Our study aims to compare the clinical and epidemiological characteristics, length of stay in the ICU, and mortality rates of COVID-19 patients admitted to the ICU who are fully vaccinated, partially vaccinated, or unvaccinated.
Patients and methodsRetrospective cohort study (March 2020–March 2022). Patients were classified into unvaccinated, fully vaccinated, and partially vaccinated groups. We initially performed a descriptive analysis of the sample, a multivariable survival analysis adjusting for a Cox regression model, and a 90-day survival analysis using the Kaplan-Meier method for the death time variable.
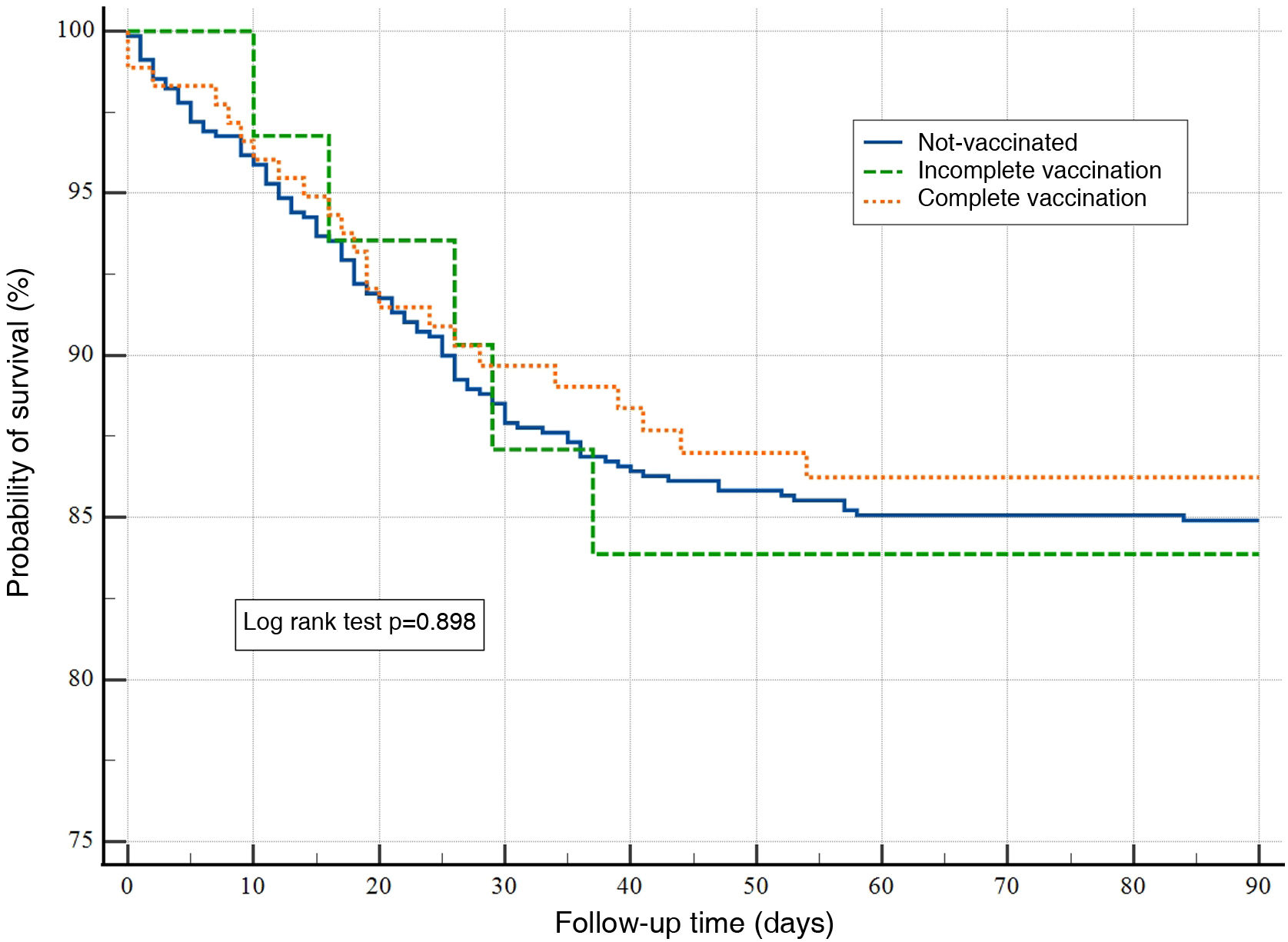
ResultsA total of 894 patients were analyzed: 179 with full vaccination, 32 with incomplete vaccination, and 683 were unvaccinated. Vaccinated patients had a lower incidence (10% vs. 21% and 18%) of severe ARDS. The survival curve did not show any differences in the probability of surviving for 90 days among the studied groups (P=.898). In the Cox regression analysis, only the need for mechanical ventilation during admission and the value of LDH (per unit of measurement) in the first 24h of admission were significantly associated with mortality at 90 days (HR: 5.78; 95% CI: 1.36–24.48); P=.01 and HR: 1.01; 95% CI: 1.00–1.02; P=.03, respectively.
