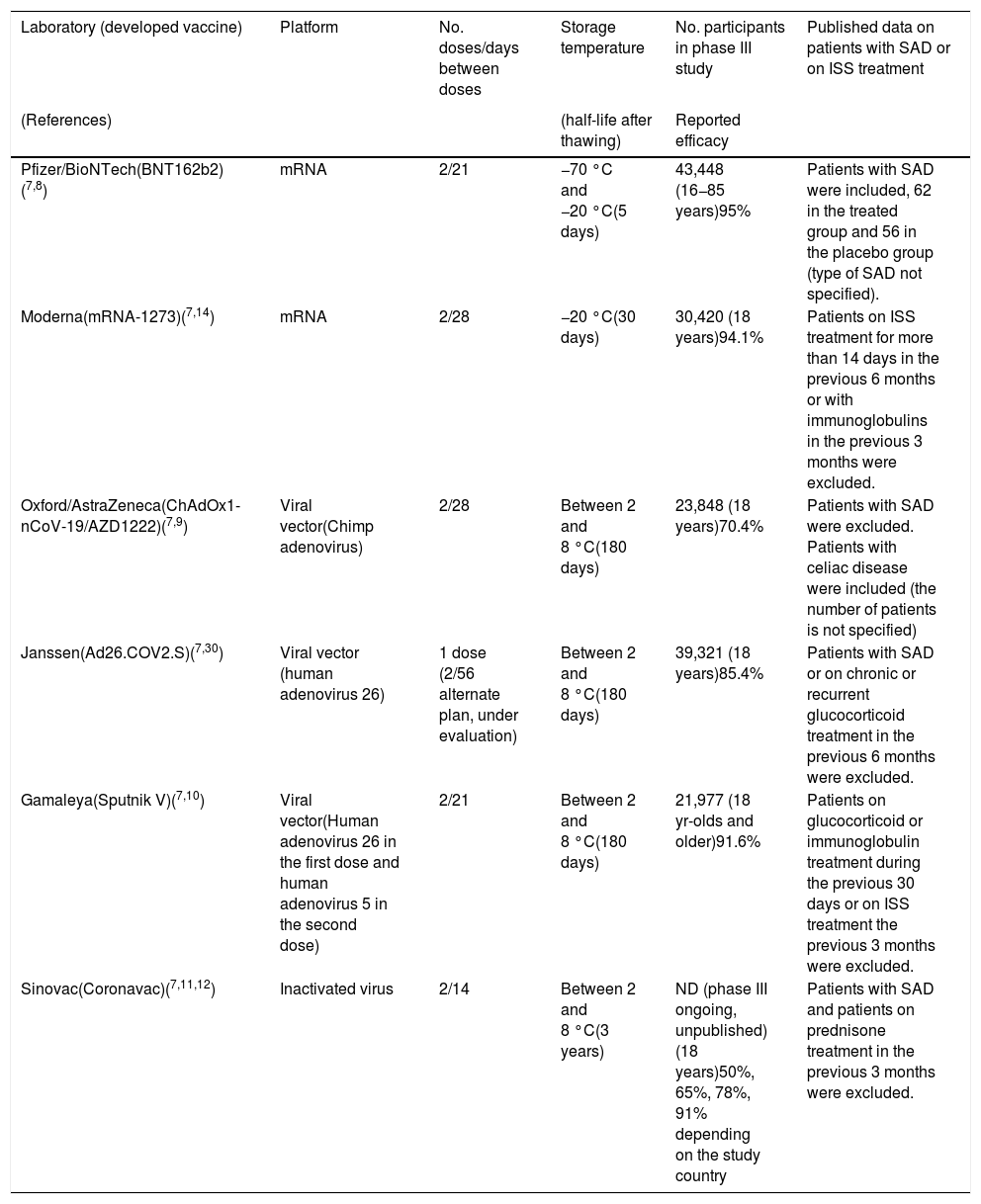
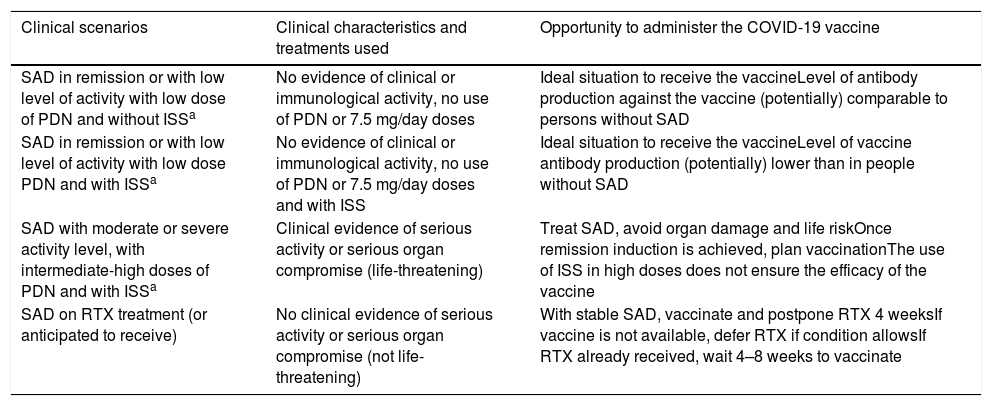
array:23 [ "pii" => "S2387020621004174" "issn" => "23870206" "doi" => "10.1016/j.medcle.2021.05.003" "estado" => "S300" "fechaPublicacion" => "2021-09-10" "aid" => "5707" "copyright" => "Elsevier España, S.L.U.. All rights reserved" "copyrightAnyo" => "2021" "documento" => "article" "crossmark" => 1 "subdocumento" => "sco" "cita" => "Med Clin. 2021;157:247-52" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "itemSiguiente" => array:19 [ "pii" => "S2387020621004095" "issn" => "23870206" "doi" => "10.1016/j.medcle.2021.08.005" "estado" => "S300" "fechaPublicacion" => "2021-09-10" "aid" => "5528" "copyright" => "Elsevier España, S.L.U." "documento" => "article" "crossmark" => 1 "subdocumento" => "pgl" "cita" => "Med Clin. 2021;157:253.e1-253.e8" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:13 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Consensus statement</span>" "titulo" => "Consensus document for the diagnosis and treatment of pyruvate kinase deficiency" "tienePdf" => "en" "tieneTextoCompleto" => "en" "tieneResumen" => array:2 [ 0 => "en" 1 => "es" ] "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "253.e1" "paginaFinal" => "253.e8" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Documento de consenso para el diagnóstico y tratamiento del déficit de piruvato quinasa" ] ] "contieneResumen" => array:2 [ "en" => true "es" => true ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "resumenGrafico" => array:2 [ "original" => 0 "multimedia" => array:8 [ "identificador" => "fig0005" "etiqueta" => "Figure 1" "tipo" => "MULTIMEDIAFIGURA" "mostrarFloat" => true "mostrarDisplay" => false "figura" => array:1 [ 0 => array:4 [ "imagen" => "gr1.jpeg" "Alto" => 2008 "Ancho" => 3167 "Tamanyo" => 486555 ] ] "detalles" => array:1 [ 0 => array:3 [ "identificador" => "at0005" "detalle" => "Figure " "rol" => "short" ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">Diagnostic algorithm for PK deficiency. It includes the differential diagnosis with other causes of congenital or acquired haemolytic anaemia and the steps to follow to confirm the diagnosis by enzymatic and/or molecular techniques.</p>" ] ] ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Marta Morado, Ana María Villegas, Silvia de la Iglesia, Jorge Martínez-Nieto, Rafael del Orbe Barreto, David Beneitez, Eduardo Salido" "autores" => array:8 [ 0 => array:2 [ "nombre" => "Marta" "apellidos" => "Morado" ] 1 => array:2 [ "nombre" => "Ana María" "apellidos" => "Villegas" ] 2 => array:2 [ "nombre" => "Silvia" "apellidos" => "de la Iglesia" ] 3 => array:2 [ "nombre" => "Jorge" "apellidos" => "Martínez-Nieto" ] 4 => array:2 [ "nombre" => "Rafael" "apellidos" => "del Orbe Barreto" ] 5 => array:2 [ "nombre" => "David" "apellidos" => "Beneitez" ] 6 => array:2 [ "nombre" => "Eduardo" "apellidos" => "Salido" ] 7 => array:1 [ "colaborador" => "on behalf of the Spanish Erythropathology Group" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S0025775320308605" "doi" => "10.1016/j.medcli.2020.10.018" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775320308605?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020621004095?idApp=UINPBA00004N" "url" => "/23870206/0000015700000005/v1_202109171210/S2387020621004095/v1_202109171210/en/main.assets" ] "itemAnterior" => array:18 [ "pii" => "S2387020621004113" "issn" => "23870206" "doi" => "10.1016/j.medcle.2021.08.006" "estado" => "S300" "fechaPublicacion" => "2021-09-10" "aid" => "5605" "documento" => "article" "crossmark" => 1 "subdocumento" => "rev" "cita" => "Med Clin. 2021;157:241-6" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "en" => array:12 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Review</span>" "titulo" => "Autoimmune polyendocrinopathy" "tienePdf" => "en" "tieneTextoCompleto" => "en" "tieneResumen" => array:2 [ 0 => "en" 1 => "es" ] "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "241" "paginaFinal" => "246" ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Síndromes pluriglandulares autoinmunes" ] ] "contieneResumen" => array:2 [ "en" => true "es" => true ] "contieneTextoCompleto" => array:1 [ "en" => true ] "contienePdf" => array:1 [ "en" => true ] "autores" => array:1 [ 0 => array:2 [ "autoresLista" => "Mercè Fernández Miró, Cristina Colom Comí, Rita Godoy Lorenzo" "autores" => array:3 [ 0 => array:2 [ "nombre" => "Mercè" "apellidos" => "Fernández Miró" ] 1 => array:2 [ "nombre" => "Cristina" "apellidos" => "Colom Comí" ] 2 => array:2 [ "nombre" => "Rita" "apellidos" => "Godoy Lorenzo" ] ] ] ] ] "idiomaDefecto" => "en" "Traduccion" => array:1 [ "es" => array:9 [ "pii" => "S0025775321001226" "doi" => "10.1016/j.medcli.2021.02.004" "estado" => "S300" "subdocumento" => "" "abierto" => array:3 [ "ES" => false "ES2" => false "LATM" => false ] "gratuito" => false "lecturas" => array:1 [ "total" => 0 ] "idiomaDefecto" => "es" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S0025775321001226?idApp=UINPBA00004N" ] ] "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020621004113?idApp=UINPBA00004N" "url" => "/23870206/0000015700000005/v1_202109171210/S2387020621004113/v1_202109171210/en/main.assets" ] "en" => array:16 [ "idiomaDefecto" => true "cabecera" => "<span class="elsevierStyleTextfn">Special article</span>" "titulo" => "Autoimmune diseases and vaccines against COVID-19. Decision making in uncertain scenarios" "tieneTextoCompleto" => true "paginas" => array:1 [ 0 => array:2 [ "paginaInicial" => "247" "paginaFinal" => "252" ] ] "autores" => array:1 [ 0 => array:4 [ "autoresLista" => "Ernesto Cairoli, Gerard Espinosa" "autores" => array:2 [ 0 => array:3 [ "nombre" => "Ernesto" "apellidos" => "Cairoli" "referencia" => array:2 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">a</span>" "identificador" => "aff0005" ] 1 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">b</span>" "identificador" => "aff0010" ] ] ] 1 => array:4 [ "nombre" => "Gerard" "apellidos" => "Espinosa" "email" => array:1 [ 0 => "gespino@clinic.cat" ] "referencia" => array:2 [ 0 => array:2 [ "etiqueta" => "<span class="elsevierStyleSup">c</span>" "identificador" => "aff0015" ] 1 => array:2 [ "etiqueta" => "*" "identificador" => "cor0005" ] ] ] ] "afiliaciones" => array:3 [ 0 => array:3 [ "entidad" => "Unidad de Enfermedades Autoinmunes, Hospital Evangélico y Centro Asistencial del Sindicato Médico del Uruguay (CASMU), Montevideo, Uruguay" "etiqueta" => "a" "identificador" => "aff0005" ] 1 => array:3 [ "entidad" => "Laboratorio de Inmunorregulación e Inflamación, Institut Pasteur de Montevideo, Montevideo, Uruguay" "etiqueta" => "b" "identificador" => "aff0010" ] 2 => array:3 [ "entidad" => "Servicio de Enfermedades Autoinmunes, Hospital Clínic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain" "etiqueta" => "c" "identificador" => "aff0015" ] ] "correspondencia" => array:1 [ 0 => array:3 [ "identificador" => "cor0005" "etiqueta" => "⁎" "correspondencia" => "Corresponding author." ] ] ] ] "titulosAlternativos" => array:1 [ "es" => array:1 [ "titulo" => "Enfermedades autoinmunes y vacunas contra la COVID-19. Toma de decisiones en escenarios de incertidumbre" ] ] "textoCompleto" => "<span class="elsevierStyleSections"><p id="par0005" class="elsevierStylePara elsevierViewall">The development of vaccines against SARS-CoV-2 (<span class="elsevierStyleItalic">severe acute respiratory syndrome coronavirus-2</span>), the causative agent of COVID-19 (<span class="elsevierStyleItalic">coronavirus disease</span>-19), is an important epidemiological tool for pandemic control. People with systemic autoimmune diseases (SAD) are at increased risk of severe forms and mortality from COVID-19.<a class="elsevierStyleCrossRefs" href="#bib0005"><span class="elsevierStyleSup">1,2</span></a> The risk factors associated with greater severity are, on the one hand, the same as in the general population, such as age over 65, male sex, high blood pressure with cardiac comorbidity and chronic lung or kidney disease.<a class="elsevierStyleCrossRef" href="#bib0010"><span class="elsevierStyleSup">2</span></a> On the other hand, there are factors inherent to SAD. In the international COVID-19 <span class="elsevierStyleItalic">Global Rheumatology Alliance physician-reported registry</span> study, the highest risk of mortality was associated with moderate-high activity, use of a dose greater than 10 mg/day of prednisone, sulfasalazine or rituximab.<a class="elsevierStyleCrossRef" href="#bib0010"><span class="elsevierStyleSup">2</span></a></p><p id="par0010" class="elsevierStylePara elsevierViewall">The increased morbidity and mortality places people with SAD among the priority groups of patients who would benefit from vaccination against COVID-19.