10 (10.0) | 0 (0.0) | 0.008 |
| Time from admission to evente, days (mean±SD) | 5.29±4.57 | 6.62±5.3 | 0.042 |
SOFA: sepsis related organ failure assessment; ICU: intensive care unit.
Complete vaccination when met the following criteria: For Pfizer vaccine, the minimum period between the two doses must be ≥19 days and the time after the 2nd dose to enter risk ≥7 days. For Moderna vaccine, a minimum period between doses of 25 days and 14 days after the 2nd dose. For AstraZeneca vaccine, a minimum period between doses of 21 days and 14 days after the 2nd dose. Finally, for Janssen vaccine, a minimum period of 14 days before infection.
Chronic medical conditions included the following: cardiovascular disease, neurologic disease, pulmonary disease, hepatic disease, endocrine disease, kidney disease, hematologic disease, malignancy and immunosuppression.
Immunocompromising conditions included active solid organ cancer (active cancer defined as treatment for the cancer or newly diagnosed cancer in the past 6 months), active hematologic cancer (such as leukemia, lymphoma, or myeloma), HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, previous splenectomy, previous solid organ transplant, immunosuppressive medication, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, scleroderma, or inflammatory bowel disease (Crohn disease or ulcerative colitis).
Vaccine breakthrough patients compared with unvaccinated cases needed less frequently ICU care (10.8% vs. 32.6%; p<0.001), high-flow oxygen (20.6% vs. 35.4%; p=0.015), and non-invasive mechanical ventilation (13.7% vs. 33.8%; p=0.001). In contrast, unvaccinated patients were more likely to have bilateral radiological infiltrates (87.7% vs. 70.6%; p=0.005), radiological worsening (35.4% vs. 21.6%; p=0.011), and higher ferritin levels, 506ng/mL (288.7, 1043.2) vs 348ng/ml (196.5,620), p=0.018, than fully vaccinated patients (Table 1).
Unvaccinated patients accounted for 77% (47/61) of cases with disease progression to mechanical ventilation or death. Mechanical ventilation or death was experienced by 14 of 102 (13.7%) vaccine breakthrough cases and 47 of 130 (36.2%) unvaccinated cases. This meant an absolute difference of −22.5% (95%CI, −17.12 to −27.87%; p<0.001). Among patients hospitalized with COVID-19, death or mechanical ventilation was associated with a lower likelihood of vaccination (aOR, 0.170; 95% CI, 0.083–0.38; p<0.001), obesity (aOR, 3.16; 95% CI, 1.59–6.27; p=0.001), and higher likelihood of previous cardiovascular disease (aOR, 3.61; 95% CI, 1.56–8.3; p=0.003). In fact, unvaccinated patients accounted for 100% (15/15) of deaths among patients with COVID-19 in this study. Death occurred in 0 of 102 (0%) vaccine breakthrough cases and 15 of 130 (11.5%) unvaccinated patients with COVID-19 (Table 2).
Characteristics of patients according to their outcome.
