Repetitive Nonsuicidal Self-Injury (R-NSSI) is complex and prevalent in adolescents. Although the reward system is a promising mechanism to explain R-NSSI, the specific processes of reward and punishment related to R-NSSI remain unclear. This study examined whether adolescents with R-NSSI displayed difficulties in both reward and punishment contexts, and further explored the role of inhibitory control in processing monetary reward and punishment.
MethodsWithin a cohort from two middle schools (N = 3,475, 48.6 % female, Mage = 12.95), a total of 187 adolescents completed three novel behavioral tasks. Specifically, in Study 1, 36 adolescents with R-NSSI and 28 without NSSI completed adapted incentive-delay tasks to evaluate sensitivity to reward and punishment. In Study 2, 27 adolescents with R-NSSI and 21 without NSSI were given novel incentive delay-two choice oddball task to evaluate the interaction between reward and inhibitory control. In Study 3, 38 adolescents with R-NSSI and 35 without NSSI completed similar task to assess the interaction between punishment and inhibitory control.
ResultsAdolescents with R-NSSI were characterized by higher levels of behavioral reward and punishment sensitivity than adolescents without NSSI. More importantly, the difference between reward and punishment in inhibitory control of R-NSSI was found. Compared to adolescents without NSSI, adolescents with R-NSSI showed lower levels of inhibitory control in response to cues depicting punishment content but not to those depicting reward content.
ConclusionsThis study provides novel experimental evidence that heightened behavioral sensitivity to both reward and punishment may be relevant trait marker in R-NSSI among adolescents, and emphasizes that punishment not reward interact with inhibitory control in the R-NSSI.
Repetitive NSSI (R-NSSI) refers to direct, broadly socially unacceptable, repetitive behavior that causes mild to moderate physical injury and lacks suicidal intent, which is especially prevalent in adolescence (Howe-Martin et al., 2012; Qian et al., 2023). In prior research, instances of NSSI occurring five or more times have been operationalized as R-NSSI (Brunner et al., 2014; Daukantaitė et al., 2021). This definition is also partly consistent with the proposed NSSI in the DSM-5 frequency criterion (i.e., engagement in NSSI for 5 or more days in the past year). R-NSSI is a serious public health problem issue due to its associated with more severe psychopathology (e.g., depression and suicidality). Given the importance of reward system in the pathogenesis and progression of NSSI (Cummings et al., 2021), identifying fundamental reward and punishment processes related to R-NSSI may help to improve our mechanistic understanding of R-NSSI and aid the development of targeted treatments.
Sensitivity to reward and punishment in R-NSSIDysfunctional responses to reward and punishment are thought to underlie many forms of psychopathology (Vollum et al., 2007). Monetary and social cues are commonly utilized as motivational incentives in research on reward/punishment processing. Monetary cues serve as classic incentives and have been extensively employed. Meanwhile, social cues, such as smiling or angry faces and positive or negative feedback, are also gaining attention. It has been observed that monetary rewards possess a stronger incentive value compared to social rewards, and they more effectively evoke motivation and emotion (Wang et al., 2020; Flores et al., 2015). Consequently, monetary cues were used for priming manipulations reward and punishment sensitivity in the current study.
Regarding reward sensitivity, the developmental neuroscience model of NSSI proposed that reward sensitivity increases following the onset of puberty, and heightened sensitivity to reward during adolescence potentiates the maintenance of learned NSSI behaviors (Cummings et al., 2021). However, there is discrepancy across studies when testing the assumption that adolescents with NSSI show elevated reward sensitivity compared to adolescents without NSSI. Some studies have reported heightened neural sensitivity to monetary reward is associated with thoughts of NSSI (Bettis et al., 2022; Poon et al., 2019), whilst others have found that adolescence with self-injury display decreased neural responses to monetary rewards (Case et al., 2021; Sauder et al., 2016). One potential explanation is prior studies ignored the heterogeneity in NSSI, and confused suicidal ideation and NSSI, which lead to challenges in interpreting whether adolescents with R-NSSI showed heightened reward sensitivity.