<a class="elsevierStyleCrossRef" href="#bib0015"><span class="elsevierStyleSup">3</span></a> To date, the data regarding their efficacy and adverse reactions are unknown since these groups of patients were excluded from the clinical studies that led to the approval of these vaccines. However, the position of different scientific societies is in favour of offering these patients vaccination against COVID-19.<a class="elsevierStyleCrossRefs" href="#bib0015"><span class="elsevierStyleSup">3–6</span></a> From a clinical perspective, the following questions are raised to guide the decisions to be made:<ul class="elsevierStyleList" id="lis0005"><li class="elsevierStyleListItem" id="lsti0005"><span class="elsevierStyleLabel">1)</span><p id="par0015" class="elsevierStylePara elsevierViewall">What types of COVID-19 vaccines are currently available and how effective are they in patients with SAD?</p></li><li class="elsevierStyleListItem" id="lsti0010"><span class="elsevierStyleLabel">2)</span><p id="par0020" class="elsevierStylePara elsevierViewall">Can COVID-19 vaccines trigger SAD or cause a disease flare-up?</p></li><li class="elsevierStyleListItem" id="lsti0015"><span class="elsevierStyleLabel">3)</span><p id="par0025" class="elsevierStylePara elsevierViewall">What potential adverse reactions can COVID-19 vaccines cause in patients with SAD?</p></li><li class="elsevierStyleListItem" id="lsti0020"><span class="elsevierStyleLabel">4)</span><p id="par0030" class="elsevierStylePara elsevierViewall">Are patients with SAD who receive adenovirus-vectored DNA vaccines at a higher risk of developing thrombosis?</p></li><li class="elsevierStyleListItem" id="lsti0025"><span class="elsevierStyleLabel">5)</span><p id="par0035" class="elsevierStylePara elsevierViewall">What is the most appropriate clinical setting to administer the COVID-19 vaccine in a patient with SAD?</p></li><li class="elsevierStyleListItem" id="lsti0030"><span class="elsevierStyleLabel">6)</span><p id="par0040" class="elsevierStylePara elsevierViewall">Is it necessary to stop immunosuppressive treatment before administering the COVID-19 vaccine?</p></li><li class="elsevierStyleListItem" id="lsti0035"><span class="elsevierStyleLabel">7)</span><p id="par0045" class="elsevierStylePara elsevierViewall"><span class="elsevierStyleItalic">Type of vaccines against COVID-19 currently available and efficacy in patients with SAD.</span></p></li></ul></p><p id="par0050" class="elsevierStylePara elsevierViewall">There are four different SARS-CoV-2 vaccine platforms evaluated in phase III clinical trials: (a) those based on messenger RNA (mRNA); (b) those using DNA via a viral vector; c) those using inactivated SARS-CoV-2 viruses; and (d) the fourth platform, consisting of vaccines using recombinant protein nanoparticles with adjuvants<a class="elsevierStyleCrossRef" href="#bib0035"><span class="elsevierStyleSup">7</span></a> (<a class="elsevierStyleCrossRef" href="#tbl0005">Table 1</a>).</p><elsevierMultimedia ident="tbl0005"></elsevierMultimedia><p id="par0055" class="elsevierStylePara elsevierViewall">mRNA-based vaccines include in their sequence the S protein (spike protein) of SARS-CoV-2 and a lipid layer of nanoparticles as a protective coating for the mRNA. This facilitates its entry into the cell cytoplasm and the initiation of translation of the mRNA into the viral protein, which then leads to the immune response generating neutralising antibodies against the S protein. This strategy is used by the <span class="elsevierStyleItalic">Pfizer-BioNTech</span> and <span class="elsevierStyleItalic">Moderna/National Institute of Allergy and Infectious Diseases</span><a class="elsevierStyleCrossRef" href="#bib0035"><span class="elsevierStyleSup">7</span></a> laboratories. The <span class="elsevierStyleItalic">Pfizer-BioNTech</span> vaccine studies included individuals aged 16 years and older and very few patients with rheumatic diseases (62 in the treated group and 56 in the placebo group), representing only 0.3% of the sample. To date, no specific data have been published for this group of patients.<a class="elsevierStyleCrossRef" href="#bib0040"><span class="elsevierStyleSup">8</span></a></p><p id="par0060" class="elsevierStylePara elsevierViewall">DNA-based vaccines use adenoviruses whose replicative capacity has been eliminated and, additionally, have had the S protein's DNA of the SARS-CoV-2 inserted. It is therefore a vaccine that introduces a replication-incompetent adenovirus but allows entry into the cell nucleus of the DNA necessary for the synthesis of the S protein, which generates the immune response. This is the strategy used (with different adenoviruses) by the laboratories <span class="elsevierStyleItalic">University of Oxford/AstraZeneca</span> (chimpanzee adenovirus), <span class="elsevierStyleItalic">Gamaleya National Research Centre for Epidemiology and Microbiology</span> (human adenoviruses 5 and 26), <span class="elsevierStyleItalic">CanSino Biological Inc/Beijing Institute of Biotechnology</span> (human adenovirus 5) and <span class="elsevierStyleItalic">Janssen Pharmaceutical Companies</span> (human adenovirus 26).<a class="elsevierStyleCrossRef" href="#bib0035"><span class="elsevierStyleSup">7</span></a> The <span class="elsevierStyleItalic">University of Oxford/AstraZeneca</span> vaccine studies included patients aged 18 years or older and excluded patients with SAD (except those with celiac disease).<a class="elsevierStyleCrossRef" href="#bib0045"><span class="elsevierStyleSup">9</span></a> Patients on glucocorticoid or immunoglobulin therapy or immunosuppressive therapy in the 3 months prior to vaccine administration were excluded from the <span class="elsevierStyleItalic">Gamaleya</span> vaccine study.<a class="elsevierStyleCrossRef" href="#bib0050"><span class="elsevierStyleSup">10</span></a></p><p id="par0065" class="elsevierStylePara elsevierViewall">The third group is made up of vaccines that use inactivated viruses as their basis. Chemically inactivated SARS-CoV-2 viral particles are used, administered together with an excipient (adjuvant) that enhances their immunogenicity. This results in an immune response with production of neutralising antibodies against the S protein and other less represented antigens, which are considered non-neutralising antibodies. This is the strategy used by the <span class="elsevierStyleItalic">SinoVac (Coronavac), Biotech</span> laboratory, whose phase I/II study results and phase III protocol have been published. This included people aged 18 years or older and excluded patients with SAD, as well as those who received prednisone in the 3 months prior to vaccine administration.<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,11,12</span></a></p><p id="par0070" class="elsevierStylePara elsevierViewall">The fourth vaccine platform is built on the basis of recombinant protein nanoparticles (subunits) associated with an adjuvant that enhances their immune response. This is the strategy used by the <span class="elsevierStyleItalic">Novavax</span> laboratory, with published data from phase I/II studies (phase III ongoing).<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,13</span></a></p><p id="par0075" class="elsevierStylePara elsevierViewall">In terms of efficacy, i.e., patients without SARS-CoV-2 infection after completing the vaccination course, the percentages are 95% for the <span class="elsevierStyleItalic">Pfizer/BioNTech</span> vaccine, 94.1% for <span class="elsevierStyleItalic">Moderna</span>, 91.6% for <span class="elsevierStyleItalic">Gamaleya</span>, 70.4% for <span class="elsevierStyleItalic">Oxford/AstraZeneca,</span> and <span class="elsevierStyleItalic">Sinovac</span> has reported variable efficacy of 50%, 65%, 78% and 91% depending on the country where the study was conducted, respectively.<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,14</span></a></p><p id="par0080" class="elsevierStylePara elsevierViewall">In terms of effectiveness in the production of antibodies in people without AAS, mRNA and DNA viral vector vaccines provide mechanisms to enhance the immune response (innate and acquired) by activation of intracellular pathways (such as interferon and <span class="elsevierStyleItalic">toll-like receptor</span> activation), effects not observed with inactivated virus vaccines. While all COVID-19 vaccine strategies generate memory cells, the levels of antibody production achieved by mRNA or viral vector DNA vaccines are (at least 4-fold) higher than those obtained by inactivated virus vaccines.