ConclusionsPatients with severe SARS-CoV-2 disease who are vaccinated against COVID-19 have a lower incidence of severe ARDS and mechanical ventilation than unvaccinated patients.
Planteamos nuestro trabajo con el objetivo de comparar las características clínico epidemiológicas, la estancia en la UCI y la mortalidad de pacientes con COVID-19 que ingresaron en la UCI con vacunación completa, incompleta o sin vacunar.
Pacientes y métodosEstudio retrospectivo de cohortes (Marzo 2020–Marzo 2022). Los pacientes fueron clasificados en pacientes no vacunados, pauta de vacunación completa y pauta de vacunación incompleta. Se realizó inicialmente un análisis descriptivo de la muestra, un análisis multivariable de la supervivencia ajustando un modelo de regresión de Cox y un análisis de supervivencia a 90 días con el método de Kaplan-Meier para la variable de tiempo de muerte.
ResultadosFueron analizados los 894 pacientes: 179 con una pauta de vacunación completa, 32 con una pauta incompleta y 683 no estaban vacunados. Los enfermos vacunados presentaron con menor frecuencia (10% frente al 21% y 18%) un SDRA grave. La curva de supervivencia, no presentó diferencias en la probabilidad de sobrevivir a los 90 días entre los grupos estudiados (P=,898). En el análisis de regresión de COX, únicamente la necesidad de VM durante el ingreso y el valor de LDH (por unidad de medida) en las primeras 24 horas de ingreso se asociaron de forma significativa con la mortalidad a los 90 días (HR: 5,78; IC95%: 1,36–24,48); P=,01 y HR: 1,01; IC95%: 1,00–1,02; P=,03 respectivamente.
ConclusionesLos pacientes vacunados frente al COVID-19 con enfermedad grave por SARS-CoV-2 presentan unas tasas de SDRA grave y de VM menores que las de aquellos pacientes no vacunados.
The COVID-19 pandemic has caused an unprecedented global health crisis.1 High rates of hospitalisation and mortality due to the disease have been reported throughout the world. Since the beginning of the pandemic, various measures have been implemented to prevent the spread of the virus, including vaccination.2
Vaccination has been recognised as an important measure to prevent the spread of the virus and reduce the severity of the illness in infected patients.3 However, uncertainty remains about the effectiveness of the vaccine in preventing admission into the intensive care unit (ICU) and mortality in patients who have already received the vaccine.4 This uncertainty is due in part to the constant evolution of the virus and the emergence of variants that may be more resistant to existing vaccines.
We believe an analysis of patients who required ICU admission due to the severity of the SARS-CoV-2 disease could help to better understand the effectiveness of the vaccine in preventing disease severity and mortality in patients with COVID-19. These analyses may also provide valuable information about patient groups that may require special attention and closer monitoring after vaccination.5
In this context we put forward this paper that has the objective of comparing the clinical-epidemiological characteristics, the stay in the ICU and the mortality of patients with COVID-19 who were admitted to the ICU with complete vaccination, incomplete vaccination, or not-vaccinated.