| No events (N=171) | Ventilation or death (N=61) | p | |
|---|---|---|---|
| Demographics and clinical background | |||
| Male sex, n (%) | 85 (49.7) | 37 (60.7) | 0.187 |
| Age, years range, n (%) | |||
| <40 | 29 (17.0) | 9 (14.8) | 0.843 |
| 40–59 | 53 (31.0) | 19 (31.1) | |
| 60–79 | 67 (39.2) | 25 (41.0) | |
| ≥80 | 22 (12.9) | 8 (13.1) | |
| Origin other than Spain (%) | 33 (19.5) | 12 (20.0) | 1.000 |
| Chronic medical conditionsa, median (IQR) | 1 (0–2) | 1 (1–3) | 0.035 |
| Hypertension, n (%) | 73 (42.7) | 27 (44.3) | 0.950 |
| Type 2 Diabetes, n (%) | 40 (23.4) | 15 (24.6) | 0.989 |
| Obesity (body mass index ≥30), n (%) | 36 (21.1) | 25 (41.0) | 0.004 |
| Current tobacco use, n (%) | 63 (36.8) | 20 (32.8) | 0.328 |
| Cardiovascular disease, n (%) | 26 (15.2) | 16 (26.2) | 0.084 |
| Chronic kidney disease, n (%) | 10 (5.8) | 6 (9.8) | 0.447 |
| Pulmonary disease, n (%) | 32 (18.7) | 13 (21.3) | 0.801 |
| Liver disease, n (%) | 6 (3.5) | 5 (8.2) | 0.259 |
| Dementia, n (%) | 7 (4.1) | 2 (3.3) | 1.000 |
| Immunocompromising conditionb, n (%) | 3 (1.8) | 4 (6.6) | 0.148 |
| COVID-19 status at admission | |||
| Complete vaccination schedule, n (%) | 88 (51.5) | 14 (23) | <0.001 |
| Days from symptom onset to admission (mean±SD) | 6.15±3.51 | 6.07±3.67 | 0.870 |
| PaO2/FiO2at admission mmHg (mean±SD) | 432±60.1 | 365.6±111.1 | <0.001 |
| CURB-65, n (%) | |||
| 0 | 30 (17.5) | 10 (16.4) | 0.018 |
| 1 | 81 (47.4) | 21 (34.4) | |
| 2 | 50 (29.2) | 18 (29.5) | |
| 3 | 10 (5.8) | 11 (18.0) | |
| 4 | 0 (0.0) | 1 (1.6) | |
| SOFA score (mean±SD) | 0.86±1.16 | 2.02±1.88 | <0.001 |
| SOFA>2, n (%) | 37 (22.0) | 33 (55.0) | <0.001 |
| Chest radiology, n (%) | |||
| No infiltrates | 24 (14.0) | 0 (0.0) | 0.001 |
| Unilateral infiltrates | 21 (12.3) | 1 (1.6) | |
| Bilateral infiltrates | 126 (73.7) | 60 (98.4) | |
| SARS-CoV-2 antibody status at admission in vaccinated (n=72)d | |||
| SARS-CoV-2 N antibody positive, n (%) | 17 (27.9) | 4 (36.4) | 0.404 |
| SARS-CoV-2 S antibody positive, n (%) | 50 (82) | 8 (72.7) | 0.362 |
| Log10 SARS-CoV-2 N antibody UI/ml median (IQR) | −0.52 (−1.04–0.31) | −1.22 (−1.69–0.75) | 0.355 |
| Log10 SARS-CoV-2 S antibody UI/ml, median (IQR) | 3.68 (2.89–4.6) | 4.12 (1.6–4.6) | 0.756 |
| SARS-CoV-2 antibody status at admission in not vaccinated (n=81)d | |||
| SARS-CoV-2 N antibody positive, n (%) | 22 (39.3) | 6 (24) | 0.139 |
| SARS-CoV-2 S antibody positive, n (%) | 24 (42.9) | 9 (36) | 0.371 |
| Log10 SARS-CoV-2 N antibody UI/ml median (IQR) | 0.03 (−0.64–0.64) | −0.48 (−1.42–0.41) | 0.355 |
| Log10 SARS-CoV-2 S antibody UI/ml, median (IQR) | 1.6 (1.06–2.86) | 1.41 (0.21–2.34) | 0.156 |
| SARS-CoV2 viral load at admissione | |||
| CT, mean (IQR) | 20 (16, 24.7) | 20 (16.5, 28) | 0.484 |
| CT higher than 30, n (%) | 49 (86) | 12 (80) | 0.412 |
| Treatments | |||
| Remdesivir, n (%) | 81 (47.4) | 21 (34.4) | 0.110 |
| Dexemethasone, n (%) | 102 (59.6) | 31 (50.8) | 0.175 |
| Dexamethasone boluses (20mg/d), n (%) | 34 (19.9) | 54 (88.5) | <0.001 |
| Tocilizumab, n (%) | 17 (9.9) | 22 (36.1) | <0.001 |
| Baricitinib, n (%) | 7 (4.1) | 2 (3.3) | 1.000 |
| HBPM prophylaxis (%) | 154 (90.6) | 31 (50.8) | <0.001 |
| Clinical evolution | |||
| Hospital mean stay (mean±SD) | 6.8±4.86 | 15.75±7.16 | <0.001 |
| Complications during hospitalizationc, n (%) | 45 (26.3) | 33 (54.1) | <0.001 |