</p><p id="par0085" class="elsevierStylePara elsevierViewall">It should be noted that the exact production of antibodies against SARS-CoV-2 in patients with SAD after vaccination is not known. The first data on the response to mRNA vaccines in patients with SAD have recently been published.<a class="elsevierStyleCrossRefs" href="#bib0075"><span class="elsevierStyleSup">15,16</span></a> The first study involving 26 patients with different autoimmune diseases, showed that the response defined by the production of IgG antibodies directed against SARS-CoV-2 protein S and neutralising antibodies was found in all patients. However, the titers were significantly lower in patients than in healthy controls. Furthermore, the authors found no differences in response to vaccines between patients treated with anti-TNF agents and those receiving disease-modifying drugs.<a class="elsevierStyleCrossRef" href="#bib0075"><span class="elsevierStyleSup">15</span></a> The second study involving 123 patients detected the generation of anti-SARS-CoV-2 antibodies in 74%.<a class="elsevierStyleCrossRef" href="#bib0080"><span class="elsevierStyleSup">16</span></a> Treatment with mycophenolate or rituximab was associated with an increased risk of not generating a response to the vaccine.<a class="elsevierStyleCrossRef" href="#bib0085"><span class="elsevierStyleSup">17</span></a><ul class="elsevierStyleList" id="lis0010"><li class="elsevierStyleListItem" id="lsti0040"><span class="elsevierStyleLabel">1)</span><p id="par0090" class="elsevierStylePara elsevierViewall"><span class="elsevierStyleItalic">Potential for COVID-19 vaccines to trigger a SAD or cause a disease flare-up.</span></p></li></ul></p><p id="par0095" class="elsevierStylePara elsevierViewall">Autoimmune diseases (Guillain-Barré syndrome, immune thrombocytopenia, and antiphospholipid syndrome, among others)<a class="elsevierStyleCrossRef" href="#bib0090"><span class="elsevierStyleSup">18</span></a> as well as the presence of antinuclear antibodies, antibodies against extractable nuclear antigens, antimitochondrial antibodies, antiphospholipid antibodies and rheumatoid factor have been described during or immediately after COVID-19.<a class="elsevierStyleCrossRef" href="#bib0090"><span class="elsevierStyleSup">18</span></a> In turn, cross-reactivity has been demonstrated between antibodies directed against the SARS-CoV-2 S protein and different tissue antigens.<a class="elsevierStyleCrossRef" href="#bib0095"><span class="elsevierStyleSup">19</span></a> This raises the hypothesis that the SARS-CoV-2 S protein could, by molecular mimicry, trigger the development of a SAD.</p><p id="par0100" class="elsevierStylePara elsevierViewall">In the same way that mRNA vaccines achieve a heightened immune response by activating additional cytoplasmic pathways, it is feasible that they may trigger a cascade of immune mechanisms leading to aberrant activation of the immune response (innate or acquired) and hypothetically induce or activate a SAD. In this sense, one of the serious adverse reactions reported in the <span class="elsevierStyleItalic">Moderna</span> vaccine has been the development of rheumatoid arthritis in one of the participants of the treated group, the only case described in the study with 30,000 volunteers included.<a class="elsevierStyleCrossRef" href="#bib0100"><span class="elsevierStyleSup">20</span></a></p><p id="par0105" class="elsevierStylePara elsevierViewall">The non-inclusion of patients with SAD in the clinical studies of the different vaccines makes it impossible to offer scientific evidence in this regard. However, the molecular mimicry mechanisms associated with the presence of the SARS-CoV-2 S protein, as well as the activation of aberrant pathways of the immune response, would leave the door open to mechanisms responsible for the activation of a SAD as a result of COVID-19 vaccination. Therefore, there is a theoretical risk of the autoimmune disease developing a flare-up or worsening after vaccination. However, the benefit of COVID-19 vaccine protection against severe disease outweighs the potential risk of disease relapse.<a class="elsevierStyleCrossRef" href="#bib0015"><span class="elsevierStyleSup">3</span></a><ul class="elsevierStyleList" id="lis0015"><li class="elsevierStyleListItem" id="lsti0045"><span class="elsevierStyleLabel">3)</span><p id="par0110" class="elsevierStylePara elsevierViewall"><span class="elsevierStyleItalic">Potential adverse reactions of COVID-19 vaccines in patients with SAD.</span></p></li></ul></p><p id="par0115" class="elsevierStylePara elsevierViewall">The most common adverse reactions (variable in frequency depending on the type of vaccine) are pain at the puncture site, erythema, headache, transient oral paraesthesia, adynamia, and adenomegaly. With the exception of the case of rheumatoid arthritis reported in the <span class="elsevierStyleItalic">Moderna</span> studies, no adverse reactions related to the development of SAD or autoinflammatory diseases have been reported.<a class="elsevierStyleCrossRefs" href="#bib0040"><span class="elsevierStyleSup">8,13</span></a> The first results of the study of vaccines in patients with SAD sponsored by the <span class="elsevierStyleItalic">"COVID-19 Global Rheumatology Alliance"</span> should be made public shortly. The study has now collected data from more than 2600 patients with SAD who have received at least one dose of the vaccine.<a class="elsevierStyleCrossRef" href="#bib0105"><span class="elsevierStyleSup">21</span></a> Another similar study is the <span class="elsevierStyleItalic">"VACOLUP study"</span>, sponsored by RESO (<span class="elsevierStyleItalic">Centre de Référence des maladies auto-immunes systémiques rares Est Sud-Ouest</span>) in France. In this case, there are updated data from 339 patients with systemic lupus erythematosus. 49% had some side effect after the first dose (81% mild-moderate) and 49% after the second dose (79% mild-moderate). Only 3% have had a disease flare-up and there have been no cases of SARS-CoV-2 infection in vaccinated patients.<a class="elsevierStyleCrossRef" href="#bib0110"><span class="elsevierStyleSup">22</span></a></p><p id="par0120" class="elsevierStylePara elsevierViewall">In addition to the adverse reactions described in the general population, the potential lung damage related to the use of vaccines against COVID-19 generates particular interest in patients with SAD, given the high prevalence of diffuse interstitial lung disease (DILD) in these patients. The damage would occur through a mechanism known as antibody-dependent enhancement (ADE), in which there would be an increase in cellular entry and replication of the virus favoured by the presence of vaccine-induced antibodies. Once the vaccinated individual is confronted with the infection for which he/she was immunised, the presence of vaccine-induced antibodies would generate an antigen-antibody reaction with immunocomplex formation and consequent tissue damage. This type of damage leads to so-called <span class="elsevierStyleItalic">vaccine antibodies enhanced respiratory disease</span> (VAERD), described during the development of respiratory syncytial virus vaccines and in models closer to COVID-19, such as vaccines against SARS-CoV-1 and MERS-CoV (<span class="elsevierStyleItalic">Middle East Respiratory Syndrome coronavirus</span>).<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,23</span></a></p><p id="par0125" class="elsevierStylePara elsevierViewall">It should be noted that the VAERD phenomenon is related to both mRNA, DNA vectors, and inactivated virus vaccines. However, the risk could potentially be higher with inactivated virus vaccines, as the patient is exposed to multiple antigens leading to the production of non-neutralising antibodies and/or the synthesis of antibodies directed against the S protein in non-neutralising conformations. This increases the diversity of targets to be recognized and would grant a greater capacity to develop ADE phenomena.<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,23</span></a><ul class="elsevierStyleList" id="lis0020"><li class="elsevierStyleListItem" id="lsti0050"><span class="elsevierStyleLabel">4)</span><p id="par0130" class="elsevierStylePara elsevierViewall"><span class="elsevierStyleItalic">Risk of thrombosis in patients with SAD who receive adenovirus-vectored DNA vaccines.</span></p></li></ul></p><p id="par0135" class="elsevierStylePara elsevierViewall">Since February 2021, several cases of cerebral venous thrombosis or splanchnic vein thrombosis together with thrombocytopenia have been reported in people who have received the <span class="elsevierStyleItalic">Oxford/AstraZeneca vaccine</span>.