Follow-up after hospital discharge of SARS-CoV-2 survivors represents a huge burden on the healthcare system. We attempt to assess the utility of symptoms and health-related quality of life questionnaire (SF-12) to identify SARS CoV2 pulmonary sequelae.
MethodsProspective, non-interventional follow-up study. A cardiopulmonary exercise test, functional respiratory test (PFT), SF12 questionnaire were performed after hospitalization at six months after the first positive PCR smear.
Results41 patients were included, female (39%), mean age 57.3±13.7 years. 70% persisted with symptoms. 46% presented a maximum oxygen consumption below 80% of predicted. SF-12 physical domain score was significantly reduced in patients with altered PFT (32.7 vs. 45.9; p<0.001) and obtained the best sensitivity and specificity to identify PFT alterations (AUC 0.862, Sensitivity 85.7%, Specificity 81.5%).
ConclusionsSF-12 questionnaire shows high sensitivity and specificity to detect SARS CoV2 survivors with pulmonary function alterations.
El seguimiento tras el alta hospitalaria de los supervivientes de SARS-CoV-2 representa una enorme carga para el sistema sanitario. Intentamos evaluar la utilidad de los síntomas y el cuestionario de calidad de vida (SF-12) para identificar los pacientes con secuelas pulmonares por SARS-CoV-2.
Materiales y métodosEstudio de seguimiento prospectivo observacional. Después de la hospitalización, a los 6 meses del primer frotis con PCR positiva se realizaron: una prueba de esfuerzo cardiopulmonar, pruebas funcionales respiratorias (PFR) y se aplicó el cuestionario SF-12.
ResultadosSe incluyó a 41 pacientes, el 39% eran mujeres, con una edad media de 57,3±13,7 años. El 70% persistía con síntomas. El 46% presentó un consumo máximo de oxígeno por debajo del 80% del predicho. La puntuación del dominio físico del SF-12 fue significativamente más baja en pacientes con PFR alteradas (32,7 vs. 45,9; p<0,001) y obtuvo la mejor sensibilidad y especificidad para identificar las alteraciones de las PFR (AUC 0,862; sensibilidad 85,7% y especificidad 81,5%).
ConclusionesEl cuestionario de calidad de vida SF-12 presenta una alta sensibilidad y especificidad para detectar a los sobrevivientes de SARS-CoV-2 con alteraciones de la función pulmonar.
Many SARS-CoV-2 survivors are showing symptoms beyond the acute phase of illness. Follow-up after hospital discharge of survivors is frequently incomplete and represents a huge burden on the healthcare system.
The persistence of symptoms developed during or after 12 weeks of SARS-CoV-2 acute infection has been defined as long COVID.1
The long covid syndrome is secondary to multiple organ dysfunction and its pathophysiology is still not fully cleared.2 Many studies describe persistent symptoms, prevalently fatigue and shortness of breath at 6 months and 1 year of follow-up.3–5 Given the burden of post SARS-CoV-2 patients that general practitioners and specialists must evaluate during the next months/years, we need quicks and suitable tools that potentially we can use telematically to identify patients who need further studies and to manage priority. In this study, we attempt to assess the utility and the diagnostic performance of health-related quality of life questionnaire SF-12 and symptoms to identify SARS-CoV-2 survivors with cardiopulmonary sequelae performing cardiopulmonary exercise tests (CPET) and static pulmonary function test (PFT).
MethodsWe performed a prospective, non-interventional follow-up study recruiting in the outpatient's post covid clinic all the consecutive patients dismissed from our institution's pneumology department, between 18 March and 30 June 2020, with a diagnosis of SARS-CoV-2 pneumonia. We included patients>18 and <75 years old without severe comorbidities.