| Radiological worsening | 35 (20.5) | 33 (54.1) | <0.001 |
| Highest CRP levels, mg/dl (median (IQR) | 7.4 [3.4,12.4] | 10.4 [5.5,18.7] | 0.003 |
| Highest D-dimer levels, ng/mL median (IQR) | 679 [441,1229] | 1547 [1068,3961] | <0.001 |
| Higher ferritin levels, ng/mL median (IQR) | 380 [204,760] | 685 [347,1332] | 0.002 |
| Lowest SaO2/FiO2mmHg (mean±SD) | 381.0±85.5 | 147.2±84.1 | <0.001 |
| PaO2/FiO2less than 200mmHg, n (%) | 11 (7.3) | 43 (81.1) | <0.001 |
| ICU admission, n (%) | 3 (1.8) | 50 (83.3) | <0.001 |
| Need for high flow oxygen, n (%) | 15 (8.8) | 52 (85.2) | <0.001 |
| Need for non-invasive mechanical ventilation, n (%) | 1 (0.6) | 57 (93.4) | <0.001 |
| Need for invasive mechanical ventilation, n (%) | 0 (0.0) | 19 (31.1) | <0.001 |
| Need for ventilation, n (%) | 0 (0.0) | 58 (95.1) | <0.001 |
| Death, n (%) | 0 (0) | 15 (24.6) | <0.001 |
| Time from admission to event, days (mean±SD) | 6.8 (4.8) | 3.3 (4.2) | <0.001 |
SOFA: sepsis related organ failure assessment; ICU: intensive care unit.
Chronic medical conditions included the following: cardiovascular disease, neurologic disease, pulmonary disease, hepatic disease, endocrine disease, kidney disease, hematologic disease, malignancy and immunosuppression.
Immunocompromising conditions included active solid organ cancer (active cancer defined as treatment for the cancer or newly diagnosed cancer in the past 6 months), active hematologic cancer (such as leukemia, lymphoma, or myeloma), HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, previous splenectomy, previous solid organ transplant, immunosuppressive medication, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, scleroderma, or inflammatory bowel disease (Crohn disease or ulcerative colitis).
Complications during hospitalization: Deep vein thrombosis or pulmonary thromboembolism, bacterial infection, acute renal failure, heart failure, stroke, acute myocardial infarction, and elevated transaminases.
According to the modified World Health Organization COVID-19 Clinical Progression Scale, the highest level (4, 5 or 6) of disease severity experienced was significantly lower among vaccine breakthrough cases than unvaccinated cases (21.5% vs. 41.4%; p<0.001) (Fig. 1).
Highest severity level experienced on the WHO COVID-19 Clinical Progression Scale during the first 35 days of hospitalization among vaccine breakthrough COVID-19 cases and unvaccinated COVID-19 cases. The highest level (4, 5 or 6) of disease severity experienced was significantly lower among vaccine breakthrough cases than unvaccinated cases (21% vs. 41.4%; p<0.001).
Obesity (41% vs. 21.1%, p=0.004) and cardiovascular disease (26.2% vs. 15.2%, p=0.084) were predominant comorbidities among patients that needed mechanical ventilation or died, compared to patients with a more favorable outcome (Table 2). As expected, among patients with less favorable clinical evolution, complete vaccination schedule was less frequent than in patients with a more favorable outcome (23% vs. 51.5%, p<0.001). Besides, patients with unfavorable outcome showed at admission lower PaO2/FiO2 (365.6±111.1mmHg vs 432±60.1mmHg, p<0.001) and higher CURB-65 (p<0.025), SOFA score (2.02 vs. 0.86, p<0.001), and frequency of bilateral infiltrates (98.4% vs. 73.7%, p<0.001). Consequently, higher frequency of high-dose dexamethasone treatment (88.5% vs. 19.9%, p<0.001) was required in these patients.
