There are limited data describing the long-term renal outcomes of critically ill COVID-19 patients with acute kidney injury (AKI) and continuous renal replacement therapy (CRRT) and invasive mechanical ventilation.
MethodsIn this retrospective observational study we analyzed the long-term clinical course and outcomes of 30 critically ill patients hospitalized with COVID-19 during the peak of highest incidence in the first wave, with acute respiratory distress syndrome (ARDS) and AKI that required CRRT. Baseline features, clinical course, laboratory data, therapies and filters used in CRRT were compared between survivors and non-survivors to identify risk factors associated with in-hospital death. Renal parameters: glomerular filtration rate, proteinuria and microhematuria were collected at 6 months after discharge.
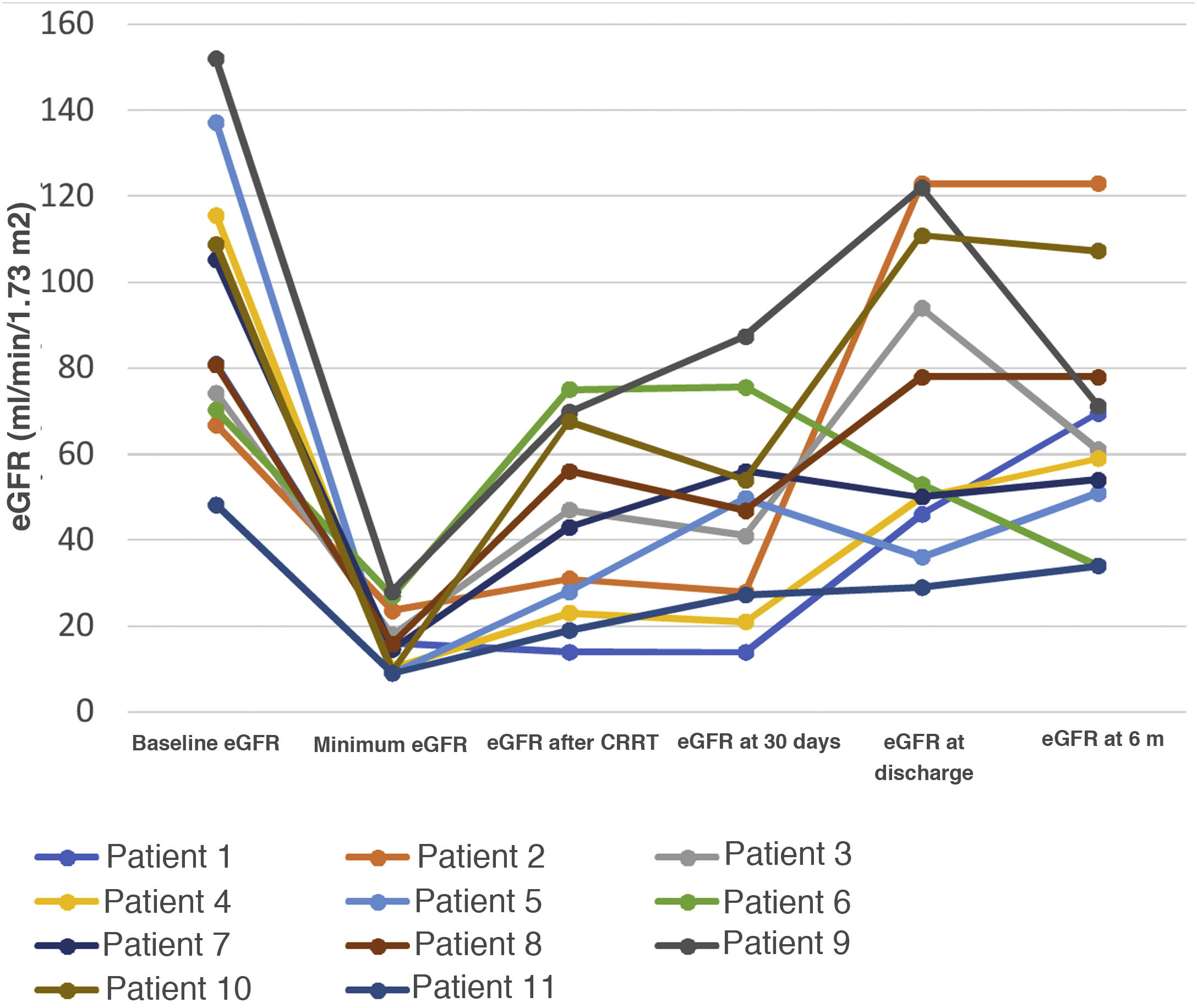
Results19 patients (63%) died and 11 were discharged. Mean time to death was 48 days (7–206) after admission. Patients with worse baseline renal function had higher mortality (P = 0.009). Patients were treated with CRRT for an average of 18.4 days. Filters with adsorptive capacity (43%) did not offer survival benefits. Regarding long-term renal outcomes, survivor patients did not receive any additional dialysis, but 9 out of 11 patients had an important loss of renal function (median of eGF of 44 (13–76) ml/min/1.73 m2) after 6 months.