MethodRetrospective cohort study of all cases who were hospitalised for SARS-CoV-2 infection and admitted to the ICU between March 2020 and March 2022. The data were obtained from the registry of the cohort of COVID-19 patients from a tertiary level hospital’s intensive medicine department. Prior approval from the Local Research Ethics Committee and the patients/legal representatives’ informed consent (written and/or telephone) was obtained.
The inclusion criteria were: over 18 years of age, confirmed diagnosis of SARS-CoV-2 disease in the first 24h of hospital admission, and need for ICU admission. The exclusion criteria were: informed consent not obtained and records with errors in the collection of the analysis variables.
Clinical-demographic variables of the patients were recorded: date of admission to the ICU, age in calendar years at the time of admission to the ICU, sex (male or female dichotomous variable), vaccination status, comorbidities (as dichotomous variables: yes or no); arterial hypertension [AHT]: diagnosed in the clinical history prior to the current admission; obesity: body mass index previously diagnosed in the clinical history or described as ≥30; diabetes mellitus: diagnosed in the clinical history prior to the current admission [no differentiation in type I or II]; dyslipidaemia: diagnosed in the clinical history as hypercholesterolemia and/or hypertriglyceridemia prior to the current admission; smoker: diagnosed in the clinical history prior to the current admission as an active smoker or found out during the patient or family anamnesis); biomarkers analysed (first value) as continuous variables collected in the first 24h of ICU admission: creatine kinase (CK in units/litre [U/L], normal reference range: 46–171), D-dimer (DD in ng/mL, normal reference range: 0–500), ferritin (in ng/mL, normal reference range: 22–322), interleukin 6 (IL-6, in pg/mL, normal reference range values < 40pg/mL), C-reactive protein (CRP in mg/dL, normal range: ≤0.5), ultrasensitive troponin I in serum (in ng/L, normal range: ≤40), lactate dehydrogenase (LDH in units/litre [U/L], normal reference range: 120–246); determination of the ratio of arterial oxygen partial pressure/fraction of inspiratory oxygen (P/F) as a continuous variable at the time of admission to the ICU; therapies required during ICU admission as dichotomous variables, yes/no; high-flow nasal cannulas (HFNC), mechanical ventilation (MV), use of the prone position as a therapeutic measure, use of corticosteroids, remdesivir, plasma, and tocilizumab as antiviral treatments, use of continuous renal replacement therapy (CRRT) due to renal failure; and evolutionary variables: days of MV in days as a continuous variable, ICU stay in days as a continuous variable.
Patients were followed up for 90 days from the time of admission into the ICU or until death (whichever occurred first).
The patients were classified as not-vaccinated patients, patients with a complete vaccination regimen, and patients with an incomplete vaccination regimen. For this, the following guidelines were followed6–11:
Complete vaccination: a patient was considered to have completed the vaccination against COVID-19 when they had received all the necessary doses, according to the type of vaccine that was administered (including booster doses when appropriate according to the vaccination schedule). Thus, complete vaccination was defined as:
- •
Pfizer-BioNTech: two doses of the vaccine administered at least 21 days apart.
- •
Moderna: two doses of the vaccine administered at least 28 days apart.
- •
AstraZeneca: two doses of the vaccine administered 4–12 weeks apart.
- •
Janssen: a single dose of the vaccine.
Incomplete vaccination: a patient was considered to have had an incomplete vaccination against COVID-19 if they have not received all the necessary doses according to the type of vaccine that was administered. Thus, incomplete vaccination was defined as:
- •
Pfizer-BioNTech: a single dose of the vaccine or two doses with an interval less than 21 days apart.
- •
Moderna: a single dose of the vaccine or two doses with an interval less than 28 days apart.
- •
AstraZeneca: a single dose of the vaccine or two doses less than 4 weeks apart or more than 12 weeks apart.
- •
Janssen: is not included as an incomplete vaccination since it is a single-dose vaccine.