According to the WHO Interim Guidance, we classify patients as SARS-CoV-2 mild Pneumonia, Severe Pneumonia and acute respiratory distress syndrome. All the patients answered the self-reported SF-12 questionnaire, performed PFT and CPET on the same day, six months after the first SARS-CoV-2 positive smear. The SF-12 is a general health-related quality-of-life survey that measures Physical Component Scale (PCS) and the Mental Component Scale (MCS), each domain is measured on a 0–100 scale. A higher score correlates with a higher quality of life. Considering that the Spanish weights of survey components are very similar to those of the original American version6 the SF-12 was scored according to normative standards established by Ware et AL.7 Spirometry and pulmonary diffusion tests were performed with the MasterScreen Diffusion (RT)/ SentrySuite®, adopting Miller Criteria for reproducibility and acceptability and using Global Lung Initiative reference values. The diffusing capacity for carbon monoxide (DLCO) was assessed with a single breath method. The results of PFT were expressed as a percentage of the predicted value according to the European Community Lung Health Survey. Abnormal PFT was defined as the presence of TLC (Total Lung Capacity), FVC (Forced Vital Capacity), FEV1 (Forced Expiratory Volume in the First second), DLCO<80% of predicted, FEV1/FVC<70 and RV (Residual Volume)>120% of predicted. According to the European Respiratory Society, we classify as an obstructive pattern a FEV1/FVC<70 and as a restrictive pattern a TLC<80% of predicted. CPET was performed using a microprocessor-controlled eddy current brake ciclo-ergometer (Viasprint 150p; Ergoline). All the patients performed, after 3min of unloaded warm-up phase, a symptoms limited exercise test with a 10/W/min ramp protocol and were encouraged to exercise up to the maximal effort. Patients wore a non-rebreathing Hans-Rudolph Mask connected to Vyntus™ CPX Metabolic Cart. Oxygen saturation with pulse oximetry and heart rate with 12 lead ECG monitoring was performed during the test. Measurements of mixed expired oxygen, mixed expired carbon dioxide and expired volume were determined at rest and for each breath throughout exercise. Oxygen uptake (VO2), carbon dioxide production (VCO2), RER (respiratory gas exchange ratio VCO2/VO2), minute ventilation, respiratory rate, tidal volume, dead space, the ratio of dead space to tidal volume (VD/VT), ventilatory equivalent for carbon dioxide and oxygen, end-tidal CO2 (petCO2) and end-tidal O2 (petO2), were determined for each breath and peak values were obtained from 8 breaths averaged data.
Maximum work rate was defined as the highest work level that was reached, VE/VCO2 slope was calculated over the linear component of VE vs. VCO2. Radiological Follow-up was performed with computed tomography or chest X-ray.
Written informed consent was obtained from all patients, and the study was approved by the ethics committee of Nuestra Señora de la Candelaria Hospital (CHUNSC_2020_50). The normality of the data distribution was determined using the Kolmogorov–Smirnov test. The correlation of different variables was analyzed using Spearman's correlation. We fitted Receiver Operating Characteristic (ROC) curves to determine the Area Under the Curve (AUC) for PCS and MCS SF-12 domains and symptoms, we evaluated the sensitivity and specificity to identify PFT alterations and percent predicted peak oxygen uptake (%pVO2)<80%. All statistical tests were performed using the open-access statistical package the jamovi project (2021). jamovi (Version 1.6) [Computer Software].
Results41 patients were included, female (39%), mean age 57.3±13.7 years (Fig. 1: Flow chart describing inclusion of patients). 22% presented acute respiratory distress syndrome, 49% severe pneumonia, 29% mild pneumonia. 70% persisted with symptoms during follow-up: dyspnoea 56.1%, fatigue 52.5%, cough 14.6%, productive cough 4.9%, loss of smell 17.1%, loss of taste 24.4%, headache 19.5%, joint pain 22%, muscle pain 12.2%, neuropathy 2.4%. 14 patients (34%) showed abnormal PFT. DLCO and TLC were finally reliable in 37 patients.
5 patients (12%) showed a restrictive pattern without DLCO alteration, 2 patients (5%) showed an obstructive pattern (1 patient with previous chronic obstructive pulmonary disease and 1 patient active smoker), 1 patient showed residual volume>120% of predicted suggestive of air trapping.
Abnormal DLCO was detected in 6 patients (15%), 4 patients with restrictive pattern and reduced TLC and 2 patients without PFT's alterations excepting TLC in the lower limit of normality.