ConclusionMortality among critically ill hospitalized patients diagnosed with COVID-19 on CRRT is extremely high (63%). Baseline renal function is a predictor factor of mortality. Filters with adsorption capacity did not modify survival. None survivor patients required long-term dialysis, but an important loss of renal function occurred after AKI episode related to COVID-19 infection.
La interacciónde COVID-19, ventilación mecánica invasiva (VMI) y fracaso renal agudo (FRA) con necesidad de terapia continua de reemplazo renal (TCRR) es conocida, pero hay pocos datos publicados sobre el pronóstico a largo plazo de este tipo de FRA.
MétodosEste estudio analiza los resultados a largo plazo de 30 pacientes ingresados en la UCI por neumonía por COVID-19, con VMI y FRA con TCRR en el pico de máxima incidencia. Comparamos las características basales, la evolución clínica y bioquímica y los diferentes filtros usados en la TCRR para identificar los factores de riesgo asociados a la muerte intrahospitalaria. Se analizaron el filtrado glomerular estimado (FGe), la proteinuria y la hematuria a los 6 meses de seguimiento de los supervivientes.
ResultadosDe los 30 pacientes, 19 fallecieron y 11 fueron dados de alta. Los pacientes con peor función renal tuvieron mayor mortalidad (p = 0,009). Los filtros usados con capacidad adsortiva no ofrecieron beneficios en cuanto a la supervivencia. De los 11 supervivientes, ninguno requirió terapia renal sustitutiva (TRS) una vez superada la infección, pero tuvieron una pérdida importante y mantenida en el tiempo de función renal (FGe de 44 ml/min/1,73 m2).
ConclusiónLa mortalidad en pacientes con neumonía por COVID-19 que requieren VMI y TCRR es extremadamente elevada (63%). Los filtros con capacidad adsortiva no modificaron la supervivencia. La función renal basal fue un factor predictor de mortalidad. En este tipo de FRA el deterioro de la función renal no se recupera, objetivándose una reducción importante del FGe a los 6 meses.
The first reports of severe acute respiratory syndrome coronavirus (SARS-CoV-2) came from Wuhan, China, in December 2019, but it quickly spread worldwide1,2. SARS-CoV-2 is a single-stranded RNA virus that primarily affects the respiratory tract of humans and animals3. Severe infection can lead to systemic disorders. The exact aetiology of acute kidney failure (AKF) in COVID-194 is not yet known. The close relationship between respiratory failure and AKF suggests that it could be due to a mechanism of ischemic acute tubular necrosis, which occurs in situations of systemic circulatory collapse. However, the mechanism appears to be more complex, with the development of cytokine storm-mediated renal injury, activation of the angiotensin II pathway, dysregulation of the complement pathway, hypercoagulation and microangiopathy5. Extracorporeal support therapies have been proposed for the removal of tissue-damaging cytokines from the bloodstream in patients with sepsis and may have beneficial effects in critically ill patients with COVID-196,7. The objective of our study was to analyse the clinical course: renal function and long-term mortality of patients admitted to the ICU with invasive mechanical ventilation (IMV) and AKF with continuous renal replacement therapy (CRRT) and the role of the different filters used in renal replacement therapies, in a tertiary hospital in Madrid, during the peak of maximum incidence in the first wave of the pandemic.