Of course, all parameters of clinical evolution, including hospital mean stay, complications, radiological worsening, ICU admission, highest D-dimer and ferritin levels, and lowest SaO2/FiO2, were worst in patients requiring ventilation or who died during the follow-up (see Table 2 for details).
In contrast, no significant differences in the positivity for SARS-CoV-2 antibody (72.7% vs. 82%, p=0.362) or SARS-CoV-2 S antibody titer (4.12 (1.6–4.6) vs 3.68(2.89–4.6); p=0.752) in vaccinated and in the positivity for SARS-CoV-2 antibody (36% vs. 42.9%, p=0.271) or SARS-CoV-2 S antibody titer (1.41 (0.21–2.34) vs 1.6(1.06–2.86); p=0.156) in non-vaccinated were detected between patients with worse or better clinical outcome.
A Cox regression multivariate analysis (Table 3) confirmed that obesity (aHR, 3.16; 95%-CI 1.59–6.3; p<0.001) and cardiovascular disease (aHR, 2.3; 95%-CI 1.02–5.2) were the main independent unfavorable factors in the clinical evolution of hospitalized COVID-19 patients. Besides, both remdesivir (aHR 0.387; 95%-CI 0.19–0.79; p<0.009) and SARS-CoV-2 vaccination (aHR 0.344; 95%-CI 0.15–0.75; p<0.008) were effective protective treatments. However, presence of SARS-CoV-2 antibodies or SARS-CoV-2 S antibody titer did not independently influence the clinical outcome of hospitalized COVID-19 patients (Table 3).
Cox regression of factors associated with ventilation or death.
| HR | 95-CI% | p | aHR | IC95% | p | |
|---|---|---|---|---|---|---|
| Age | 1.0 | 0.97–1.02 | 0.99 | |||
| Obesity | 3.08 | 1.54–6.14 | 0.001 | 3.16 | 1.59–6.3 | 0.001 |
| Cardiovascular disease | 2.5 | 1.04–6.02 | 0.041 | 2.3 | 1.02–5.2 | 0.044 |