Besides reward sensitivity, punishment sensitivity is also a crucial, adaptive guide to human behavior. Individual with higher punishment sensitivity is characterized by sensitivity to aversive stimulus, and is easy to evoked by negative feelings such as fear, anxiety, and loss (Bijttebier et al., 2009). The negative reinforcement model proposes that the avoidance of negative affect is the prepotent motive behind NSSI, and reduction of emotional distress thereby increases the likelihood of future NSSI (Nock & Prinstein, 2004). Specifically, adolescents with high punishment sensitivity tend to show avoidant motivations, experience more negative emotions and psychological distress in response to the negative feedback (e.g., punishment, peer reject). Then, adolescents may adopt NSSI as a coping strategy for regulating aversive emotional experiences. This may result in a negative feedback loop contributing to the presence of R-NSSI. Despite the significance of punishment sensitivity to developing R-NSSI among adolescents, only a few studies focused on punishment processing specifically in youth with NSSI. For example, one of the studies has directly compared children with NSSI to healthy controls in the processing of guessing tasks, and found greater neural response to losses versus rewards than children with no history of NSSI (Tsypes et al., 2018). Furthermore, Pollak et al. (2022) found that heighten neural reactivity to social punishment predicted greater NSSI engagement 1 year later among adolescents with high peer rejection. However, whether punishment sensitivity contributes to the R-NSSI remains unclear.
The interplay between inhibitory control and sensitivity to reward/punishment in R-NSSIInhibitory control refers the ability deliberately override an automatic, dominant or prepotent response (Miyake et al., 2000), has been suggested to play a crucial role in NSSI (McHugh et al., 2019). Laboratory studies found that self-injurious adolescents possessed weaker behavioral inhibitory control, and focused on inhibitory control as a behavioral marker for NSSI (Fikke et al., 2015; Liu et al., 2022). According to the dual systems model, adolescent behaviors have been attributed to two competing brain systems: the cognitive control system associated with the prefrontal cortex (e.g., inhibitory control) and the socioemotional system involved in the striatum (e.g., reward sensitivity). Specifically, the prefrontal cortex develops slowly and is relatively weak, whereas striatum peaks during adolescence, explaining the increase in risk-taking behaviors (Casey et al., 2019; Shulman et al., 2016). Moreover, the striatum not only processes rewards but also responds to punishments, indicating that both types of motivational incentives are crucial in driving behavior (Kohls et al., 2013; Lindquist et al., 2016). This is supported by evidence showing that adolescents demonstrate greater striatum activation in response to both reward and punishment stimuli (Telzer, 2016), indicating that these stimuli serve as significant motivational drivers in risk-taking behaviors. Thus, both reward and punishment stimuli may be key motivational drivers in adolescent risk behaviors, with behavior breaking down when heightened sensitivity to these stimuli overpowers the immature cognitive control system. Based on dual systems perspective and considering inhibitory control deficits in NSSI, when identifying reward and punishment sensitivity as risk factors to driven the NSSI, it is important to take into account the levels of inhibitory control contribute to NSSI. Furthermore, it is possible that difficulties with inhibitory control would explain additional variance in differing who engage in R-NSSI among those with elevated reward/punishment sensitivity.
However, prior studies have provided mixed evidence on the interaction between inhibitory control and reward sensitivity in relation to NSSI. For example, one study identified an association between the interplay of reward sensitivity and inhibitory control with NSSI (Jenkins et al., 2013), whereas another study found that reward sensitivity did not moderate the relationship between inhibitory control and NSSI (Burke, 2020). Furthermore, in examining the interplay between inhibitory control and punishment sensitivity, a study found that inhibitory deficits during a punishment context (negative feedback) were associated with more frequent NSSI among adults (Allen et al., 2019). In sum, existing literature does not provide a clear picture of how the interplay between reward/punishment sensitivity and inhibition control relate to R-NSSI among adolescents. Therefore, further investigation is needed to clarify whether adolescents with R-NSSI display deficits in the process of interaction between reward/punishment sensitivity and inhibitory control.
Current studyTo identify the features of reward/punishment sensitivity, and the interaction between inhibitory control and reward/punishment sensitivity in R-NSSI, we compared two groups of adolescents (R-NSSI vs. no-NSSI) on three novel experiments. In the first experiment, we aim to investigate differences between adolescents with R-NSSI and those without NSSI in terms of sensitivity to reward and punishment, it was hypothesized adolescents with R-NSSI would display higher sensitivity to monetary reward (Hypothesis 1a) and punishment (Hypothesis 1b) than adolescents without NSSI. In the second experiment, we aim to explore whether group differences in the interaction between reward sensitivity and inhibitory control, it was hypothesized adolescents with R-NSSI would expand more cognitive effort for high-value reward stimulus (Hypothesis 2). In the third experiment, we aim to explore whether group differences in the interaction between punishment sensitivity and inhibitory control, it was hypothesized adolescents with R-NSSI would expand more cognitive effort for high-value punishment stimulus (Hypothesis 3).
