Post-COVID olfactory dysfunction continues to be studied due to the controversy of the mechanisms involved. The aim was to investigate the olfactory dysfunctions in association with other post-COVID symptoms.
Material and methodsObservational, descriptive and single-center study. The patients had confirmed mild COVID-19 and subjective olfactory dysfunction of more than a month of evolution, which was assessed by Sniffin’ Sticks Olfactory Test.
ResultsA total of 86 patients participated. The mean age was 37.2 years (SD 9.82). 70.9% reported parosmia and 46.5% symptoms of brain fog. A pathological test result was obtained in 72.1% of the participants. The most failed pen was 11 (apple) in 76.7%. Anosmia of pen 15 (anise) was reported more frequently in 24.4% and cacosmia of pen 9 (garlic) in 27.9%. We observed a significant association between patients who reported parosmias and brain fog (RR 2.18; p=0.018), also between parosmia and phantosmia (RR 6.042; p<0.001).
ConclusionThere is some pathological selectivity for certain test pens, a higher prevalence of cognitive symptoms and many patients with combined parosmia and brain fog.
La alteración olfatoria post-COVID continúa en estudio por la controversia sobre los mecanismos implicados. El objetivo de este estudio es caracterizar las alteraciones olfatorias y su relación con otros síntomas post-COVID.
Material y métodosEstudio unicéntrico, observacional y descriptivo. Los pacientes tuvieron infección por COVID-19 leve confirmada y disfunción olfatoria subjetiva de más de un mes de evolución, evaluada con el Sniffin’ Sticks Olfatory Test.
ResultadosSe seleccionaron 86 pacientes. La edad media fue de 37,2 años (DE 9,82). El 70,9% refirieron parosmia y el 46,5% niebla mental. Se obtuvo un test patológico en el 72,1% de los participantes. El lápiz más fallado fue el número 11 (manzana), en el 76,7%. La anosmia fue reportada más frecuentemente con el lápiz 15 (anís) y la cacosmia con el lápiz 9 (ajo) en el 27,9%. Observamos una asociación significativa entre pacientes que refieren parosmias y niebla mental (RR 2,18; p=0,018) y entre parosmia y fantosmia (RR 6,042; p<0,001).
ConclusiónSe observa anosmia y cacosmia selectiva para algunos olores testados. Hay una alta prevalencia de síntomas cognitivos, más frecuentes en pacientes con parosmia.
One of the most frequent symptoms of COVID-19 is olfactory dysfunction.1 The incidence of this complication is highly variable in studies published to date, with the latest studies reporting a range of 19–36% depending on the severity of COVID-19, reaching up to 50% in nonhospitalized patients.2 Therefore, it is possible that olfactory involvement is more frequent in those who have had the mild form of the disease.2,3
Most published studies used subjective scales to measure the degree of olfactory disorder, which can influence the variability in the results. Among the objective tests for assessing smell is the Sniffin’ Sticks Olfactory Test, which was designed and validated more than 20 years ago.4 Its use in patients with COVID-19 has produced results inconsistent with the responses to olfaction questionnaires, giving us a more objective view of the patient's actual clinical condition.5
The objective of this study was to describe the characteristics of the persistent olfactory dysfunction in patients who have had a mild COVID-19 infection using, in addition to patient history, an objective and validated test such as the Sniffin’ Sticks Olfactory Test and to describe the relationship between this symptom and others observed in persistent COVID, such as brain fog.
Materials and methodsThis is a descriptive observational study conducted in the University Hospital Complex of Toledo, a tertiary care center with a patient population of approximately 478,000 individuals. This study was conducted following the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the University Hospital of Toledo. Informed consent was obtained from all participants.
Inclusion and exclusion criteriaThe inclusion criteria were as follows: age between 16 and 50 years (the upper age limit was selected to exclude patients with presbyosmia),4 a positive COVID-19 test (PCR test, rapid antigen test, antibody test or immune cell profiling) and subjective olfactory dysfunction more than one month after COVID-19 recovery.