Initially, a descriptive analysis of the sample was carried out where the categorical variables are presented as the value and its percentage; and as median and its 25−75 percentiles (p25−75) for continuous quantitative variables. Comparisons of percentages between groups were made using contingency tables and χ2, for the analysis of continuous variables, the Kruskal-Wallis test was used.
Subsequently, a multivariate analysis of survival was carried out adjusting a Cox regression model (method: forward; introduction of variables to the model if P<.05 and exclusion of variables if P>.2; status: death in the ICU at 90 days; using as covariates: age, sex, vaccination status, period of time, biomarkers analysed, need for MV and use of corticosteroids). For all analyses, the statistical significance was set at P<.05.
A 90-day survival analysis was performed using the Kaplan-Meier method for the time to death variable (log rank test).
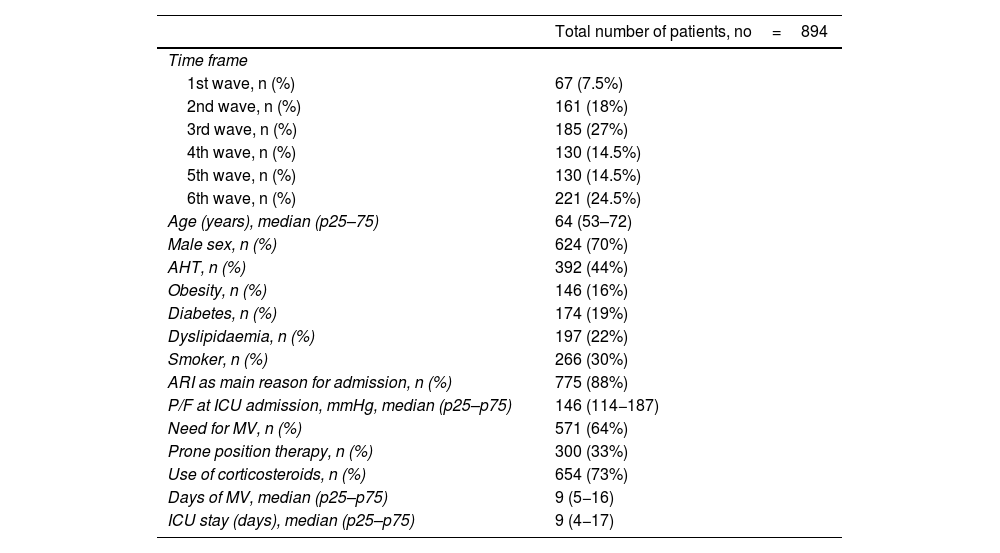
ResultsDuring the period analysed, a total of 911 patients were admitted to the ICU - COVID-19. 894 patients met the inclusion criteria and were analysed. Their main characteristics are shown in Table 1.
Main variables of the analysed cohort.
| Total number of patients, no=894 | |
|---|---|
| Time frame | |
| 1st wave, n (%) | 67 (7.5%) |
| 2nd wave, n (%) | 161 (18%) |
| 3rd wave, n (%) | 185 (27%) |
| 4th wave, n (%) | 130 (14.5%) |
| 5th wave, n (%) | 130 (14.5%) |
| 6th wave, n (%) | 221 (24.5%) |
| Age (years), median (p25–75) | 64 (53–72) |
| Male sex, n (%) | 624 (70%) |
| AHT, n (%) | 392 (44%) |
| Obesity, n (%) | 146 (16%) |
| Diabetes, n (%) | 174 (19%) |
| Dyslipidaemia, n (%) | 197 (22%) |
| Smoker, n (%) | 266 (30%) |
| ARI as main reason for admission, n (%) | 775 (88%) |
| P/F at ICU admission, mmHg, median (p25–p75) | 146 (114−187) |
| Need for MV, n (%) | 571 (64%) |
| Prone position therapy, n (%) | 300 (33%) |
| Use of corticosteroids, n (%) | 654 (73%) |
| Days of MV, median (p25–p75) | 9 (5−16) |
| ICU stay (days), median (p25–p75) | 9 (4−17) |
P/F: ratio between arterial oxygen partial pressure and the fraction of inspiratory oxygen; HFNC: high flow oxygen therapy; MV: mechanical ventilation; ARI: acute respiratory failure; ICU: Intensive Care Unit.