All the patients performed a radiological follow-up test: 20 patients (48.8%) performed a computed tomography, 21 patients (51.2%) performed a chest X-ray. 20 patients (48.8%) showed mild radiological sequelae during follow-up. 2 patients without previously known pulmonary disease persisted with extensive pulmonary fibrosis.
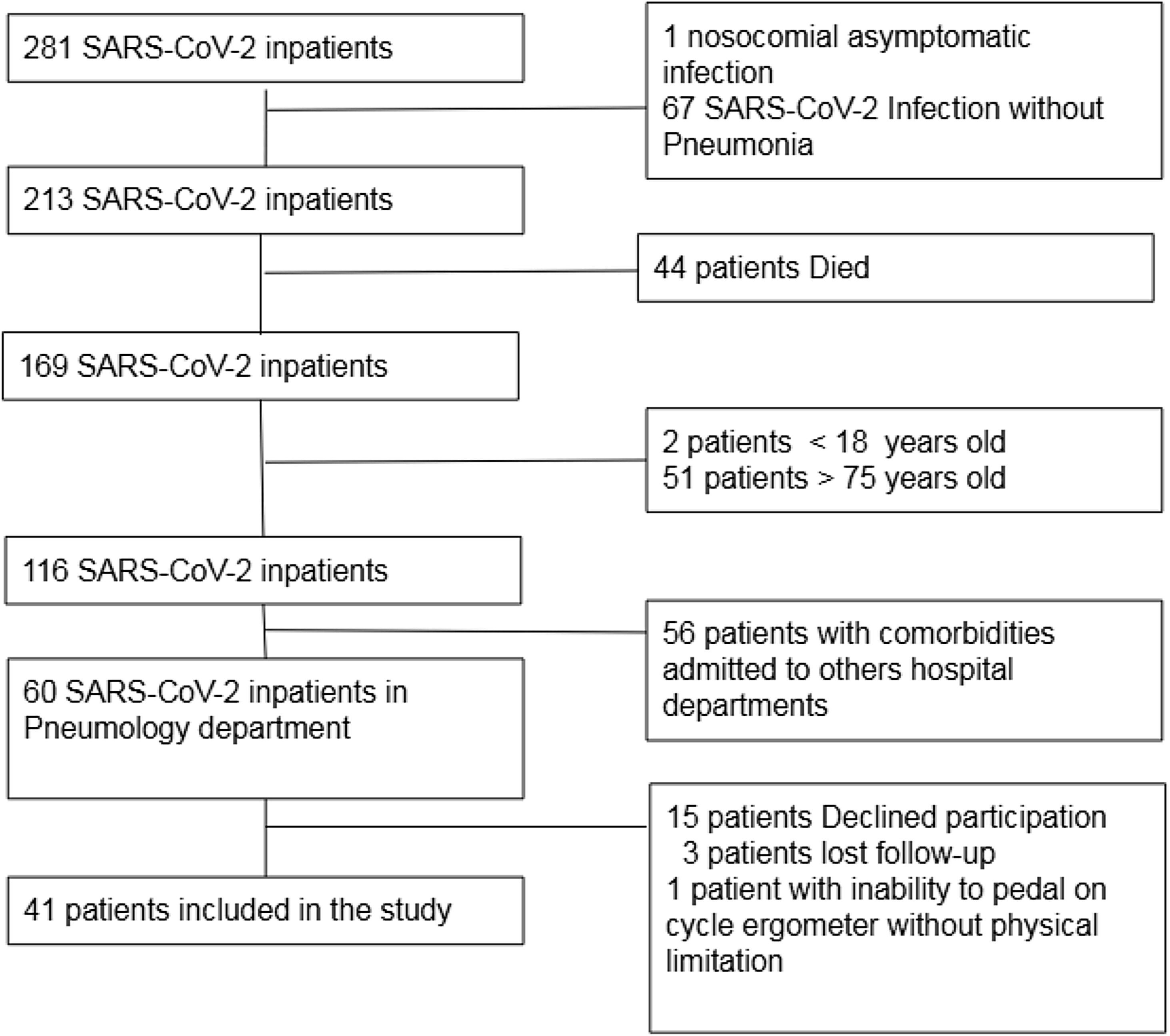
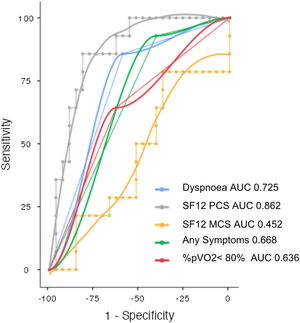
Both SF-12 physical and mental domain scores were reduced in SARS-CoV-2 survivors (41.4±10.4; 47.5±11.5). SF-12 physical domain score was significantly reduced in patients with altered PFT comparing to patients with normal PFT (32.7 vs. 45.9; p<0.001), no significant difference of Mental domain score between normal and altered PFT groups was found (46.5 vs. 49.4; NS) (Table 1). Patients with altered PFT presented higher prevalence of symptoms (92.9 vs. 59.3; p 0.025) especially dyspnoea (85.7 vs. 40.7; p 0.004) and a not significant reduction of %pVO2 (74 vs. 84; p 0.054). SF-12 physical domain score obtained the best sensitivity and specificity to identify PFT alterations (AUC 0.862, Sensitivity 85.7%, Specificity 81.5%, Cutpoint 37.61) (Fig. 2). Dyspnoea obtained a good sensitivity with a lower specificity to detect PFT alterations (AUC 0.725, Sensitivity 85.71%, Specificity 59.26%) (Fig. 2).
Clinical characteristics, static and dynamic pulmonary function tests characteristics of COVID-19 patients at 6 months follow-up.
| Normal PFT – n 27 (65.9%) | Altered PFT – n 14 (34.1%) | P | |
|---|---|---|---|
| Mild pneumonia – no. (%) | 9 (33.3) | 3 (21.4) | 0.427 |
| Severe pneumonia – no. (%) | 14 (51.9) | 6 (42.9) | 0.585 |
| ARDS – no. (%) | 4 (14.8) | 5 (35.7) | 0.125 |
| Mean age – yr (±SD) | 55.0 (13.7) | 61.7 (13.1) | 0.144 |
| Female sex – no. (%) | 13 (48.1) | 3 (21.4) | 0.096 |
| Mean body mass index – kg/m2 (±SD) | 30.7 (7.7) | 29.9 (4.9) | 0.716 |
| Obesity – no. (%) | 13 (48.1) | 8 (57.1) | 0.585 |
| Hypertension – no. (%) | 12 (44.4) | 8 (57.1) | 0.440 |
| Previous pulmonary disease – no. (%) | 0 | 5 (35.7) | <0.001 |
| Diabetes mellitus, type 2 – no. (%) | 6 (22.2) | 8 (57.1) | 0.025 |
| Ischemic cardiomyopathy – no. (%) | 2 (8) | 0 | 0.22 |
| Dyslipidemia – no. (%) | 9 (33.3) | 6 (42.9) | 0.548 |