Material and methodsStudy designRetrospective, observational, analytical, single center.
Inclusion criteria- •
Adults >18 years with COVID-19 pneumonia, in need of IMV, hospitalised in ICU from 10th–31st March 2020.
- •
AKF requiring CRRT.
Demographic data, clinical, laboratory and radiological patterns, treatment regimens and mortality were recorded.
The diagnostic method for SARS-CoV-2 was through PCR test (Thermofisher Multiplex RT-PCR) with a sample of nasopharyngeal swab on admission to the emergency department. Lab controls included complete blood count, coagulation times and biochemistry. These parameters were analysed at admission, 7 and 30 days after discharge or at death. Kidney function data were collected in survivors 6 months after hospital discharge.
Treatment regimenThroughout the outbreak, the treatment regimen was modified according to the protocols issued by the hospital committee. Thus, patients were initially treated with lopinavir/ritonavir plus hydroxychloroquine and interferon beta, restricting the use of methylprednisolone or dexamethasone for patients who developed acute respiratory distress syndrome (ARDS), with PaO2/FiO2 < 200 or orotracheal intubation requirement.
From 21st March 2020 the use of azithromycin was also added to the previous regimen for patients admitted to the ward and tocilizumab for patients with ARDS and interleukin 6 (IL-6) levels >40 pg/mL.
Patients admitted to the ICU also received prophylactic anticoagulation with low molecular weight heparin (enoxaparin) at a dose of 4000 IU/day SC.
The study was approved by the Clinical Research Ethics Committee of our hospital.
Definitions and measurementsRenal function was measured by estimated glomerular filtration rate (eGFR), serum creatinine (Cr) before admission, at admission, maximum peak and at 30 days after admission, at hospital discharge or before death and at 6 months after discharge in surviving patients.
The eGFR was calculated using the abbreviated equation Modification of Diet in Renal Disease (MDRD-4).
A dipstick urinalysis was performed to screen for urinary abnormalities in the survivors’ group as an initial evaluation of proteinuria and haematuria.
CRRT protocolIndications for CRRT were AKF AKI 3 (acute kidney injury) by creatinine levels, according to the KDIGO guidelines (The Kidney Disease Improving Global Outcomes)8 or any AKF AKI 1 or 2 that due to volume overload or metabolic acidosis or hyperkalaemia did not respond to initial conservative treatment, subsequently requiring renal replacement therapy (RRT). In non-oliguric AKF, defined as volume of diuresis >500 ml/day, continuous veno-venous haemodialysis (CVVHD) was indicated and in oliguric AKF with diuresis volume <500 ml/day continuous veno-venous hemofiltration (CVVH) was used.
The so-called standard filters for the CRRT were: polysulfone AV600® (Fresenius Medical Care Bad Homburg, Germany) and the AN69 HF M100® (Baxter, IL, USA), which is a copolymer of acrylonitrile and sodium methallyl sulfonate. The filter used for CRRT, which also associates cytokine and endotoxin adsorption capacity, is called oXiris® (Baxter, IL, USA), which is a modified AN69 ST that associates heparin and polyethyleneimine, grafted onto the filter capillaries that confer the adsorptive capacity of this membrane.
The standard initial clearance dose was between 25−30 ml/kg/h, and the daily negative balance by ultrafiltration was determined at the discretion of the attending physician based on the haemodynamic status and needs of the patient. We associated 1% sodium heparin perfusion administered prefilter for circuit anticoagulation at doses of 250−500 U/h.
As a central access route, the right internal jugular vein or the femoral vein was preferred, with priority being given to jugular access in patients requiring pronation.