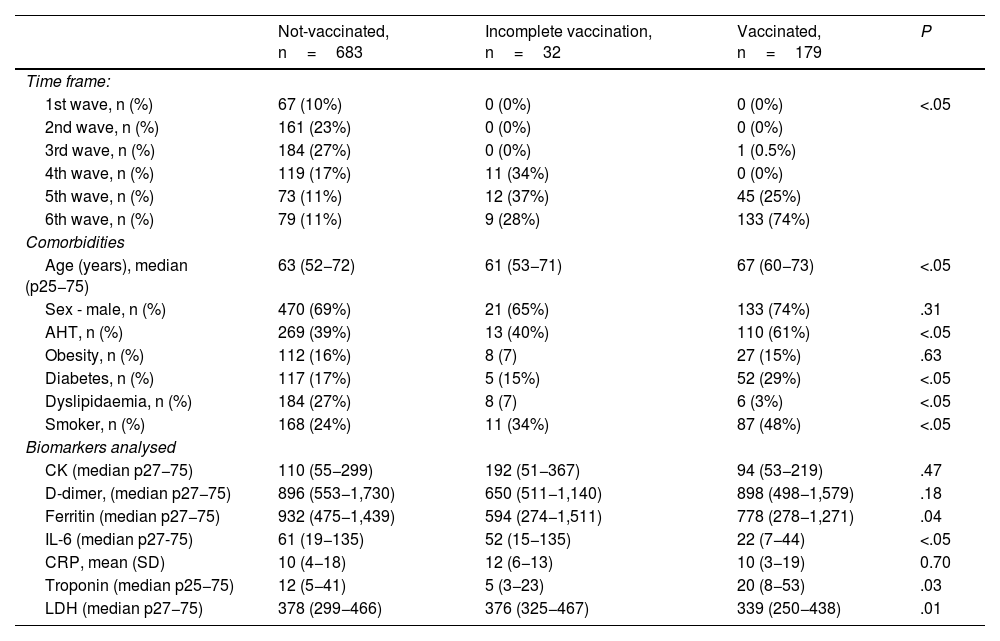
It was considered that 179 patients fulfilled the complete vaccination factor, 32 had an incomplete regimen, and 683 were not vaccinated. A heterogeneous distribution was evidenced (P<.05) between the three groups of patients and the studied time period (waves), as well as significant differences in the mean age of the patients, comorbidities and biomarkers analysed (Table 2).
Comparison of the clinical-epidemiological characteristics of the patients categorised according to groups studied.
| Not-vaccinated, n=683 | Incomplete vaccination, n=32 | Vaccinated, n=179 | P | |
|---|---|---|---|---|
| Time frame: | ||||
| 1st wave, n (%) | 67 (10%) | 0 (0%) | 0 (0%) | <.05 |
| 2nd wave, n (%) | 161 (23%) | 0 (0%) | 0 (0%) | |
| 3rd wave, n (%) | 184 (27%) | 0 (0%) | 1 (0.5%) | |
| 4th wave, n (%) | 119 (17%) | 11 (34%) | 0 (0%) | |
| 5th wave, n (%) | 73 (11%) | 12 (37%) | 45 (25%) | |
| 6th wave, n (%) | 79 (11%) | 9 (28%) | 133 (74%) | |
| Comorbidities | ||||
| Age (years), median (p25−75) | 63 (52−72) | 61 (53−71) | 67 (60−73) | <.05 |
| Sex - male, n (%) | 470 (69%) | 21 (65%) | 133 (74%) | .31 |
| AHT, n (%) | 269 (39%) | 13 (40%) | 110 (61%) | <.05 |
| Obesity, n (%) | 112 (16%) | 8 (7) | 27 (15%) | .63 |
| Diabetes, n (%) | 117 (17%) | 5 (15%) | 52 (29%) | <.05 |
| Dyslipidaemia, n (%) | 184 (27%) | 8 (7) | 6 (3%) | <.05 |
| Smoker, n (%) | 168 (24%) | 11 (34%) | 87 (48%) | <.05 |
| Biomarkers analysed | ||||
| CK (median p27−75) | 110 (55−299) | 192 (51−367) | 94 (53−219) | .47 |
| D-dimer, (median p27−75) | 896 (553−1,730) | 650 (511−1,140) | 898 (498−1,579) | .18 |
| Ferritin (median p27−75) | 932 (475−1,439) | 594 (274−1,511) | 778 (278−1,271) | .04 |
| IL-6 (median p27-75) | 61 (19−135) | 52 (15−135) | 22 (7−44) | <.05 |
| CRP, mean (SD) | 10 (4−18) | 12 (6−13) | 10 (3−19) | 0.70 |
| Troponin (median p25−75) | 12 (5−41) | 5 (3−23) | 20 (8−53) | .03 |
| LDH (median p27−75) | 378 (299−466) | 376 (325−467) | 339 (250−438) | .01 |
SD: standard deviation; AHT: arterial hypertension; LDH: lactate dehydrogenase; CRP: C-reactive protein.