The exclusion criteria were as follows: a previously diagnosed olfactory or taste dysfunction and hospitalization with acute COVID-19, a previous diagnosis with a relevant neurological or otorhinolaryngological disorders and any dysfunction on neurological examination.
Volunteers who met the abovementioned criteria were recruited over a period of 5 months in the second half of 2021.
ProceduresAll participants underwent a clinical interview in which variables related to olfactory alterations were collected, such as allergies, diabetes mellitus, active smoking, history of recent traumatic brain injury (TBI), neurological and otorhinolaryngological history, the presence of parosmia, foods that had a greatly altered smell, the presence of phantosmia (olfactory hallucinations) and symptoms related to brain fog. A neurological examination was also performed, and the Sniffin’ Sticks Olfactory Test was used to evaluate olfactory function in a quiet and well-ventilated room.
The “Sniffin’ Sticks” (Wedel, Germany, 1997) used in the test are felt-tipped pens that contain a selection of scents.4 It has been validated in various countries, including Spain.6 Patients were instructed not to wear perfume, smoke, eat or drink any substance other than water at least one hour before participating in the study. The set used was the Sniffin’ Sticks 16 (n=16) odor identification set. For each stimulus, the participant is provided with a written list of four response alternatives from which to choose the most appropriate item for identification. The cutoff values were those validated in the general population (anosmia≤7, hyposmia≤8 and ≤11 and normosmia≥12). In addition, to obtain more clinical information, patients indicated which pencils did not smell at all and which had an unknown and unpleasant smell.
Statistical analysisWe used the SPSS v24.0 software (IBM). Quantitative variables are described with the mean and standard deviation, while qualitative variables are described with the frequency and percentage.
To analyze the possible association of demographic variables (sex and age) and clinical variables (presence of allergies, headache, smoking status, brain fog, parosmia and phantosmia) with the result of the Sniffin’ Sticks Olfactory Test, a bivariate analysis was performed between patients with normal and pathological test results using Fisher's exact test for qualitative variables and Student's t test for age.
In addition to comparing patients classified with normal or pathological olfaction, the numerical score of the test and its association with the presence of parosmia and/or phantosmia was analyzed using Student's t test.
Given that age has been mentioned in previous studies as a confounding factor, despite excluding patients older than 50 years (who tend to have a greater incidence of presbyosmia), the Pearson correlation was calculated between age and score.
In addition, a bivariate analysis was performed between the subjective perception of olfactory disorder (parosmia and phantosmia) and the presence of brain fog using Fisher's exact test. The alpha level for statistical significance was 0.05.
ResultsSample characteristicsA total of 86 patients with confirmed COVID-19 and a subjective perception of olfactory dysfunction for more than one month participated in the study, of which 84.9% were women and 15.1% were men. The mean age was 37.2 years (SD 9.82).
The data showed 58.1% of the participants were nonsmokers, and only 36% had some type of allergy, mainly to pollen, grasses and the Arizona cypress, but none presented with allergic rhinitis. None of the participants had diabetes, a relevant otorhinolaryngological history or a recent TBI that could justify their olfactory disorder.
Regarding patient history, 69.8% had no neurological history, and headache was the main reason for consultation among those who had visited a neurologist (25.6%).
Regarding the symptomatology associated with olfactory dysfunction, the vast majority (70.9%) reported some type of parosmia, and more than a third of the patients (36%) described some phantosmia, especially in the first weeks of infection. Almost half had symptoms describing brain fog (46.5%).
Olfactory resultsThe mean time from the onset of the perceived olfactory disorder until consultation was 304.2 days (SD 160.17). The average score of the Sniffin’ Sticks Olfactory Test was 9.40 points (SD 3.01). A total of 72.1% of the participants achieved a pathological result in the test, with 41.9% being classified with hyposmia and 30.2% with anosmia.