| Symptoms – no. (%) | 16 (59.3) | 13 (92.9) | 0.025 |
| Dyspnoea – no. (%) | 11 (40.7) | 12 (85.7) | 0.004 |
| Asthenia – no. (%) | 13 (50) | 8 (57.1) | 0.491 |
| SF-12 mental domain score (±SD) | 46.5 (11.5) | 49.4 (11.4) | 0.452 |
| SF-12 physical domain score (±SD) | 45.9 (8.9) | 32.7 (7.3) | <0.001 |
| TLC<80% of predicted normal – no. (%) | 0 | 8 (57.1) | <0.001 |
| DLCO<80% of predicted normal – no. (%) | 0 | 6 (42.9) | 0.002 |
| FVC<80% of predicted normal – no. (%) | 3 (11.1) | 5 (35.7) | 0.059 |
| FEV1<80% of predicted normal – no. (%) | 0 | 3 (21.4) | 0.012 |
| FEV1/FVC<70% – no. (%) | 0 | 4 (28.6) | 0.003 |
| RER-mean (± SD) | 1.16 (0.05) | 1.11 (0.09) | 0.023 |
| Peak VO2%pred-mean (± SD) | 0.84 (0.16) | 0.74 (0.13) | 0.054 |
| Breath reserve<20% of predicted normal – no. (%) | 5 (18.5) | 5 (35.7) | 0.224 |
| Vd/Vt exercise peak>20% – no. (%) | 1 (3.7) | 3 (21.4) | 0.152 |
| VCO2slope>30 – no. (%) | 9 (33.3) | 6 (42.9) | 0.548 |
| Work % predicted mean (± SD) | 97.8 (26.4) | 74.0 (17.2) | 0.030 |
| Work<80% – no. (%) | 8 (29.6) | 8 (57.1) | 0.087 |
| SaO2<95% with exercise no. (%) | 0 | 3 (21.4) | 0.012 |
Comparison between groups with Normal or altered pulmonary function test (PFT). ARDS=Acute Respiratory Distress Syndrome; FVC=Forced Vital Capacity; FEV1=Forced Expiratory Volume in One Second; TLC=Total Lung Capacity; DLCO: carbon monoxide diffusion capacity. Peak VO2: peak oxygen uptake; RER=Respiratory Gas Exchange Ratio VCO2/VO2; SaO2=Oxygen Saturation.
Operating characteristic (ROC) and area under the curve (AUC) for detecting abnormal pulmonary function tests in post COVID patients. SF12-PCS (Physical Component Scale), SF-12 MCS (Mental Component Scale), Dyspnoea, presence of any symptoms, percent predicted peak oxygen uptake<80% (%pVO2<80%).
46% of SARS-CoV-2 survivors presented a maximum oxygen consumption below 80% of predicted. 37% of survivors presented inefficiency ventilatory data and of this subgroup 73% persisted with dyspnoea, 26% without PFT alterations.
Comparing groups of patients with normal percent predicted peak oxygen uptake (%pVO2>80%) vs. reduced (%pVO2<80%), no significant differences of SF-12 physical domain score (40.5 vs. 42.2; p 0.617), SF-12 mental domain scores (48.4 vs. 46.6; p 0.622), dyspnoea (50% vs. 63.1%; p 0.397), any symptoms (72.7% vs. 68%; p 0.763) were found.