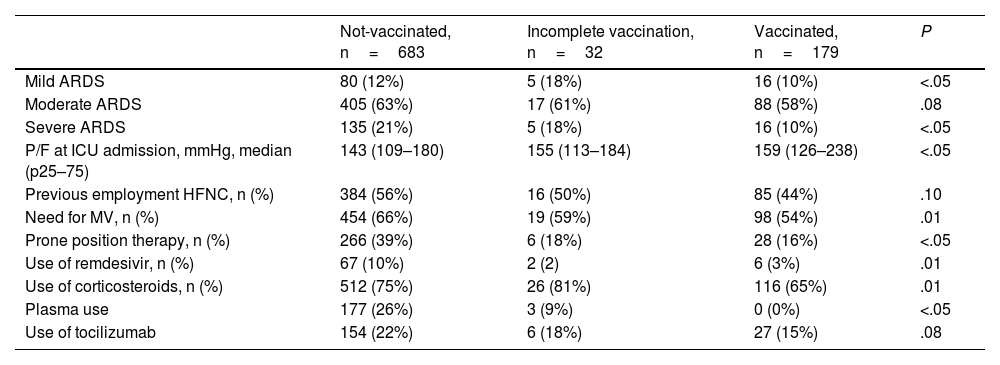
Vaccinated patients presented severe acute respiratory distress syndrome (ARDS) less frequently (10% vs. 21% and 18%) compared to unvaccinated or incompletely vaccinated patients; significant differences were found in the use of MV and different therapies between the studied groups (Table 3)
Comparative analysis between groups of the main variables associated with acute respiratory failure and therapeutic actions in the ICU.
| Not-vaccinated, n=683 | Incomplete vaccination, n=32 | Vaccinated, n=179 | P | |
|---|---|---|---|---|
| Mild ARDS | 80 (12%) | 5 (18%) | 16 (10%) | <.05 |
| Moderate ARDS | 405 (63%) | 17 (61%) | 88 (58%) | .08 |
| Severe ARDS | 135 (21%) | 5 (18%) | 16 (10%) | <.05 |
| P/F at ICU admission, mmHg, median (p25–75) | 143 (109–180) | 155 (113–184) | 159 (126–238) | <.05 |
| Previous employment HFNC, n (%) | 384 (56%) | 16 (50%) | 85 (44%) | .10 |
| Need for MV, n (%) | 454 (66%) | 19 (59%) | 98 (54%) | .01 |
| Prone position therapy, n (%) | 266 (39%) | 6 (18%) | 28 (16%) | <.05 |
| Use of remdesivir, n (%) | 67 (10%) | 2 (2) | 6 (3%) | .01 |
| Use of corticosteroids, n (%) | 512 (75%) | 26 (81%) | 116 (65%) | .01 |
| Plasma use | 177 (26%) | 3 (9%) | 0 (0%) | <.05 |
| Use of tocilizumab | 154 (22%) | 6 (18%) | 27 (15%) | .08 |
ARDS: acute respiratory distress syndrome; P/F: ratio between arterial oxygen pressure and the fraction of inspiratory oxygen; HFNC: high flow oxygen therapy; MV: mechanical ventilation; CRRT: continuous renal replacement therapy.