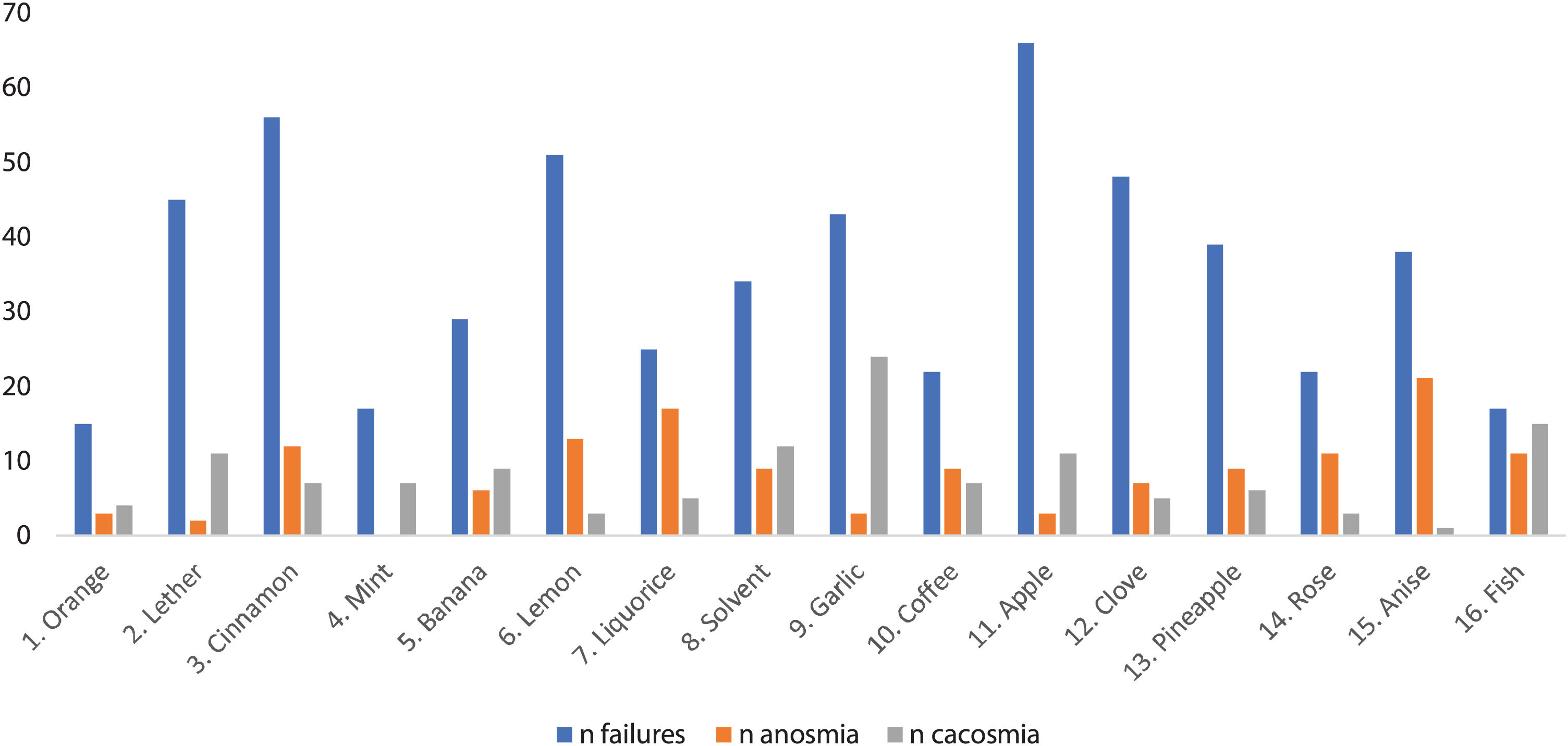
Fig. 1 shows the pens according to the number of patients who failed, reported anosmia or an unpleasant odor them. In the test, the most failed pen was number 11 (apple) (76.7% of the participants), and the least failed pen was number 1 (orange) (17.4%).
In our sample, up to 58.1% of the participants claimed to have anosmia with some of the pencils, and 55.8% claimed to have sensed an unpleasant and unrecognizable odor. The pen with which patients most frequently reported anosmia was number 15 (anise) (24.4% of the participants), while pen number 9 (garlic) was the most frequently identified as having an unpleasant smell (27.9%). In the clinical interview, approximately one-third of the patients stated that they had perceived altered olfaction, mostly cacosmia, of coffee and chocolate, followed by some type of fruit, meat, fish and dairy.