Study 1Study 1 was to examine whether two groups of adolescents (R-NSSI vs. no-NSSI) differed in sensitivity to reward and punishment.
MethodsParticipants and procedureTo obtain a representative cohort from the adolescence, a total of 3475 students (48.6 % female, Mage = 12.95) in 7th and 8th grade were randomly selected from two public middle schools located in North China. All participants were Chinese, the median household income of families was approximately $1222 per month (range $153 to $6112). The median education level for both fathers and mothers were middle school. After the schools were contacted, the teachers, potential participants, and their parents/caregivers received information about the study's objectives. Each participant received a gift of stationery after the survey. All participants and their parents/caregivers provided written consent prior to the start of the study. The participants completed questionnaires in their classrooms during regular school hours. In the past six months, 30.1 % of adolescents (n = 1046; 69.7 % female, Mage = 13.01, S.D. = 0.78) reported at least one instance of NSSI, with a subset prevalence of 14.85 % for R-NSSI (n = 516; 66.1 % female, Mage = 13.07, S.D. = 0.81).
To qualify for the study, the inclusion criteria for both groups included a) no history of Axis I mental disorders, b) no current psychoactive medications, and c) no history of concussions. Potential participants' eligibility was verified by school mental health educators, ensuring that none had previously been diagnosed with or received treatment for any Axis I disorders. The inclusion criteria for the R-NSSI group included a history of engaging in R-NSSI at least five times (e.g., cutting or burning themselves) in past six months (Daukantaitė et al., 2021; Manca et al., 2014). The individuals who engaged in NSSI were provided with referrals to campus psychological services for assistance with NSSI. Ethical approval was granted by the Institutional Review Board of the Faculty of Psychology, Beijng Normal University. Eligible individuals were invited to Studies 1, 2 and 3, which took place in a laboratory setting.
The final (sub)sample (N = 64) included 28 adolescents without NSSI (35.71 % female, Mage = 13.11) and 36 with R-NSSI (66.67 % female, Mage = 12.94) in Study 1.