Statistical analysisAll statistical analyses were performed using SPSS 21.0 software (SPSS; Chicago, IL, USA). Qualitative variables are presented with their frequency distribution. Quantitative variables, with their mean and standard deviation (SD) or median and interquartile range (IQR) according to their distribution. The association between qualitative variables was analysed with the χ2 test (chi square) or Fisher's exact test. Quantitative variables were analysed using Student's t-test (in comparisons of one variable with two categories) and/or analysis of variance (ANOVA or Friedman test). The mean differences due to the individual or main effect of each factor and/or the effect of their interactions have been assessed using this method. The distribution of the variables was verified in all cases with the theoretical models and the hypothesis of homogeneity of variance has been tested. Survival analysis (log rank) was used to determine the influence of different factors and filters on hospital death. A p value < 0.05 was considered to be statistically significant.
ResultsDuring the period from 10th to 31st March 2020, 137 critically ill patients in need of IMV due to COVID-19 were hospitalised in the different ICU rooms (7 in total) at the Hospital General Universitario Gregorio Marañón (HGUGM). A total of 42 patients (30.6%) developed AKF, 30 of them requiring CRRT (22%). The 30 patients included had a mean age of 62 ± 11 (range: 31–78), with 70% male.
Most of the patients were hypertensive (73%), with 57% receiving renin angiotensin aldosterone system (RAAS) inhibitors, 50% had dyslipidaemia, 43% were obese, and 20% had diabetes. The most common symptoms at emergency admission were fever (83%), cough (57%) and asthenia (57%). 33% of the patients had gastrointestinal symptoms.
On admission from the emergency department, 15 of the 30 patients (50%) had a baseline oxygen saturation <95%, with radiological patterns already pathological in 97% of the patients, showing bilateral peripheral infiltrates in 21 and unilateral in 8 of them. Only one of these 30 patients had an absence of infiltrates at the time of emergency department visit, which we have defined as a normal X-ray.
There were no significant differences in clinical, demographic or radiological values at admission between the survivors and the deceased.
Treatment regimenAll patients received treatment with hydroxychloroquine and lopinavir/ritonavir. Nineteen (63%) also associated azithromycin, 29 (97%) corticosteroids, 13 (36%) interferon® and 24 (80%) tocilizumab.
No significant differences were found between the treatment administered between the survivors and the deceased.
Clinical course and outcomesPatients underwent follow-up until 30th December 2020, with a median time of 116 days (7–262).
Eleven patients were discharged, and 19 patients (63%) died during hospitalization.
The average time of hospitalization until death was 48 days (7–206), and the mean time in CRRT was 18.4 days (1–41).
The mean Apache II score on admission to the ICU was 14.2 ± 4.7, without differences between the Apache II score of the survivors (12.7 ± 5.2) and of the deceased (15.3 ± 3.9) (p = 0.1).
Mortality in the ICU of patients with IMV without AKF during the same period of time was 37%, rising to 43% in the subgroup of patients who developed AKF without the need for extrarenal clearance (mean creatinine 1.37 ± 0.74 mg/dl). Both groups had a similar score on the Apache II: 13.74 ± 4.2 and 12.4 ± 3.4, respectively.
During ICU admission, lymphopenia, anaemia, dimer D and C-reactive protein (CRP) increased progressively, but without differences between the survivors and the deceased. Only dimer D and procalcitonin at 30 days were higher in patients who died (Table 1).
Laboratory values at the emergency department, at 7 and 30 days (at discharge or before death).