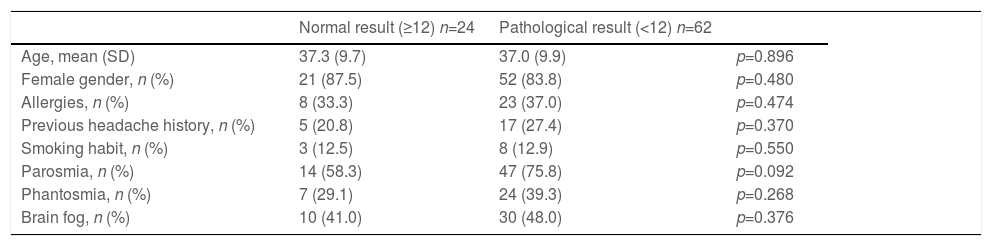
Statistical group comparisonThe bivariate analysis revealed no significant association between the test result (pathological or normal) and the demographic (sex and age) and clinical variables (presence of allergy, exposure to smoke or smoking habit, headache, phantosmia or parosmia) (Table 1).
Statistical group comparison.
| Normal result (≥12) n=24 | Pathological result (<12) n=62 | ||
|---|---|---|---|
| Age, mean (SD) | 37.3 (9.7) | 37.0 (9.9) | p=0.896 |
| Female gender, n (%) | 21 (87.5) | 52 (83.8) | p=0.480 |
| Allergies, n (%) | 8 (33.3) | 23 (37.0) | p=0.474 |
| Previous headache history, n (%) | 5 (20.8) | 17 (27.4) | p=0.370 |
| Smoking habit, n (%) | 3 (12.5) | 8 (12.9) | p=0.550 |
| Parosmia, n (%) | 14 (58.3) | 47 (75.8) | p=0.092 |
| Phantosmia, n (%) | 7 (29.1) | 24 (39.3) | p=0.268 |
| Brain fog, n (%) | 10 (41.0) | 30 (48.0) | p=0.376 |
The test score was not associated with age (p=0.623), the perception of phantosmia (p=0.096) or brain fog (p=0.732).
A total of 59% of the 61 patients who reported parosmia in the clinical interview had associated symptoms of brain fog, and the association between these symptoms was significant (RR 2.18; 95% CI 1.087–4.088; p=0.018). There was also a significant association between participants who reported parosmia and those who reported phantosmia (RR 6.042; 95% CI 1.559–23.418; p<0.001). The presence of brain fog was not related to the age of the participants (p=0.314) or the presence of phantosmia (p=0.069).
DiscussionOf the 86 patients recruited with a subjective perception of olfactory disorder lasting more than one month, 27.9% achieved a normal result in the Sniffin’ Sticks test. This is consistent with previous publications5 that have described a dissociation between personal perception and the results of olfactory tests. However, it should be noted that unlike in other studies, the patients in our sample had a prolonged duration of symptom presentation at the time of consultation (mean time 304.2 days, SD 160.17).
Female predominance was revealed among our patients (84.9%). According to previous studies, olfactory loss postviral prevails among female patients. Increase in numbers of female patients can be attributed by gender-related variation in the inflammatory process and greater tendency of females to volunteer for studies.5
It is worth noting the large percentage (70.9%) of patients in the sample who reported having parosmia, usually unpleasant (cacosmia), with food. In addition, the percentage of patients with reported parosmia was higher for those with a pathological test result (75.8% vs. 58.3%), although in our study, this difference was not significant.
It should also be mentioned that approximately half of the cohort had symptoms of brain fog (46.5%), a higher percentage than that described in studies that also included patients without olfactory dysfunction (5%).2 In addition, in our sample, we observed a significant association between patients who reported parosmia and the presence of brain fog. These two findings may lead to new research directions on the association between persistent post-COVID olfactory dysfunction and brain fog, which could yield interesting results regarding the possible pathophysiological mechanisms underlying this association.