MeasurementsNonsuicidal self-injuryNSSI was measured using the Deliberate Self-harm Inventory (DSHI, Bjärehed et al., 2012). The scale includes nine items and each of the items is rated on a seven-point scale of 0 (never) to 6 (more than five times), reflecting participants’ frequency of self-injury behaviors over the past six months. Herein, the Cronbach's alpha coefficient was 0.87.
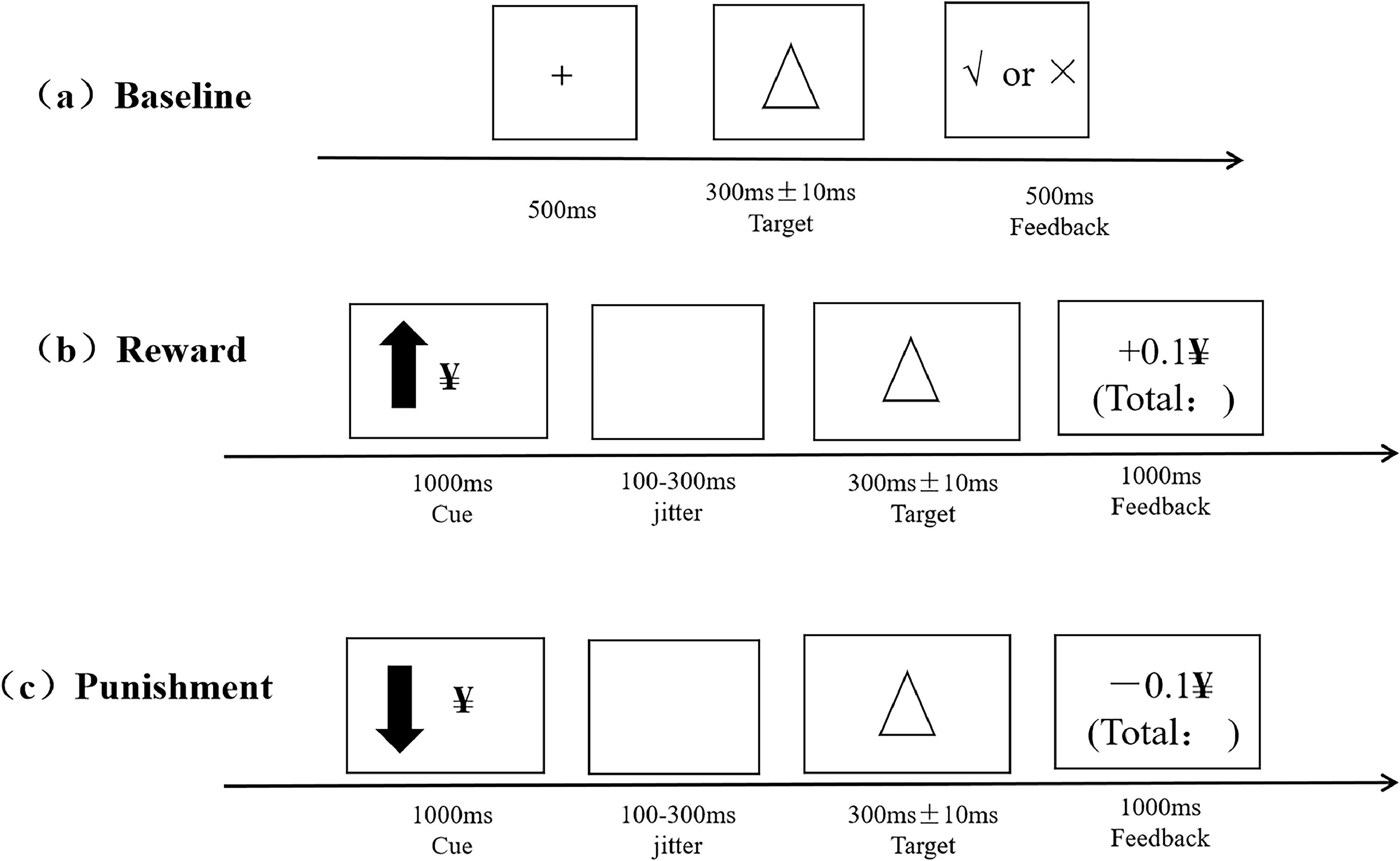
Revised monetary incentive delay taskThe assessment of reward and punishment sensitivity was performed using the Revised MID (Ait Oumeziane et al., 2019). The entire tasks lasted for 30–35 min and were divided into three phases: Baseline measurement, formal tasks regarding the monetary reward and punishment sensitivity, and post-experiment subjective ratings concerning motivation for the reward and punishment. An overview of the trial structure is shown in Fig. 1. Detailed descriptions of tasks appear in the Supplementary.
Data analysisFirst, manipulation check on subjective ratings (results were reported in Supplementary materials). Then, a 2 × 2 repeated-measures analysis of variance (ANOVA) was used to examine the effects of group (no-NSSI vs. R-NSSI) and reward/punishment cue magnitude (high vs. low) on participants’ response times (RTs, only accurate trials) and accuracy (ACC) performance. In addition to classic RTs and ACC, RT cost under varied experimental conditions was calculated and served as behavioral indices of reward and punishment sensitivity. The RT cost was defined by the reaction time incentive from baseline task to formal tasks. For example, participant's high reward sensitivity = RT baseline – RT high reward, increased reaction time incentive indicated higher reward sensitivity. Thus, RT cost was compared among no-NSSI and R-NSS groups under varied task conditions.
Analyses were conducted using SPSS 21.0. The Greenhouse–Geisser correction was applied to the degrees of freedom of the F ratio and the p values in all analyses. Significant interactions were followed up with independent t tests to compare the effects of reward/punishment sensitivity between the no-NSSI and R-NSSI groups. The comparative analyses were subjected to Bonferroni adjustment, with p-values below 0.05 denoting significant differences. Additionally, both ηp2 and Cohen's d were reported for effect size. For sample size calculations in each study, see Supplementary Materials.