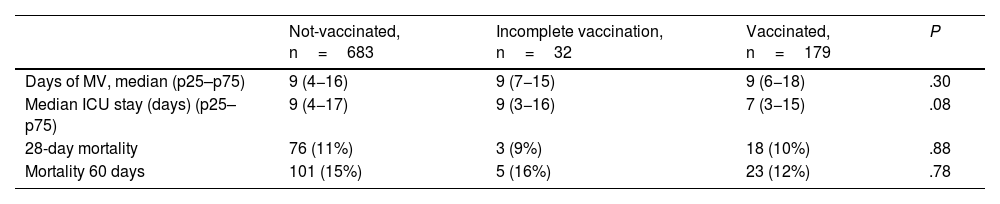
No significant differences were found in the days of MV and the stay in the ICU (Table 4). There were also no differences in mortality at 28 and 60 days. No analysed patient died during the period of 60 and 90 days follow-up. The survival curve did not express differences in the probability of survival at 90 days between the groups studied (log rank test P=.898) (Fig. 1).
Main progressive variables analysed between the different groups categorised by vaccination level.
| Not-vaccinated, n=683 | Incomplete vaccination, n=32 | Vaccinated, n=179 | P | |
|---|---|---|---|---|
| Days of MV, median (p25–p75) | 9 (4−16) | 9 (7−15) | 9 (6−18) | .30 |
| Median ICU stay (days) (p25–p75) | 9 (4−17) | 9 (3−16) | 7 (3−15) | .08 |
| 28-day mortality | 76 (11%) | 3 (9%) | 18 (10%) | .88 |
| Mortality 60 days | 101 (15%) | 5 (16%) | 23 (12%) | .78 |
MV: mechanical ventilation; ICU: Intensive Care Unit.
In the COX regression analysis, only the need for MV during hospitalisation and the LDH value (per unit of measurement) in the first 24h of admission were significantly associated with 90-day mortality (hazard ratio [HR]: 5.78; 95% confidence interval [95% CI]: 1.36–24.48); P=.01 and HR: 1.01; 95% CI: 1.00–1.02; P=.03, respectively.
DiscussionVaccines against COVID-19 have been shown to be highly effective in preventing severe illness, hospitalisation, and death from COVID-19 in clinical studies. However, no vaccine is 100% effective, and some vaccinated patients may still contract the disease and develop a severe and life-threatening condition.12 The length of stay in the ICU and the need for MV are important measures to assess the severity of the disease in patients with severe COVID-19.
In this context, we present a comparative study of the most important characteristics and the progression data of patients admitted to an ICU in a tertiary hospital during the six waves of the pandemic recognised up to now in Spain.