| Baseline | Day 7 | Day 30 | |
|---|---|---|---|
| Lymphocyte count (⋅109) | |||
| Total (n = 30) | 0.71 ± 0.31 | 0.46 ± 0.29 | 0.77 ± 0.46 |
| Survivors (n = 11) | 0.69 ± 0.23 | 0.51 ± 0.31 | 0.8 ± 0.42 |
| Deceased (n = 19) | 0.73 ± 0.38 | 0.43 ± 0.29 | 0.74 ± 0.50 |
| Haemoglobin (g/l) | |||
| Total (n = 30) | 13.8 ± 2.4 | 11.1 ± 2.1 | 8.3 ± 0.5 |
| Survivors (n = 11) | 13.8 ± 2.6 | 11.7 ± 2.7 | 8.3 ± 0.5 |
| Deceased (n = 19) | 13.8 ± 2.3 | 10.8 ± 1.5 | 8.2 ± 0.8 |
| D-dimer (ng/mL) | |||
| Total (n = 30) | 524 (272−847) | 2040 (830−3607) | 2314 (1140−3288) |
| Survivors (n = 11) | 376 (252−801) | 996 (473−1930) | 1228 (815−1928) |
| Deceased (n = 19) | 601 (307−895) | 3199 (975−4823) | 2825 (975−4823) |
| p = 0.036 | |||
| Serum procalcitonin (μg/l) | |||
| Total (n = 30) | 0.39 (0.17−1.75) | 0.83 (0.29−2.81) | 0.46 (0.17−1.77) |
| Survivors (n = 11) | 0.23 (0.09−0.66) | 0.90 (0.42−1.40) | 0.30 (0.42−1.40) |
| Deceased (n = 19) | 0.54 (0.20−6.71) | 0.81 (0.24−5.36) | 2.08 (0.36−2.62) |
| p = 0.029 | |||
| C-reactive protein (mg/dl) | |||
| Total (n = 30) | 17.6 (11.3−26.9) | 4.3 (2.2−12.8) | 7.8 (2.4−14.9) |
| Survivors (n = 11) | 13.8 (7.4−19.7) | 3.95 (2.7−25.3) | 3.7 (1.3−10) |
| Deceased (n = 19) | 18.3 (10−28.1) | 8.0 (5.9−6.4) | 3.7 (1.9−8.6) |
Concomitant infections were found in 5 of the survivors (3 catheter-associated bacteraemia and 2 bacteraemia with fungemia) and in 8 of the deceased patients (1 catheter-associated bacteraemia, 2 fungemias and 5 bacteremias with fungemia).
The oXiris filter, with adsorptive properties, did not improve survival (log Rank = 0.945, p = 0.833) of our patients. Nor did we find any significant difference in terms of mortality between the different therapeutic regimens used.
Renal function and CRRTAKF occurred on average on day 15 ± 8 from the onset of symptoms, with Cr figures at ICU admission of 1.6 ± 1.2 mg/dl, and peak Cr of 4.1 ± mg/dl.
86.6% of patients were classified by Cr level as AKI 3 according to the KDIGO guidelines8, while the remaining 13.4% had AKF classified as AKI 2 or AKI 1, although all would fall into the AKI category 3 due to the need for RRT, in our case in continuous therapy mode.
Patients with AKI 1 required CRRT due to oliguria and ion channel abnormalities, and those with AKI 2 due to volume overload refractory to treatment with diuretics.
Twenty-four patients had RRT in CVVH mode and 6 patients in CVVHD mode.
Fourteen of the 19 patients who died required CRRT at the time of death due to lack of renal function recovery.
The description of the CRRT of survivors and deceased is shown in Table 2.
CRRT parameters.
| Survivors (n = 11) | Deceased(n = 19) | p | |
|---|---|---|---|
| CRRT modality, n (%) | |||
| CVVHD | 2 (18%) | 4 (21%) | 1.00 |
| CVVH | 9 (82%) | 16 (84%) | 1.00 |
| Central venous line | |||
| Internal jugular vein | 7 (64%) | 6 (32%) | |
| Femoral vein | 4 (36%) | 13 (68%) | 0.13 |
| CRRT oXiris, n (%) | 5 (45%) | 8 (42%) | 1.00 |
| oXiris / number of sessions per patient | 8 (3−13) | 10 (7−24) | |
| Filter half-life, days | 1.19 ± 0.23 | 1.33 ± 0.47 | 0.59 |
| Standard CRRT, n (%) | 6 (54%) | 11 (58%) | 1.00 |
| Standard filter/number of sessions per patient | 21 (8−30) | 18 (8−24) | |
| Filter half-life, days | 1.56 ± 0.24 | 1.58 ± 0.47 | 1.00 |
With respect to baseline patterns, comorbidities, Apache II Score or treatments administered, no significant differences were found between the use of the standard filter vs oXiris (Table 3).