<a class="elsevierStyleCrossRefs" href="#bib0120"><span class="elsevierStyleSup">24–26</span></a> This atypical location of thrombosis and thrombocytopenia after the administration of the vaccine has been called <span class="elsevierStyleItalic">vaccine-induced immune thrombotic thrombocytopenia</span> (VITT). Its pathogenetic mechanism is similar to that of <span class="elsevierStyleItalic">heparin induced thrombocytopenia</span> (HIT) characterised by the presence of antibodies targeting platelet factor 4 that trigger platelet activation (among other mechanisms), resulting in thrombosis together with a decrease in platelet numbers.<a class="elsevierStyleCrossRefs" href="#bib0120"><span class="elsevierStyleSup">24–26</span></a> The European Medicines Agency (EMA) has reported on the plausibility of the relationship between the administration of this vaccine and this adverse reaction without having been able to identify the risk factors that predispose its occurrence. Overall, the EMA establishes that the estimated risk of this adverse reaction is 1 case per 100,000 people who have received the first dose of the <span class="elsevierStyleItalic">Oxford/AstraZeneca vaccine</span>.<a class="elsevierStyleCrossRef" href="#bib0135"><span class="elsevierStyleSup">27</span></a></p><p id="par0140" class="elsevierStylePara elsevierViewall">The data published as of April 2021 includes 39 patients who received the <span class="elsevierStyleItalic">Oxford/AstraZeneca</span> vaccine and none of them had a previous history of SAD, thrombocytopenia or thrombosis.<a class="elsevierStyleCrossRefs" href="#bib0120"><span class="elsevierStyleSup">24–26</span></a> The study to rule out other causes of thrombosis and thrombocytopenia revealed 2 cases with antiphospholipid antibodies, one of them with von Willebrand's disease and a heterozygous factor V Leiden mutation. The other case had anticardiolipin antibodies. In the series including 23 patients (the largest series), antinuclear antibodies were negative in all patients and lupus anticoagulant was present in 5 of the 10 cases in which it was measured. The authors relate this finding to the state of disseminated intravascular coagulation that existed in these patients.<a class="elsevierStyleCrossRefs" href="#bib0120"><span class="elsevierStyleSup">24–26</span></a> More recently, 6 cases of VITT have been described in association with the <span class="elsevierStyleItalic">Janssen</span> adenovirus vector vaccine, and the same pathogenic mechanism has been proposed by the EMA.<a class="elsevierStyleCrossRef" href="#bib0140"><span class="elsevierStyleSup">28</span></a></p><p id="par0145" class="elsevierStylePara elsevierViewall">The development of these thromboses after the administration of both vaccines has been interpreted by regulatory agencies as an adverse reaction probably related to adenovirus vector vaccines. On the other hand, since none of the cases had a previous history of SAD, thrombocytopenia, or thrombosis, it can be inferred that there is no evidence to suggest that this type of vaccine may represent an increased thrombotic risk in patients with SAD. However, more pharmacovigilance studies are necessary to reaffirm or refute the existence of this risk.<ul class="elsevierStyleList" id="lis0025"><li class="elsevierStyleListItem" id="lsti0055"><span class="elsevierStyleLabel">5)</span><p id="par0150" class="elsevierStylePara elsevierViewall"><span class="elsevierStyleItalic">Most appropriate clinical setting for COVID-19 vaccine administration.</span></p></li></ul></p><p id="par0155" class="elsevierStylePara elsevierViewall">Ideally, patients with SAD should receive any vaccine during remission or a period of low activity, or before planned immunosuppressive therapy, in particular B-cell depletion therapy.<a class="elsevierStyleCrossRef" href="#bib0145"><span class="elsevierStyleSup">29</span></a> Regarding the timing of vaccination and the type of ongoing immunosuppressive therapy, the general recommendation is not to change the treatment of SAD, since there is no evidence in this regard.<a class="elsevierStyleCrossRefs" href="#bib0025"><span class="elsevierStyleSup">5,6</span></a> Patients with SAD could be divided into four scenarios according to their clinical situation at the time of offering the vaccine (<a class="elsevierStyleCrossRef" href="#tbl0010">Table 2</a>):<ul class="elsevierStyleList" id="lis0030"><li class="elsevierStyleListItem" id="lsti0060"><span class="elsevierStyleLabel">•</span><p id="par0160" class="elsevierStylePara elsevierViewall">Patient with disease in remission or low disease activity, on low-dose glucocorticoids and no immunosuppressants. This group is in the ideal situation, where the disease has no activity and glucocorticoid levels are low (≤7.5 mg/day). It is the best scenario to administer the vaccine and achieve antibody levels comparable to people without SAD.</p></li><li class="elsevierStyleListItem" id="lsti0065"><span class="elsevierStyleLabel">•</span><p id="par0165" class="elsevierStylePara elsevierViewall">Patient with disease in remission or low disease activity, on low-dose glucocorticoid therapy, conventional immunosuppressive and/or biologic therapy or Janus kinase inhibitors. In this group of patients, immunosuppression levels could determine the lower production of antibodies or lower development of memory cells. However, this condition should not preclude vaccination, considering the pandemic scenario, where the potential benefits outweigh the possible adverse reactions.</p></li><li class="elsevierStyleListItem" id="lsti0070"><span class="elsevierStyleLabel">•</span><p id="par0170" class="elsevierStylePara elsevierViewall">Patient with moderate or severe activity, in treatment with intermediate or high doses of glucocorticoids, with conventional immunosuppressants and/or biological treatment or Janus kinase inhibitors or considering any of these treatments. In this situation it is reasonable to treat SAD first and postpone vaccination as high doses of immunosuppressants are commonly used, which can significantly decrease the production of antibodies induced by COVID-19 vaccines. In addition, the potential adverse reactions to vaccination may overlap with the patterns of disease activity, which may make it difficult to differentiate which symptoms are due to which cause.<a class="elsevierStyleCrossRef" href="#bib0015"><span class="elsevierStyleSup">3</span></a></p></li><li class="elsevierStyleListItem" id="lsti0075"><span class="elsevierStyleLabel">•</span><p id="par0175" class="elsevierStylePara elsevierViewall">Patient on (or scheduled to receive) rituximab. The use of rituximab is associated with an overall decrease in immunoglobulins (especially IgG isotype), as well as lower levels of specific antibodies after vaccination.<a class="elsevierStyleCrossRefs" href="#bib0085"><span class="elsevierStyleSup">17,29</span></a> Regarding the use of rituximab as part of the treatment, the following should be considered: (1) if clinically feasible and the vaccine is available, vaccinate the patient and defer rituximab for 4 weeks; (2) if the patient has already received rituximab, a reasonable option would be to vaccinate against COVID-19 4–8 weeks after receiving it; (3) if the vaccine is not yet available and it is clinically safe to defer rituximab, rituximab administration should be delayed or an alternative treatment plan should be used; (4) if the vaccine is not yet available and it is not safe to defer rituximab for 4 weeks, rituximab should be administered and the vaccine should be offered when available.<a class="elsevierStyleCrossRef" href="#bib0030"><span class="elsevierStyleSup">6</span></a></p></li><li class="elsevierStyleListItem" id="lsti0080"><span class="elsevierStyleLabel">6)</span><p id="par0180" class="elsevierStylePara elsevierViewall"><span class="elsevierStyleItalic">Need for discontinuation of immunosuppressive treatment prior to administration of COVID-19 vaccine.</span></p></li></ul></p><elsevierMultimedia ident="tbl0010"></elsevierMultimedia><p id="par0185" class="elsevierStylePara elsevierViewall">There is no consensus on this point. Recommendations from the American College of Rheumatology suggest discontinuing treatment with methotrexate, Janus kinase inhibitors, abatacept, and intravenous cyclophosphamide pulses for up to one week after each dose of the vaccine is administered (as long as the disease is stable and if clinically feasible).