DiscussionThe persistence of symptoms after SARS-CoV-2 hospitalization is highly frequent, 70% of our patients persist symptomatic at 6 months of follow-up and many (46%) presented impaired cardiopulmonary capacity. The real challenge for physicians working in an overloaded health care system is to detect patients with cardiopulmonary sequelae and decide who needs priority follow up studies. Cardiopulmonary capacity in SARS-CoV-2 survivors generally deteriorates and frequently this is not secondary only to cardiac or pulmonary sequelae and could be explained by several other factors like the high prevalence of obese patients, critical illness myopathy and an exaggerated hiperventilatory response during exercise.8,9 In our study patients with altered PFT presented a significantly higher prevalence of symptoms, mainly dyspnoea. SF-12 physical score showed high sensitivity and specificity to detect patients with pulmonary function alterations but didn’t show accuracy as a diagnostic tool to identify cardiopulmonary impairment defined as a %pVO2<80%.
PFTs alterations are generally mild, but some patients, especially with post-SARS CoV-2 pulmonary fibrosis presented moderate-severe alteration of DLCO and TLC. Although cardiopulmonary deterioration is multifactorial, symptomatic patients, especially with dyspnoea and with a significant reduction of SF-12 physical score need further studies, including PFT with DLCO and TLC. The main limitations of our study are a missing baseline assessment of cardiopulmonary function and PFT before SARS-CoV-2 infection, the presence of patients with previous pulmonary disease and the small numbers of patients.
ConclusionsShort Form 12 Health Survey could be a quick and useful tool to detect post COVID pulmonary function sequelae that we can use telematically and could prioritize follow-up studies.
Authors’ contributionsAll authors have contributed to the redaction of this manuscript.
Conflicts of interestThe authors do not have conflicts of interest to declare. There are no relationships with industry.