Demographic, clinical data, radiological patterns and treatment administered between patients with CRRT with standard filter vs oXiris.
| Standard filter | oxiris | p | |
|---|---|---|---|
| (n = 17) | (n = 13) | ||
| Age, years | 65 ± 7 | 58 ± 14 | 0.11 |
| Males | 11 (65%) | 10 (77%) | 0.69 |
| AKF stage at the start of CRRT | |||
| AKI 1 | 2 (13%) | 0 (0%) | |
| AKI 2 | 0 (0%) | 2 (18%) | 0.12 |
| AKI 3 | 15 (87%) | 11 (82%) | |
| Cr peak | 4.3 ± 1.7 | 3.8 ± 1.5 | 0.39 |
| Hypertension | 12 (71%) | 10 (77%) | 1.00 |
| RAASi | 7 (41%) | 10 (77%) | 0.07 |
| Diabetes | 3 (18%) | 3 (23%) | 1.00 |
| Heart disease | 1 (6%) | 2 (15%) | 0.38 |
| Dyslipidemia | 9 (53%) | 6 (46%) | 0.44 |
| Obesity | 5 (29%) | 8 (61%) | 0.13 |
| Previous chronic kidney disease | 2 (13%) | 5 (33%) | 0.39 |
| Fever | 13 (76%) | 12 (92%) | 0.35 |
| Cough | 8 (47%) | 9 (69%) | 0.28 |
| Fatigue | 8 (47%) | 9 (69%) | 0.28 |
| Diarrhoea, nausea or vomiting | 5 (29%) | 5 (38%) | 0.70 |
| Apache II Score | 14.4 ± 5.7 | 13.8 ± 3.1 | 0.07 |
| Time from emergency to ICU admission, days | 3.2 ± 3.2 | 1.5 ± 1.5 | 0.06 |
| Baseline oxygen saturation | 85.1 ± 10.9 | 82.1 ± 10.2 | 0.48 |
| Bilateral infiltrates - ground glass | 11 (65%) | 10 (77%) | 0.46 |
| Unilateral infiltrate | 5 (29%) | 3 (23%) | 0.46 |
| Normal chest x-ray | 1 (6%) | 0 (0%) | 0.46 |
| Treatments | |||
| Corticosteroids | 16 (94%) | 13 (100%) | 1.00 |
| Beta-interferon | 9 (53%) | 8 (61%) | 0.72 |
| Azithromycin | 10 (59%) | 9 (69%) | 0.70 |
| Tocilizumab | 13 (76% | 11 (84%) | 0.67 |
| Hydroxychloroquine | 17 (100%) | 13 (100%) 13 (100%) | 1.00 |
| Lopinavir/ritonavir | 17 (100%) | 1.00 | |
| Mortality | 11 (65%) | 8 (61%) | 1.00 |
The evolution of laboratory parameters between patients treated with oXiris vs standard filters at admission, at 7 and at 30 days did not show significant differences either (Table 4).
Laboratory findings on admission to the emergency department, 7 and 30 days after the clinical onset with the different filters.
| Baseline | Day7 | Day 30 | |
|---|---|---|---|
| Lymphocyte count (×109/l) | |||
| Standard filter (n = 17) | 0.73 ± 0.34 | 0.44 ± 0.29 | 0.70 ± 0.60 |
| oXiris filter (n = 13) | 0.68 ± 0.28 | 0.49 ± 0.31 | 0.80 ± 0.32 |
| Haemoglobin (g/l) | |||
| Standard filter (n = 17) | 13.8 ± 2.3 | 11.1 ± 1.9 | 8.2 ± 0.8 |
| oXiris filter (n = 13) | 13.7 ± 2.5 | 11.3 ± 2.3 | 8.2 ± 0.7 |
| D-dimer (ng/mL) | |||
| Standard filter (n = 17) | 307 (252−756) | 1618 (680−7704) | 2326 (877−4271) |
| oXiris filter (n = 13) | 673 (399−871) | 2040 (844−3430) | 1788 (1230−3205) |
| Serum procalcitonin (μg/l) | |||
| Standard filter (n = 17) | 0.2 (0.1−6.7) | 0.8 (0.2−1.9) | 0.4 (0.2−1.9) |
| oXiris filter (n = 13) | 0.5 (0.4−1.7) | 0.9 (0.3−1.6) | 0.6 (0.2−2.2) |
| C-reactive protein (mg/dl) | |||
| Standard filter (n = 17) | 14.4 (5.8−24.3) | 5.3 (3.2−24.6) | 8.0 (1.5−16.4) |
| oXiris filter (n = 13) | 18.8 (13.9−25.8) | 2.9 (1.6−7.1) | 7.8 (2.8−10.4) |
| p < 0.05 |
Basal renal function was worse in the deceased than in the survivors (eGFR 66.5 ± 22.4 vs 94.6 ± 31.9 ml/min/1, 73 m2 (p = 0.009), with eGFR ≤ 60 ml/min/1,73 m2 in 7 of the 30 patients.