<a class="elsevierStyleCrossRef" href="#bib0015"><span class="elsevierStyleSup">3</span></a> This recommendation is based on the results of the immune response to other vaccines such as influenza or pneumococcal vaccines in patients with SAD on immunosuppressive therapy. However, other scientific societies recommend that treatment should not be discontinued when administering the COVID-19 vaccine.<a class="elsevierStyleCrossRefs" href="#bib0025"><span class="elsevierStyleSup">5,6</span></a></p><span id="sec0005" class="elsevierStyleSection elsevierViewall"><span class="elsevierStyleSectionTitle" id="sect0005">Variables to consider when making decisions regarding vaccination against COVID-19</span><p id="par0190" class="elsevierStylePara elsevierViewall">As a general rule, none of the available and approved COVID-19 vaccines are based on live attenuated SARS-CoV-2 viruses and are therefore not formally contraindicated in patients with SAD. It can be inferred that, in some cases, the response to the vaccine will be less intense and therefore protection will be weaker. This underlines the need to maintain physical protective measures, such as face mask use, hand hygiene and continued physical distancing, as vaccination does not prevent infection, but prevents severe forms of infection and therefore does not exempt the patient from complying with physical preventive measures. In the same way, vaccination against COVID-19 of SAD patient cohabitants is a way to reduce the risks of infection.<a class="elsevierStyleCrossRef" href="#bib0015"><span class="elsevierStyleSup">3</span></a></p><p id="par0195" class="elsevierStylePara elsevierViewall">The act of recommending vaccination against COVID-19 should be a shared decision between the physician and the patient, who should assess whether the benefits outweigh the potential risks of infection. In cases of patients with SAD who have already had COVID-19 symptoms, it seems reasonable to suggest the same regimen as for the general population and to wait 6 months before administering the vaccine.</p><p id="par0200" class="elsevierStylePara elsevierViewall">The scientific societies that have taken a position on this issue agree to offer vaccination against COVID-19 to all patients with SAD, with any of the vaccination platforms approved by the drug regulatory agencies and emphasise that the potential benefits of avoiding a severe form of COVID-19 outweigh the risks of potential adverse reactions.<a class="elsevierStyleCrossRefs" href="#bib0015"><span class="elsevierStyleSup">3–6</span></a> With all this information, when prescribing vaccination against COVID-19 in patients with SAD, it is important to consider the following factors that can potentially influence both the levels of protection and the development of adverse reactions:<ul class="elsevierStyleList" id="lis0035"><li class="elsevierStyleListItem" id="lsti0085"><span class="elsevierStyleLabel">1)</span><p id="par0205" class="elsevierStylePara elsevierViewall">The degree of SAD activity and the type and intensity of immunosuppressive treatment used at the time of offering vaccination.</p></li><li class="elsevierStyleListItem" id="lsti0090"><span class="elsevierStyleLabel">2)</span><p id="par0210" class="elsevierStylePara elsevierViewall">Patients with risk factors associated with severe COVID-19 could benefit from the use of mRNA or viral vector DNA vaccines, since they offer additional intracellular mechanisms to enhance the immune response. This would allow for higher antibody titres, considering that due to age or immunosuppressive treatment they might develop a less intense immune response against SARS-CoV-2.</p></li><li class="elsevierStyleListItem" id="lsti0095"><span class="elsevierStyleLabel">3)</span><p id="par0215" class="elsevierStylePara elsevierViewall">Inactivated virus vaccines potentially have a higher risk of developing lung damage due to VAERD, a fact to be considered in patients with SAD and respiratory comorbidity such as the presence of ILD. This could be an important factor in the choice of mRNA or viral vector DNA vaccines, which induce the production of neutralising antibodies in a specific way and have a lower risk of inducing non-neutralising antibodies (VAERD-associated).</p></li><li class="elsevierStyleListItem" id="lsti0100"><span class="elsevierStyleLabel">4)</span><p id="par0220" class="elsevierStylePara elsevierViewall">mRNA, viral vector DNA, inactivated virus or recombinant protein nanoparticles vaccines could be suitable options in patients with SAD in remission or with low activity, absence, or low dose of immunosuppressants and absence of ILD.</p></li><li class="elsevierStyleListItem" id="lsti0105"><span class="elsevierStyleLabel">5)</span><p id="par0225" class="elsevierStylePara elsevierViewall">To date, there is not enough scientific evidence to support modifying the treatment of SAD before or after the administration of the COVID-19 vaccine.</p></li><li class="elsevierStyleListItem" id="lsti0110"><span class="elsevierStyleLabel">6)</span><p id="par0230" class="elsevierStylePara elsevierViewall">Once the vaccination schedule of the chosen option has been completed, since it is not possible to predict the titre of neutralising antibodies against COVID-19 and to determine with certainty the level of protection that the patient will have, it is suggested to assess the magnitude of the response acquired after vaccination. Future studies will probably show whether this is possible by detecting in serum (e.g., by ELISA techniques) IgG isotype antibodies against the S protein of SARS-CoV-2 3–6 months after administration of the vaccine.</p></li><li class="elsevierStyleListItem" id="lsti0115"><span class="elsevierStyleLabel">7)</span><p id="par0235" class="elsevierStylePara elsevierViewall">Last but not least, vaccination against COVID-19 in patients with SAD does not exempt them from maintaining the recommended physical and social protective measures, as the levels of protection that the new COVID-19 vaccines will offer in patients with SAD are unknown.</p></li></ul></p><p id="par0240" class="elsevierStylePara elsevierViewall">Promoting vaccination against COVID-19 in patients with SAD is putting the high risk of developing a severe form of the infection ahead of the potential risk of side effects. It should be noted that these serious side effects are very rare. However, due to the scarcity of specific evidence, the patient must be involved in the decision to receive the vaccine. In this process, the patient should be advised by the treating physician, who will inform him/her of the benefit-risk ratio derived from his/her disease, the degree of activity and the type of underlying treatment. In any case, it is very important that the patient continues to maintain the general measures that have proven to be effective in preventing the spread of the virus, as well as maintaining the treatment of his/her SAD.</p></span><span id="sec0010" class="elsevierStyleSection elsevierViewall"><span class="elsevierStyleSectionTitle" id="sect0010">Funding</span><p id="par0245" class="elsevierStylePara elsevierViewall">This article has not received any type of funding.</p></span><span id="sec0015" class="elsevierStyleSection elsevierViewall"><span class="elsevierStyleSectionTitle" id="sect0015">Conflict of interests</span><p id="par0250" class="elsevierStylePara elsevierViewall">The authors declare no conflict of interest.</p></span></span>" "textoCompletoSecciones" => array:1 [ "secciones" => array:4 [ 0 => array:2 [ "identificador" => "sec0005" "titulo" => "Variables to consider when making decisions regarding vaccination against COVID-19" ] 1 => array:2 [ "identificador" => "sec0010" "titulo" => "Funding" ] 2 => array:2 [ "identificador" => "sec0015" "titulo" => "Conflict of interests" ] 3 => array:1 [ "titulo" => "References" ] ] ] "pdfFichero" => "main.pdf" "tienePdf" => true "fechaRecibido" => "2021-02-21" "fechaAceptado" => "2021-05-13" "NotaPie" => array:1 [ 0 => array:2 [ "etiqueta" => "☆" "nota" => "<p class="elsevierStyleNotepara" id="npar0010">Please cite this article as: Cairoli E, Espinosa G. Enfermedades autoinmunes y vacunas contra la COVID-19. Toma de decisiones en escenarios de incertidumbre. Med Clin (Barc). 2021;157:247–252.</p>" ] ] "multimedia" => array:2 [ 0 => array:8 [ "identificador" => "tbl0005" "etiqueta" => "Table 1" "tipo" => "MULTIMEDIATABLA" "mostrarFloat" => true "mostrarDisplay" => false "detalles" => array:1 [ 0 => array:3 [ "identificador" => "at0005" "detalle" => "Table " "rol" => "short" ] ] "tabla" => array:2 [ "leyenda" => "<p id="spar0010" class="elsevierStyleSimplePara elsevierViewall">MRNA: Messenger RNA; SAD: systemic autoimmune disease; ISS: immunosuppressant; ND: no data.