When characterizing the persistent olfactory dysfunction produced by COVID-19, the results indicate that this disorder could be accompanied by a certain element of selectivity for certain odors. As seen in Fig. 1, the patients tended to indicate anosmia for pen 15 (anise) and cacosmia for pen 9 (garlic). We also observed a certain connection with some foods, as approximately one-third of the patients stated that they had a disordered perception, mostly manifesting as cacosmia of coffee and chocolate, followed by some type of fruit, meat, fish and dairy. These results could suggest the existence of an affinity by the virus for a specific olfactory receptor, for example, OR1G1 and OR52D1 in the case of anise and many others in the case of fruity and sweet odors7 which would explain the frequent mention of the latter in the interview and the fact that the most failed pen was 11 (apple). This selectivity could be the cause of the fluctuation of the olfactory symptoms reported by the patients; rather than an authentic clinical fluctuation, the sensation could be a variation of the exposed odor.
According to the results of this study, the olfactory damage produced by SARS-CoV-2 is independent of other variables that are associated with olfactory disorder, including age. This suggests that the pathological results obtained in this study were produced by the action of the virus and not by a previous predisposition related to other variables.
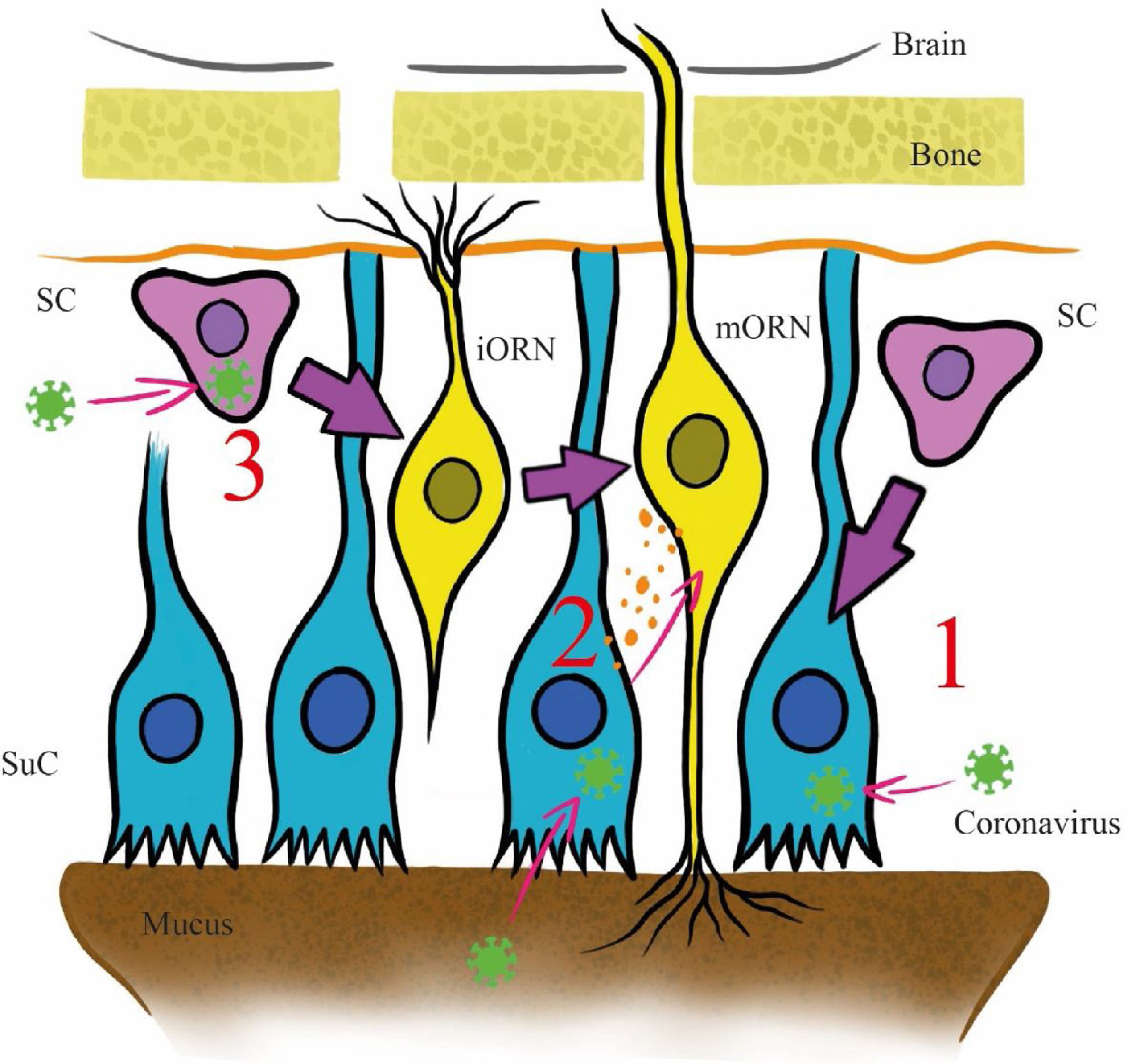
The pathophysiology of the olfactory disorder in COVID-19 has remained unresolved until now. Three main mechanisms have been postulated in the literature to explain it (Fig. 2). All hypotheses are based on the high affinity with which the viral spike protein binds to the entry-point protein angiotensin-converting enzyme 2 (ACE2).8 This protein is present in the sustentacular cells of the olfactory mucosa and the injury to these cells could explain the cases of transient olfactory dysfunction, as the duration of the symptoms coincides with the recovery time (approximately two weeks), excluding axonal destruction9 (Fig. 2, Path 1).
Proposed invasion pathways for SARS-CoV-2 in the olfactory epithelium. Abbreviation: SC: stem cell; SuC: sustentacular cell; iORN: immature olfactory receptor; mORN: mature olfactory receptor. Pathway 1: The virus invades sustentacular cells via ACE2 receptors. Pathway 2: the virus reaches the olfactory receptors from the sustentacular cells through exosomes. Pathway 3: the virus invades stem cells before they differentiate into olfactory receptor.