ResultsReward sensitivityRegarding ACC, the main effect of reward magnitude obtained marginal significance, F (1, 62) = 2.91, p = 0.09, ηp2 = 0.05. ACC was significantly higher with the high reward (M = 0.56, S.E. = 0.01) than with the low reward (M = 0.54, S.E. = 0.002) in both groups. The main effect of group obtained marginal significance, F (1, 62) = 3.52, p = 0.07, ηp2 = 0.05, with the no-NSSI group (M = 0.56, S.E. = 0.01) performing better than the R-NSSI group (M = 0.53, S.E. = 0.01). Regarding RTs, there was a main effect of reward magnitude, F (1, 62) = 3.74, p = 0.06, ηp2 = 0.06. RTs was significantly quicker with the high reward (M = 101.87, S.E. = 4.31) than with the low reward (M = 105.15, S.E. = 4.68) in both groups. The other main effects and interaction effects were not significant (ps > 0.05) (online Supplementary Table S1).
To further compare the sensitivity of the monetary reward between two groups, RT cost was calculated for each reward magnitude. R-NSSI group showed higher RT cost for high and low reward cues than no-NSSI group (tHigh (62) =−2.87, p < 0.01; tLow (62) =−2.54, p < 0.01), which indicated that adolescents with R-NSSI showed increased reward sensitivity to both high and low reward monetary cues.
Punishment sensitivityRegarding ACC, there was a main effect of group, F (1, 62) = 4.79, p < 0.05, ηp2 = 0.07, with the no-NSSI group (M = 0.58, SE = 0.01) performing better than the R-NSSI group (M = 0.55, S.E. = 0.01). There was a significant interaction between group and punishment magnitude, F (1, 62) = 4.92, p < 0.05, ηp2 = 0.07. Follow-up t-tests showed that individuals with no-NSSI performed better than a R-NSSI group under high punishment (t (62) = 2.99, p < 0.005) but not low punishment (t (62)=0.84, p = 0.40). Regarding RTs, there was a main effect of punishment magnitude, F (1, 62) = 4.27, p < 0.05, ηp2 = 0.06. RTs was significantly quicker with the high punishment (M = 96.14, S.E. = 3.55) than with the low punishment (M = 99.58, SE = 3.74) in both groups. The other main effects and interaction effects were not significant (ps > 0.05) (online Supplementary Table S2).
To further compare the sensitivity of the monetary punishment between two groups, RT cost was calculated for each punishment magnitude. R-NSSI group showed higher RT cost for high and low punishment cues than no-NSSI group (tHigh (62) =−1.98, p < 0.05; tLow (62) =−1.90, p = 0.06), which indicated that adolescents with R-NSSI showed increased punishment sensitivity to both high and low punishment monetary cues.
Study 2To further address the role of inhibitory control in the reward/punishment processing among adolescence with R-NSSI, the second and third studies were conducted. Study 2 was to examine differences in interaction between inhibitory control and reward sensitivity in two groups of adolescents (R-NSSI vs. no-NSSI).
MethodsParticipantsThe final (sub)sample (N = 48) included 21 adolescents without NSSI (55.9 % female, Mage = 13.33) and 27 with R-NSSI (54.3 % female, Mage = 13.49).