At 6 months after discharge, renal function had changed from baseline parameters at hospital admission: 9 of the 11 survivors had a mean eGFR loss of 44 (IR 13–76) ml/min/1.73 m2 (Fig. 1), 1 patient had albuminuria < 1 g/day and 3 patients had microhaematuria on urinalysis.
DiscussionThe main objective of this study was to analyse the clinical course: long-term renal function (6 months) and mortality in a series of patients hospitalized in the ICU with COVID-19 pneumonia requiring IMV and AKF who required CRRT at the peak of the pandemic's incidence.
Our main findings were: 1) that mortality in this type of patient was extremely high (63%); 2) that only renal function at admission was associated with increased mortality, finding no association with other general risk factors, such as hypertension, obesity or diabetes; 3) that filters with adsorptive capacity in our cohort did not modify survival, and 4) that survivors did not require long-term RRT, but neither did they fully recover their initial renal function, maintaining at 6 months a significant reduction in renal function compared to baseline.
The development of AKF in critically ill patients with COVID-19 infection differs among published series. In the Chinese series of Guan et al.9, of the 5.5% of patients who ended up in the ICU, 0.5% developed AKF, but in series described in the US and Europe10,11 the incidence of AKF in critically ill COVID-19 patients is around 20–40%, with the development of AKF being a marker and a negative prognostic factor in survival12. In 5700 patients with COVID-19 in a New York cohort the development of AKF in those hospitalised in the ICU was 36%, much higher than that published in China and other different areas (0.5–29%)13,14. In the ICU of HGUGM the percentage was the same as that described in the New York series, 36.6%.
The percentage of RRT requirement in the critically ill COVID-19 patient ranged from 20% in the Chinese series with CRRT15 to 31%, without specifying whether the technique was continuous or intermittent in the American cohort16, and 22% in our series.
A recently published meta-analysis17 shows a high prevalence of urinary abnormalities and renal dysfunction in patients with COVID-19: 4.5% AKF, and 57% of patients with proteinuria during hospitalization. Our data show that patients who survive COVID-19 infection maintain long-term loss of renal function, despite most of them returning to normal urinary sediment levels: only one patient remained with proteinuria and 3 patients with microhaematuria.
Between 5 and 10% of patients with COVID-19 require ICU admission with IMV due to progression to severe pneumonia with ARDS. In these patients the immune response is exaggerated, with the onset of a “cytokine storm” that develops into a systemic inflammatory response syndrome (SIRS). In the absence of effective therapy, the indications for CRRT should be those widely accepted for AKF treatment, such as restoring immune homeostasis, removing inflammatory mediators that cause SIRS, preventing and correcting volume overload when diuretics are ineffective. In the absence of established treatment options for COVID-19, the pathophysiological rationale could support the use of sorbent cartridges, which could remove or reduce by adsorptive mechanism the released cytokines and thereby decrease lung and kidney damage18. Evidence for these treatments is currently limited. A retrospective case series of 50 critically ill COVID-19 patients treated with CRRT and Cytosorb cartridge has recently been published®19. These patients showed a reduction in inflammatory parameters after use of the filter, but no control group was included for comparison. Another membrane with adsorptive capacity is the AN69 ST oXiris, whose heparin- and polyethyleneimine-impregnated surface offers the possibility of adsorbing endotoxins and cytokines through ionic interactions. In our series we used this type of membrane in 13 of the patients. However, we found no clinical, laboratory, or survival differences compared to patients in whom we used standard filters. Our patients were probably already in an advanced stage of the disease, with IMV and a requirement for CRRT.