</p>" "tablatextoimagen" => array:1 [ 0 => array:2 [ "tabla" => array:1 [ 0 => """ <table border="0" frame="\n \t\t\t\t\tvoid\n \t\t\t\t" class=""><thead title="thead"><tr title="table-row"><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col">Laboratory (developed vaccine) \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col">Platform \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col">No. doses/days between doses \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col">Storage temperature \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col">No. participants in phase III study \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col">Published data on patients with SAD or on ISS treatment \t\t\t\t\t\t\n \t\t\t\t\t\t</th></tr><tr title="table-row"><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">(References) \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black"> \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black"> \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">(half-life after thawing) \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Reported efficacy \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black"> \t\t\t\t\t\t\n \t\t\t\t\t\t</th></tr></thead><tbody title="tbody"><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Pfizer/BioNTech(BNT162b2)(<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,8</span></a>) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">mRNA \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2/21 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">−70 °C and −20 °C(5 days) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">43,448 (16−85 years)95% \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Patients with SAD were included, 62 in the treated group and 56 in the placebo group (type of SAD not specified). \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Moderna(mRNA-1273)(<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,14</span></a>) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">mRNA \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2/28 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">−20 °C(30 days) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">30,420 (18 years)94.1% \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Patients on ISS treatment for more than 14 days in the previous 6 months or with immunoglobulins in the previous 3 months were excluded. \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Oxford/AstraZeneca(ChAdOx1-nCoV-19/AZD1222)(<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,9</span></a>) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Viral vector(Chimp adenovirus) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2/28 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Between 2 and 8 °C(180 days) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">23,848 (18 years)70.4% \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Patients with SAD were excluded. Patients with celiac disease were included (the number of patients is not specified) \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Janssen(Ad26.COV2.S)(<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,30</span></a>) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Viral vector (human adenovirus 26) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">1 dose (2/56 alternate plan, under evaluation) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Between 2 and 8 °C(180 days) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">39,321 (18 years)85.4% \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Patients with SAD or on chronic or recurrent glucocorticoid treatment in the previous 6 months were excluded. \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Gamaleya(Sputnik V)(<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,10</span></a>) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Viral vector(Human adenovirus 26 in the first dose and human adenovirus 5 in the second dose) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2/21 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Between 2 and 8 °C(180 days) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">21,977 (18 yr-olds and older)91.6% \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Patients on glucocorticoid or immunoglobulin treatment during the previous 30 days or on ISS treatment the previous 3 months were excluded. \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Sinovac(Coronavac)(<a class="elsevierStyleCrossRefs" href="#bib0035"><span class="elsevierStyleSup">7,11,12</span></a>) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Inactivated virus \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">2/14 \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Between 2 and 8 °C(3 years) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">ND (phase III ongoing, unpublished) (18 years)50%, 65%, 78%, 91% depending on the study country \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Patients with SAD and patients on prednisone treatment in the previous 3 months were excluded. \t\t\t\t\t\t\n \t\t\t\t</td></tr></tbody></table> """ ] "imagenFichero" => array:1 [ 0 => "xTab2696996.png" ] ] ] ] "descripcion" => array:1 [ "en" => "<p id="spar0005" class="elsevierStyleSimplePara elsevierViewall">Main characteristics of vaccines against COVID-19.</p>" ] ] 1 => array:8 [ "identificador" => "tbl0010" "etiqueta" => "Table 2" "tipo" => "MULTIMEDIATABLA" "mostrarFloat" => true "mostrarDisplay" => false "detalles" => array:1 [ 0 => array:3 [ "identificador" => "at0010" "detalle" => "Table " "rol" => "short" ] ] "tabla" => array:3 [ "leyenda" => "<p id="spar0020" class="elsevierStyleSimplePara elsevierViewall">SAD: systemic autoimmune disease; PDN: prednisone; ISS: immunosuppressants; RTX rituximab.</p>" "tablatextoimagen" => array:1 [ 0 => array:2 [ "tabla" => array:1 [ 0 => """ <table border="0" frame="\n \t\t\t\t\tvoid\n \t\t\t\t" class=""><thead title="thead"><tr title="table-row"><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Clinical scenarios \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Clinical characteristics and treatments used \t\t\t\t\t\t\n \t\t\t\t\t\t</th><th class="td" title="\n \t\t\t\t\ttable-head\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t" scope="col" style="border-bottom: 2px solid black">Opportunity to administer the COVID-19 vaccine \t\t\t\t\t\t\n \t\t\t\t\t\t</th></tr></thead><tbody title="tbody"><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">SAD in remission or with low level of activity with low dose of PDN and without ISS<a class="elsevierStyleCrossRef" href="#tblfn0005"><span class="elsevierStyleSup">a</span></a> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">No evidence of clinical or immunological activity, no use of PDN or 7.5 mg/day doses \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Ideal situation to receive the vaccineLevel of antibody production against the vaccine (potentially) comparable to persons without SAD \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">SAD in remission or with low level of activity with low dose PDN and with ISS<a class="elsevierStyleCrossRef" href="#tblfn0005"><span class="elsevierStyleSup">a</span></a> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">No evidence of clinical or immunological activity, no use of PDN or 7.5 mg/day doses and with ISS \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Ideal situation to receive the vaccineLevel of vaccine antibody production (potentially) lower than in people without SAD \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">SAD with moderate or severe activity level, with intermediate-high doses of PDN and with ISS<a class="elsevierStyleCrossRef" href="#tblfn0005"><span class="elsevierStyleSup">a</span></a> \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Clinical evidence of serious activity or serious organ compromise (life-threatening) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">Treat SAD, avoid organ damage and life riskOnce remission induction is achieved, plan vaccinationThe use of ISS in high doses does not ensure the efficacy of the vaccine \t\t\t\t\t\t\n \t\t\t\t</td></tr><tr title="table-row"><td class="td-with-role" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t ; entry_with_role_rowhead " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">SAD on RTX treatment (or anticipated to receive) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">No clinical evidence of serious activity or serious organ compromise (not life-threatening) \t\t\t\t\t\t\n \t\t\t\t</td><td class="td" title="\n \t\t\t\t\ttable-entry\n \t\t\t\t " align="left" valign="\n \t\t\t\t\ttop\n \t\t\t\t">With stable SAD, vaccinate and postpone RTX 4 weeksIf vaccine is not available, defer RTX if condition allowsIf RTX already received, wait 4–8 weeks to vaccinate \t\t\t\t\t\t\n \t\t\t\t</td></tr></tbody></table> """ ] "imagenFichero" => array:1 [ 0 => "xTab2696997.png" ] ] ] "notaPie" => array:1 [ 0 => array:3 [ "identificador" => "tblfn0005" "etiqueta" => "a" "nota" => "<p class="elsevierStyleNotepara" id="npar0005">Includes synthesis ISS, biologics, and Janus kinase inhibitors.