| Remdesivir | 0.35 | 0.17–0.75 | 0.006 | 0.387 | 0.19–0.79 | 0.009 |
| Vaccinated | 0.41 | 0.15–1.1 | 0.078 | 0.344 | 0.15–0.75 | 0.008 |
| SARS-CoV-2 S-Ab positive | 0.58 | 0.46–1.37 | 0.219 | |||
| SARS-CoV-2 S-Ab UI/ml | 1.0 | 1.00–1.00 | 0.82 |
HR: hazard ratio; aHR: adjusted hazard ratio.
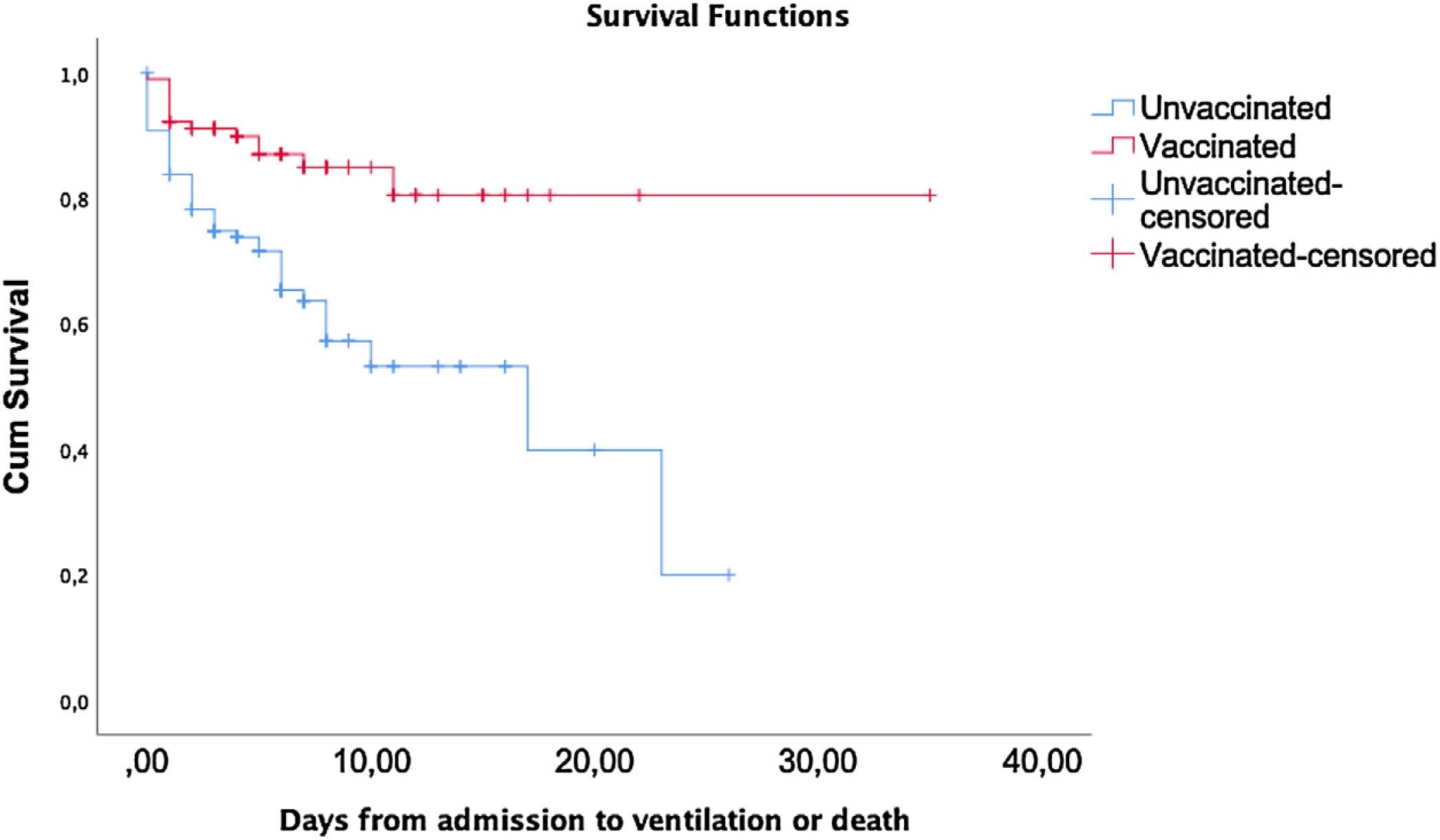
Survival analysis of the outcome (ventilation or death) according to the vaccination status is shown in Fig. 2. A statistically significant difference was found by the Long-Rank method (p<0.001) between unvaccinated and vaccinated patients.
DiscussionThis comparative analysis of the clinical outcome of fully vaccinated and unvaccinated adult patients hospitalized for COVID-19, most of them infected with the SARS-CoV-2 delta variant during the sixth wave in Spain, clearly shows that a complete vaccination schedule of either mRNA or adenovirus vaccines protected patients from progression to severe disease or death, compared to unvaccinated patients. Even though, as expected,9 vaccinated patients were older and suffered from more comorbidities such as hypertension, cardiovascular disease or chronic kidney failure, which are well-established risk factors for a fatal outcome associated with coronavirus diseases.12,13 Although these results are consistent with a reduced risk of developing severe COVID-19 among patients with vaccine breakthrough infections, in our series this protective immunity induced by the vaccine was not associated with either the presence or the titer of SARS-CoV-2 spike protein antibodies, pointing a role for immune-protective mechanisms other than the humoral ones.