The stem cells of the olfactory epithelium expresses ACE2, but its presence in the olfactory receptor neurons of the mucosa remains highly controversial.9 Another postulated mechanism involves a transneuronal invasion from the sustentacular cells to the olfactory receptors of the mucosa through exosomes, as has been previously documented with other viruses, and subsequent transmission to the central nervous system (CNS), where ACE2 receptors have been identified9 (Fig. 2, Path 2). In the third mechanism, the virus invades stem cells before they differentiate into olfactory receptor neurons and from there reach the CNS9 (Fig. 2, Path 3).
Given that the selected participant sample recovered from mild-moderate COVID-19 without requiring hospitalization or demonstrating substantial systemic involvement at other levels as well as the persistence of symptoms beyond the time of sustentacular cell regeneration and their likely selective nature due to possible involvement of olfactory receptors or nerve clusters, the data of this study could support the hypothesis of involvement of the CNS by the intranasal route, as previously shown with other coronaviruses in studies with mice.10 This hypothesis could also be supported by the high prevalence of cognitive symptoms in this participant sample.
Nevertheless, there are several notable limitations. Generalization of our results should be done with care because small sample size, the single-center nature of the study and few studies with objective evaluations in non-severe COVID-19 patients to compare. In addition, the number of participants in the study required use of a bivariate analysis of the association between variables. Larger studies that could allow for a multivariate analysis should be considered in the future.
ConclusionThe results of our study indicate that there is some pathological selectivity for certain test pens (anosmia with anise and cacosmia with garlic), a higher prevalence of cognitive symptoms and many patients with combined parosmia and brain fog. These data, in addition to the prolonged time of olfactory dysfunction in our sample greater than the regeneration time of the sustentacular cells, could lead to discovery of an affinity of the virus for certain olfactory receptors or association structures of nerve pathways. However, due to our limitations these findings should be interpreted with caution and should be corroborated in future studies with more specific tests.
Informed consentInformed consent was obtained from all participants.
FundingThe authors declared no financial support with respect to the research, authorship, and/or publication of this article.
Conflicts of interestThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
This paper and the research behind it would not have been possible without the exceptional support of the Neurology Department at the University Hospital of Toledo and Long COVID support groups.