</p>" ] ] ] "descripcion" => array:1 [ "en" => "<p id="spar0015" class="elsevierStyleSimplePara elsevierViewall">Clinical Scenarios of Patients with Systemic Autoimmune Disease at the Time of COVID-19 Vaccine Administration.</p>" ] ] ] "bibliografia" => array:2 [ "titulo" => "References" "seccion" => array:1 [ 0 => array:2 [ "identificador" => "bibs0005" "bibliografiaReferencia" => array:30 [ 0 => array:3 [ "identificador" => "bib0005" "etiqueta" => "1" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Risk of death among people with rare autoimmune diseases compared to the general population in England during the 2020 COVID-19 pandemic" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "E. Peach" 1 => "M. Rutter" 2 => "P. Lanyon" 3 => "M.J. Grainge" 4 => "R. Hubbard" 5 => "J. Aston" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "Rheumatology (Oxford)" "fecha" => "2021" "volumen" => "60" "paginaInicial" => "1902" "paginaFinal" => "1909" ] ] ] ] ] ] 1 => array:3 [ "identificador" => "bib0010" "etiqueta" => "2" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "A.S. Strangfeld" 1 => "M. Schafer" 2 => "M.A. Gianfrancesco" 3 => "S. Lawson-Tovey" 4 => "J.W. Liew" 5 => "L. Ljung" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1136/annrheumdis-2020-219498" "Revista" => array:2 [ "tituloSerie" => "Ann Rheum Dis" "fecha" => "2021" ] ] ] ] ] ] 2 => array:3 [ "identificador" => "bib0015" "etiqueta" => "3" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases – version 1" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "J. Curtis" 1 => "A.R. Johnson" 2 => "D.D. Anthony" 3 => "R.J. Arasaratnam" 4 => "L.R. Baden" 5 => "A.R. Bass" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1002/art.41734" "Revista" => array:2 [ "tituloSerie" => "Arthritis Rheumatol" "fecha" => "2021" ] ] ] ] ] ] 3 => array:3 [ "identificador" => "bib0020" "etiqueta" => "4" "referencia" => array:1 [ 0 => array:1 [ "referenciaCompleta" => "SER - Sociedad Española de Reumatología: <a target="_blank" href="https://www.ser.es/comunicado-de-la-ser-sobe-la-vacunacion-para-la-covid-19-en-pacientes-con-enfermedades-autoinmunes-sistemicas-eas/">https://www.ser.es/comunicado-de-la-ser-sobe-la-vacunacion-para-la-covid-19-en-pacientes-con-enfermedades-autoinmunes-sistemicas-eas/</a>. [Accessed 17 February 2021]." ] ] ] 4 => array:3 [ "identificador" => "bib0025" "etiqueta" => "5" "referencia" => array:1 [ 0 => array:1 [ "referenciaCompleta" => "Canadian Rheumatology Association recommendation on COVID-19 vaccination in persons with autoimmune rheumatic disease. Published on February 12 2021. En <a target="_blank" href="http://www.rheum.ca">www.rheum.ca</a>. (Accessed 14 February 2021]." ] ] ] 5 => array:3 [ "identificador" => "bib0030" "etiqueta" => "6" "referencia" => array:1 [ 0 => array:1 [ "referenciaCompleta" => "British Society for Rheumatology. Principles for COVID-19 vaccination in musculoskeletal and rheumatology for clinicians. Version 1, 14th January 2021). (<a target="_blank" href="http://rheumatology.org.uk">rheumatology.org.uk</a>, Accessed 3 February 2021)." ] ] ] 6 => array:3 [ "identificador" => "bib0035" "etiqueta" => "7" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Looking beyond COVID-19 vaccine phase 3 trial" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:3 [ 0 => "J.H. Kim" 1 => "F. Marks" 2 => "J.D. Clements" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1038/s41591-021-01230-y" "Revista" => array:6 [ "tituloSerie" => "Nat Med" "fecha" => "2021" "volumen" => "27" "paginaInicial" => "205" "paginaFinal" => "211" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/33469205" "web" => "Medline" ] ] ] ] ] ] ] ] 7 => array:3 [ "identificador" => "bib0040" "etiqueta" => "8" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Safety and efficacy of the BNT162b mRNA COVID-19 vaccine" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "F.P. Polack" 1 => "S.J. Thomas" 2 => "N. Kitchin" 3 => "J. Absalon" 4 => "A. Gurtman" 5 => "S. Lockhart" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "N Eng J Med" "fecha" => "2020" "volumen" => "383" "paginaInicial" => "2603" "paginaFinal" => "2612" ] ] ] ] ] ] 8 => array:3 [ "identificador" => "bib0045" "etiqueta" => "9" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Safety and efficacy of the ChAd0x1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa and the UK" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "M. Voysey" 1 => "S.A. Costa Clemens" 2 => "S.A. Madhi" 3 => "L.Y. Weckx" 4 => "P.M. Folegatti" 5 => "P.K. Aley" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/S0140-6736(20)32661-1" "Revista" => array:6 [ "tituloSerie" => "Lancet" "fecha" => "2021" "volumen" => "397" "paginaInicial" => "99" "paginaFinal" => "111" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/33306989" "web" => "Medline" ] ] ] ] ] ] ] ] 9 => array:3 [ "identificador" => "bib0050" "etiqueta" => "10" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "D.Y. Logunov" 1 => "I.D. Dolzhikova" 2 => "D.V. Shcheblyakov" 3 => "O.V. Zubkova" 4 => "A.S. Dzharullaeva" 5 => "A.V. Kovyrshina" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/S0140-6736(21)00234-8" "Revista" => array:6 [ "tituloSerie" => "Lancet" "fecha" => "2021" "volumen" => "397" "paginaInicial" => "671" "paginaFinal" => "681" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/33545094" "web" => "Medline" ] ] ] ] ] ] ] ] 10 => array:3 [ "identificador" => "bib0055" "etiqueta" => "11" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Safety, tolerability and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomized, double-blind, placebo-controlled, phase 1-2 clinical trial" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "Y. Zhang" 1 => "G. Zeng" 2 => "H. Pan" 3 => "C. Li" 4 => "Y. Hu" 5 => "K. Chu" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1016/S1473-3099(20)30843-4" "Revista" => array:6 [ "tituloSerie" => "Lancet Infect Dis" "fecha" => "2021" "volumen" => "21" "paginaInicial" => "181" "paginaFinal" => "192" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/33217362" "web" => "Medline" ] ] ] ] ] ] ] ] 11 => array:3 [ "identificador" => "bib0060" "etiqueta" => "12" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Double-blind, randomized, placebo controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professional with the adsorbed COVID-19 (inactivated) vaccine" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "R. Palacios" 1 => "E. González Patiño" 2 => "R. de Oliveira Piorelli" 3 => "M.T.R.P. Conde" 4 => "A.P. Batista" 5 => "G. Zeng" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1186/s13063-020-04775-4" "Revista" => array:6 [ "tituloSerie" => "Trials" "fecha" => "2020" "volumen" => "21" "numero" => "1" "paginaInicial" => "853" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/33059771" "web" => "Medline" ] ] ] ] ] ] ] ] 12 => array:3 [ "identificador" => "bib0065" "etiqueta" => "13" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "C. Keech" 1 => "G. Albert" 2 => "I. Cho" 3 => "A. Robertson" 4 => "P. Reed" 5 => "S. Neal" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1056/NEJMoa2026920" "Revista" => array:6 [ "tituloSerie" => "N Engl J Med" "fecha" => "2020" "volumen" => "383" "paginaInicial" => "2320" "paginaFinal" => "2332" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/32877576" "web" => "Medline" ] ] ] ] ] ] ] ] 13 => array:3 [ "identificador" => "bib0070" "etiqueta" => "14" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "L.R. Baden" 1 => "H.M. El Sahly" 2 => "B. Essink" 3 => "K. Kotloff" 4 => "S. Frey" 5 => "R. Novak" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "N Eng J Med" "fecha" => "2021" "volumen" => "384" "paginaInicial" => "403" "paginaFinal" => "416" ] ] ] ] ] ] 14 => array:3 [ "identificador" => "bib0075" "etiqueta" => "15" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "U.M. Geisen" 1 => "D.K. Berner" 2 => "F. Tran" 3 => "M. Sümbül" 4 => "L. Vullriede" 5 => "M. Ciripoi" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1136/annrheumdis-2021-220272" "Revista" => array:2 [ "tituloSerie" => "Ann Rheum Dis" "fecha" => "2021" ] ] ] ] ] ] 15 => array:3 [ "identificador" => "bib0080" "etiqueta" => "16" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "B.J. Boyarsky" 