First of all, we would like to highlight the heterogeneous distribution of the patients analysed under the various waves of the pandemic and the different treatments that were received depending on the stage of the pandemic in which the patients were admitted. This situation is to be expected since medical knowledge and experience in managing the disease and the availability of treatments improved over time. However, despite being expected, we consider it important to consider the unequal distribution of vaccinated and unvaccinated patients in the ICU when interpreting the data and results of all research related to COVID-19.13,14
In our results we found a significant disparity in age and comorbidities between the groups compared. These data are in line with previous articles that showed that, compared with unvaccinated patients, vaccinated patients requiring intensive care and MV were older on average and had a higher prevalence of comorbidities such as hypertension and diabetes. However, no significant differences were found in the duration of MV or hospital stay between the two groups.15,16 It has been shown that older people and those with certain comorbidities may have a weaker immune response to the vaccine, which may reduce their protection against severe disease. We must also consider the possibility that the vaccine’s protection may decrease over time, which may be especially relevant for older people who, even they were considered as fully vaccinated, they received the vaccine some time ago.17
In terms of the length of ICU stay and the need for MV in vaccinated and unvaccinated patients with severe COVID-19, heterogeneous results are found in published papers. A major retrospective study that analysed three groups of patients between the ages of 18 and 65, hospitalised for COVID-19 between September and December 2021: not-vaccinated, partially vaccinated, and fully vaccinated; in which a total of 854 patients were included, with a mean age of 47.9±10.6 years and 474 patients (55.5%) were men: 230 patients (26.9%) were fully vaccinated, 97 (11.3%) were partially vaccinated, and 527 (61.7%) were not-vaccinated. Of the fully vaccinated patients, 67% (n=153) were vaccinated with CoronaVac and 33% (n=77) were vaccinated with Pfizer-BioNTech. All patients (n=97) with a single dose were vaccinated with Pfizer-BioNTech. One hundred and thirteen (13.2%) patients were transferred to the ICU. One hundred (11.7%) patients were intubated and 77 (9%) patients died. Advanced age (p=0.028, 95% CI=1.00-1.07, OR=1.038) and a higher Charlson comorbidity index (CCI) (P<.001, 95% CI=1.20–1.69, OR=1,425) were associated with increased mortality while being fully vaccinated (P=.008, 95% CI=0.23–0.80, OR=0.435) was associated with survival in the multivariate analysis. Full-dose vaccination reduced the need for ICU admission by 49.7% (95% CI=17–70) and mortality in 56.5% (95% CI=20–77). When the fully vaccinated group was evaluated, it was found that death was observed more frequently in patients with CCI>3 (19.1 vs. 5.8%, P<.01, OR=3.7).18
Another study, carried out in the United Kingdom, compared the length of stay in the ICU and the need for MV in patients with severe COVID-19 who had been vaccinated and those who had not been vaccinated; the results indicated that, although the length of ICU stay was similar between the two groups, as is the case in our paper, vaccinated patients were less likely to require MV and had a lower mortality rate compared with patients who were not vaccinated.19 In the same vein, a different study conducted in Spain found similar results, with vaccinated patients with severe COVID-19 requiring less MV and having a lower mortality rate compared to not-vaccinated patients.20
As seen, despite vaccination against COVID-19, some severely ill patients may continue to require MV and be hospitalised in the ICU for as long as not-vaccinated patients. The pathophysiological explanation of these findings is complex, involving many variables: firstly, the presence of SARS-CoV-2 virus variants that can partially escape the immune response induced by the different vaccines; these variants may have a higher viral load and trigger a more intense inflammatory response in the body, which may prolong the need for MV and ICU stay.21,22 The presence of comorbidities in vaccinated patients that contribute to their severe disease (old age, obesity, diabetes) and may not be fully protected by the vaccine.23,24 Also, it’s possible that the vaccine is not effective in some patients with a compromised immune system, such as those receiving immunosuppressive therapy. In these patients, the immune response may be insufficient to control infection and prevent the need for MV and ICU stay.25
The purpose of vaccination against COVID-19 is to improve the outcome of the disease by reducing its severity and improving the immune response. Vaccinated patients with severe COVID-19 may experience a more rapid and effective immune response than not-vaccinated patients, reducing the viral load in the body and the systemic inflammation. The adaptive immune response generated in vaccinated patients reduces the virus replication and the release of more viral particles in the body, thereby lowering the viral load and preventing organ tissue damage. The reduced viral load and decreased inflammation are important to prevent lung damage, which is the main cause of the need for MV in patients with severe COVID-19.26,27
Our study has the limitations of a retrospective analysis of a single centre. Furthermore, it is important to note that the definition of complete and incomplete vaccination may change over time in different regions in our setting as vaccination recommendations and protocols are updated. Each country may have its own definitions and vaccination guidelines. Therefore, our results may not be valid to be extrapolated to other centres.
ConclusionsPatients vaccinated against COVID-19 with severe SARS-CoV-2 disease have lower rates of severe ARDS and MV than not-vaccinated patients. However, no differences were found in MV days, ICU days, and mortality.
FundingThis work has not received any type of funding.
Conflict of interestsThe authors declare that they have no conflict of interest.