The current expert opinion is to use such adjuvant therapies in early stages, prior to the need for IVM.
The absence of serial IL-6 determinations in all patients, together with the tocilizumab treatment and the difficulty of interpreting IL-6 levels once this antibody is administered, do not allow us to draw definitive conclusions in the case of COVID-19 infection regarding the adsorptive capacity of the filters, and in particular of oXiris.
However, the results we obtained changed our clinical practice with regard to its use.
Two clinical trials are currently underway: NCT 04597034, which compares the efficacy of oXiris versus AN69 in COVID-19, and NCT 04478539, which studies this membrane as a treatment modality for COVID-19.
With regard to mortality in patients intubated by COVID-19 with CRRT, although there are few and inconsistent published data, the percentage of mortality in this type of patient varies from 39% described by Eriksson et al.20 to 90.7% in the group of Zahid et al.21.
Hirsch et al.5 described that, in a series of 285 patients with CRRT, 157 died and 9 were discharged; the mortality rate (55%) was similar to that of our group, but 118 patients remained on RRT at the end of follow-up. Baseline renal function was also a predictor of mortality. What seems clear is that mortality is much higher in patients requiring RRT compared to those who develop ARF but do not require clearance, as shown by our ICU data (43 and 63%, respectively), with no difference in the Apache II Score.
The published findings on the impact of renal function on prognosis in COVID-19 infection are corroborated by data published in OpenSAFELY.
OpenSAFELY analyses the factors associated with death from COVID-19. Primary care records of 17,278,392 adults were associated with 10,926 COVID-19-related deaths. The most important factor associated with mortality was previous chronic kidney disease22.
Other authors, such as Cheng et al.23 confirm that having AKF, either from the moment of admission or developing it during the course of admission, is a predictor of mortality, adjusted for common comorbidities such as hypertension, diabetes mellitus or dyslipidaemia. These findings are similar to our results, in which renal function at admission was associated with higher mortality, compared to classic risk factors in the general population, such as hypertension, diabetes, and obesity. Although the limitation of the sample size and the low prevalence of these risk factors in our series must also be taken into account in order to be able to draw conclusions.
LimitationsOur study has limitations, among which we highlight the following: the number of patients, the fact that it is single-centre and retrospective, so our results may contain significant biases; however, it is novel in that there is little literature on the long-term renal prognosis of this type of AKF in this setting, and on the use of oXiris-type sorbents in these patients, and therefore we wanted to describe our experience with this filter, which, after our use, was authorised by the health authorities (FDA) as a treatment for COVID-19.
ConclusionsThe mortality of patients admitted to the ICU who require organ support treatments such as IMV and CRRT during COVID-19 infection is extremely high, with baseline renal function being a predictor of mortality.
Filters with cytokine and endotoxin adsorption capacity did not modify survival in our series.
Patients who overcome the infection do not require RRT but have a significant reduction in glomerular filtration rate at 6 months of SARS-COV-2 infection.
Ethical considerationsThe research was conducted in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki). The local Ethics Committee of the Hospital General Universitario Gregorio Marañón approved the study in view of its retrospective nature and the fact that all procedures being performed were part of routine care.
FundingNo funding has been received for the development of this article.
Author contributionsRM, AM, and MG organized and designed the study, with full access to study data, and are responsible for the integrity and accuracy of the data analysis. RM and MG collected, analysed and interpreted the results. The remaining authors reviewed and wrote the manuscript.
Conflict of interestsPRB has received conference fees from BAXTER. The rest of the authors state that there is no conflict of interest.
I would like to express special gratitude to the nursing department of the HGUGM specialised in CRRT, which did an extraordinary and exemplary job, under extremely complex conditions.