1 => "J.A. Ruddy" 2 => "C.M. Connolly" 3 => "M.T. Ou" 4 => "W.A. Werbel" 5 => "J.M. Garonzik-Wang" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1136/annrheumdis-2021-220289" "Revista" => array:2 [ "tituloSerie" => "Ann Rheum Dis" "fecha" => "2021" ] ] ] ] ] ] 16 => array:3 [ "identificador" => "bib0085" "etiqueta" => "17" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "The effect of disease-modifying antirheumatic drugs on vaccine immunogenicity in adults" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:3 [ 0 => "A.L. Day" 1 => "K.L. Winthrop" 2 => "J.R. Curtis" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "Clev Clin J Med" "fecha" => "2020" "volumen" => "87" "paginaInicial" => "695" "paginaFinal" => "703" ] ] ] ] ] ] 17 => array:3 [ "identificador" => "bib0090" "etiqueta" => "18" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "COVID-19 and autoimmunity" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "M. Ehrenfeld" 1 => "A. Tincani" 2 => "L. Andreoli" 3 => "M. Cattalini" 4 => "A. Greenbaum" 5 => "E. Kanduc" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:5 [ "tituloSerie" => "Autoimmunity Rev" "fecha" => "2020" "volumen" => "19" "numero" => "8" "paginaInicial" => "102597" ] ] ] ] ] ] 18 => array:3 [ "identificador" => "bib0095" "etiqueta" => "19" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:2 [ 0 => "A. Vojdani" 1 => "D. Kharrazian" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Revista" => array:3 [ "tituloSerie" => "Clin Immunol" "fecha" => "2020" "volumen" => "217" ] ] ] ] ] ] 19 => array:3 [ "identificador" => "bib0100" "etiqueta" => "20" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Vaccines and Related Biological Products Advisory Committee Meeting. FDA Briefing Document Moderna COVID-19 Vaccine" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:1 [ 0 => "US Food and Drug Administration (FDA)" ] ] ] ] ] "host" => array:1 [ 0 => array:1 [ "Libro" => array:1 [ "fecha" => "2020" ] ] ] ] ] ] 20 => array:3 [ "identificador" => "bib0105" "etiqueta" => "21" "referencia" => array:1 [ 0 => array:1 [ "referenciaCompleta" => "GRA COVID19 Vax Survey. Available at: <a target="_blank" href="https://vdash.rheum-covid.org">https://vdash.rheum-covid.org</a>. [Accessed 25 April 2021]." ] ] ] 21 => array:3 [ "identificador" => "bib0110" "etiqueta" => "22" "referencia" => array:1 [ 0 => array:1 [ "referenciaCompleta" => "The Vacolup study. Available at: <a target="_blank" href="https://maladie-autoimmune.fr/vacolup/">https://maladie-autoimmune.fr/vacolup/</a>. [Accessed 25 April 2021]." ] ] ] 22 => array:3 [ "identificador" => "bib0115" "etiqueta" => "23" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:4 [ 0 => "W.S. Lee" 1 => "A.K. Wheatley" 2 => "S.J. Kent" 3 => "B.J. DeKosky" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1038/s41564-020-00789-5" "Revista" => array:6 [ "tituloSerie" => "Nat Microbiol" "fecha" => "2020" "volumen" => "5" "paginaInicial" => "1185" "paginaFinal" => "1191" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/32908214" "web" => "Medline" ] ] ] ] ] ] ] ] 23 => array:3 [ "identificador" => "bib0120" "etiqueta" => "24" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:6 [ 0 => "A. Greinacher" 1 => "T. Thiele" 2 => "E. Warkentin" 3 => "K. Weisser" 4 => "P.A. Kyrle" 5 => "S. Eichinger" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1056/NEJMoa2104840" "Revista" => array:3 [ "tituloSerie" => "N Engl J Med" "fecha" => "2021" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/32222134" "web" => "Medline" ] ] ] ] ] ] ] ] 24 => array:3 [ "identificador" => "bib0125" "etiqueta" => "25" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "N.H. Schultz" 1 => "I.H. Sorvoll" 2 => "A.E. Michelsen" 3 => "L.A. Munthe" 4 => "F. Lund-Johansen" 5 => "M.T. Ahlen" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1056/NEJMoa2104882" "Revista" => array:3 [ "tituloSerie" => "N Engl J Med" "fecha" => "2021" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/32222134" "web" => "Medline" ] ] ] ] ] ] ] ] 25 => array:3 [ "identificador" => "bib0130" "etiqueta" => "26" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "M. Scully" 1 => "D. Singh" 2 => "R. Lown" 3 => "A. Poles" 4 => "T. Solomon" 5 => "M. Levi" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1056/NEJMoa2105385" "Revista" => array:3 [ "tituloSerie" => "N Engl J Med" "fecha" => "2021" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/32222134" "web" => "Medline" ] ] ] ] ] ] ] ] 26 => array:3 [ "identificador" => "bib0135" "etiqueta" => "27" "referencia" => array:1 [ 0 => array:1 [ "referenciaCompleta" => "AstraZeneca’s Covid-19 vaccine: benefits and risks in context. Available at: <a target="_blank" href="https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-benefits-risks-context">https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-benefits-risks-context</a>. [Accessed 24 April 2021]." ] ] ] 27 => array:3 [ "identificador" => "bib0140" "etiqueta" => "28" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Thrombotic thrombocytopenia after Ad26.COV2.S vaccination – response from the manufacturer" "autores" => array:1 [ 0 => array:2 [ "etal" => false "autores" => array:3 [ 0 => "J. Sadoff" 1 => "K. Davis" 2 => "M. Douoguih" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1056/NEJMc2106075" "Revista" => array:3 [ "tituloSerie" => "N Engl J Med" "fecha" => "2021" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/32222134" "web" => "Medline" ] ] ] ] ] ] ] ] 28 => array:3 [ "identificador" => "bib0145" "etiqueta" => "29" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "2019 update of EULAR recommendations for vaccinations in adult patients with autoimmune inflammatory rheumatic diseases" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "V. Furer" 1 => "C. Rondaan" 2 => "M.W. Heijstek" 3 => "N. Agmon-Levin" 4 => "S. van Assen" 5 => "M. Bijl" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1136/annrheumdis-2019-215882" "Revista" => array:6 [ "tituloSerie" => "Ann Rheum Dis" "fecha" => "2020" "volumen" => "79" "paginaInicial" => "39" "paginaFinal" => "52" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/31413005" "web" => "Medline" ] ] ] ] ] ] ] ] 29 => array:3 [ "identificador" => "bib0150" "etiqueta" => "30" "referencia" => array:1 [ 0 => array:2 [ "contribucion" => array:1 [ 0 => array:2 [ "titulo" => "Safety and efficacy of single – dose Ad26.COV2.S vaccine against Covid-19" "autores" => array:1 [ 0 => array:2 [ "etal" => true "autores" => array:6 [ 0 => "J. Sadoff" 1 => "G. Gray" 2 => "A. Vandebosch" 3 => "V. Cárdenas" 4 => "G. Shukarev" 5 => "B. Grinsztejn" ] ] ] ] ] "host" => array:1 [ 0 => array:2 [ "doi" => "10.1056/NEJMoa2101544" "Revista" => array:3 [ "tituloSerie" => "N Engl J Med" "fecha" => "2021" "link" => array:1 [ 0 => array:2 [ "url" => "https://www.ncbi.nlm.nih.gov/pubmed/32222134" "web" => "Medline" ] ] ] ] ] ] ] ] ] ] ] ] ] "idiomaDefecto" => "en" "url" => "/23870206/0000015700000005/v1_202109171210/S2387020621004174/v1_202109171210/en/main.assets" "Apartado" => array:4 [ "identificador" => "44145" "tipo" => "SECCION" "en" => array:2 [ "titulo" => "Special article" "idiomaDefecto" => true ] "idiomaDefecto" => "en" ] "PDF" => "https://static.elsevier.es/multimedia/23870206/0000015700000005/v1_202109171210/S2387020621004174/v1_202109171210/en/main.pdf?idApp=UINPBA00004N&text.app=https://www.elsevier.es/" "EPUB" => "https://multimedia.elsevier.es/PublicationsMultimediaV1/item/epub/S2387020621004174?idApp=UINPBA00004N" ]
Journal Information
Vol. 157. Issue 5.
Pages 247-252 (September 2021)
Vol. 157. Issue 5.
Pages 247-252 (September 2021)
Special article
Autoimmune diseases and vaccines against COVID-19. Decision making in uncertain scenarios
Enfermedades autoinmunes y vacunas contra la COVID-19. Toma de decisiones en escenarios de incertidumbre
Visits
7592
a Unidad de Enfermedades Autoinmunes, Hospital Evangélico y Centro Asistencial del Sindicato Médico del Uruguay (CASMU), Montevideo, Uruguay
b Laboratorio de Inmunorregulación e Inflamación, Institut Pasteur de Montevideo, Montevideo, Uruguay
c Servicio de Enfermedades Autoinmunes, Hospital Clínic, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
This item has received
Article information
These are the options to access the full texts of the publication Medicina Clínica (English Edition)
Subscriber
Subscribe
Purchase
Contact
Phone for subscriptions and reporting of errors
From Monday to Friday from 9 a.m. to 6 p.m. (GMT + 1) except for the months of July and August which will be from 9 a.m. to 3 p.m.
Calls from Spain
932 415 960
Calls from outside Spain
+34 932 415 960
E-mail