The physiological mechanisms by which SARS-CoV-2 vaccines protect COVID-19 patients from an unfavorable clinical course are mostly unknown. However, it has been well established that, in unvaccinated patients, the cytokine storm triggered by the virus is a deadly unregulated systemic inflammatory response. This results from the release of large amounts of pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) by damaged infected tissue and local immune cells that attract many other immune effectors that contribute to perpetuate and worsen the inflammatory situation.14 The uncontrolled cytokine response is one of the main mechanisms for the acute respiratory distress syndrome (ARDS), responsible for the mortality in COVID-19 patients.15,16 Although most COVID-19 vaccines are designed to elicit neutralizing antibodies (NAbs) against the SARS-CoV-2 spike protein, vaccines seem to be additionally modulating the release of cytokines, favoring the development of non-inflammatory and more effective anti-viral cellular responses.17,4 Apparently, vaccine-induced immune response to the virus is modulating in quantity and/or quality the cytokine storm in most breakthrough infected patients and reducing the initial inflammatory reaction. It is known that vaccinated subjects have a lower viral load, which could be associated with the lower inflammatory response.18,19 However, the fact that some viral variants can widely evade humoral immunity makes it evident that cellular responses induced by vaccines cause strong cross-protection against variants of concern (VOCs) and support the concept that cellular responses mostly contribute to the control of the disease.20
Clinical trials evaluating SarCoV-2 vaccines efficacy have focused on the disease severity, mortality, infection, transmission, or other surrogate endpoints, but the cytokine storm incidence has not been studied.21 Despite the fact that more than 80% of patients in our series received the last dose of the complete regimen 5 months before hospitalization, and therefore could have suffered a drop in their antibody titer, fully vaccinated patients had lower ferritin levels as well as less radiological progression, less need for ventilation and less high-dose corticosteroids. This suggests that vaccines, in the long-term, may be modulating ‘the cytokine storm’ and, therefore, reducing the appearance of ARDS. Still, further studies will be needed to study in depth the interplay between the vaccines and inflammatory response, and verify if the vaccination program is an effective strategy to prevent or modify the cytokine storm.22,23
The results of this study indicate that, in order to be able to predict the vaccine-induced protection from adverse clinical evolution of hospitalized COVID-19 patient, measurement of spike-specific neutralizing antibodies is not enough and should be complemented with functional test to evaluate memory T cell response that, in addition, could contribute to ‘personalize’ their risk management and therapeutic approach. Besides, such information should contribute to define the relationship between humoral and cellular immunity in predicting the clinical outcome of hospitalized patients. However, the greater complexity and cost and the lack of standardization about measuring cellular immune responses hinder the generalization of these methodologies. Currently, ELISpot and intracellular cytokine staining, which are well-established, accurate and sensitive techniques, remain somewhat time-consuming and expensive.23 Therefore, the development of rapid and standardized cellular assays is needed. Some rapid whole-blood peptide-stimulation assays have recently been marketed and have demonstrated their efficacy in determining the T cell response against Sars-Cov-2 in recently vaccinated patients.24 However, future studies will be necessary to explore the usefulness of this methodology in hospitalized COVID-19 patients, whether they are vaccinated or not.
Although the small number of patients included in this study who required hospitalization for COVID-19, either fully vaccinated or unvaccinated, could be a limitation to obtain solid conclusions, the clinical relevance of this study is sustained on the exclusion of patients hospitalized for reasons unrelated to COVID-19 that tested positive in the meanwhile. Thus, our results sustain that: (1) SARS-CoV-2 vaccines protect from severe COVID-19 and death, but patients with insufficient immunogenicity, such as the elderly, or those with obesity or cardiovascular disease,25 continue to be at risk of severe disease even if they are fully vaccinated, and therefore it is very important to insist on infection prevention measures; and (2) vaccination but not antibody titters seems to protect from adverse events, pointing a role of immune-protective mechanisms other than humoral response. Therefore, total benefits of vaccination clearly exceed those estimated from prevention of infection.
Ethical considerationsPatient's written consent was obtained for this study. The study was approved by local Ethics Committee (‘Comité Ético de Investigación Clínica del Hospital General Universitario Reina Sofía de Murcia’) and conformed principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestAuthors declare that they have no competing interests.
Maria Dolores Hernández (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Gabriel Puche (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Paula Carter (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Mónica Martinez (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Antonia Alcaraz (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Maria Luz Nuñez (Microbiology Service. Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Natalia Sancho (Laboratory Medicine Department, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Mari Carmen Villalba (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Alfredo Cano (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Angeles Muñoz (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)
Carlos Báguena (Infectious Disease Unit, Reina Sofia University Hospital and Biomedical Research Institute of Murcia (IMIB), Murcia, Spain)