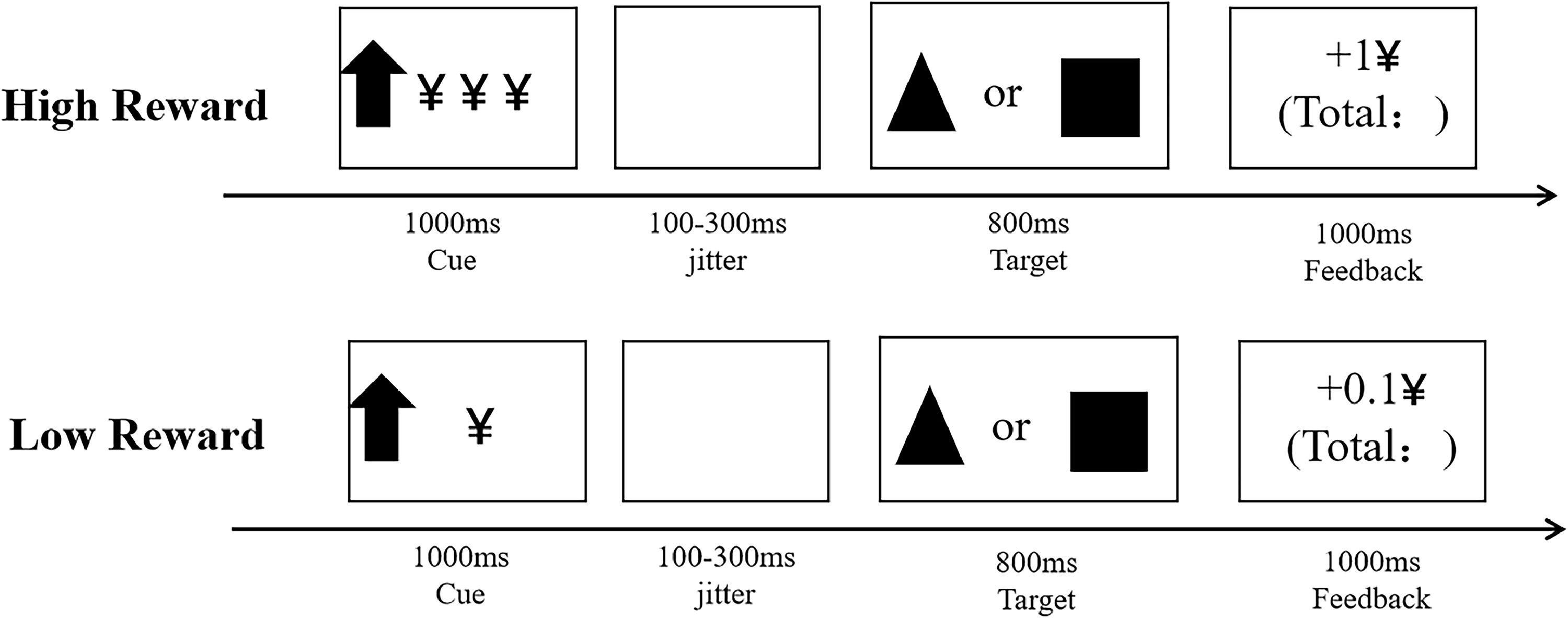
Integrated monetary incentive delay and two-choice oddball task (MID−Oddball task)The MID−Oddball task is a performance-based measure that records inhibitory control toward monetary reward cues based on how quickly and accurately participants identified the different targets presented on a computer screen. This new task was created by referring Integrated Monetary Incentive Delay Go/No-Go paradigm (Demurie et al., 2016). The entire tasks lasted for 25–35 min. An overview of the trial structure is shown in Fig. 2. Detailed descriptions of task appear in Supplementary.
Data analysisFirst, manipulation check on subjective ratings (results reported in the Supplementary materials). Then, a 2 × 2 × 2 repeated-measures analysis of variance (ANOVA) was used to examine the effects of group (no-NSSI vs. R-NSSI), reward magnitude (high, low) and stimulus type (standard, deviant) on both RTs (only accurate trials were included when calculating RTs) and ACC performance. Third, the ACC cost was defined as the accuracy reduction from standard to deviant trials, and lower accuracy reductions reflected higher inhibitory control. The RT cost was defined by the reaction time delay from standard to deviant trials (standard – deviant), and increased reaction time delays indicated lower inhibitory control. Both ACC cost and RT cost typically serve as behavioral indices of inhibitory control (Yuan et al., 2012). Thus, in this experiment, under high reward and under low reward conditions, ACC cost and RT cost (standard – deviant High/ Low reward) served as behavioral indices of successful inhibit high/low monetary reward. A 2 × 2 repeated-measures analysis of variance (ANOVA) was used to examine the effects of group (no-NSSI vs. R-NSSI) and reward magnitude (high, low) on ACC cost and RT cost performance.
ResultsManipulations of reward magnitude and inhibitory controlFor ACC, there was a main effect of stimulus type, F (1, 46) = 114.08, p < 0.001, ηp2 = 0.71, ACC was significantly lower with the deviant stimuli (M = 0.85, S.E. = 0.01) than with the standard stimuli (M = 0.97, S.E. = 0.003) in both groups. Furthermore, there was a significant interaction between stimulus type and reward magnitude, F (1, 46) = 6.21, p < 0.05, ηp2 = 0.12. Follow-up t tests showed that in the condition of standard stimuli, ACC was significantly higher with the high reward cue (M = 0.97, S.E. = 0.02) than with the low reward cue (M = 0.96, S.E. = 0.03), t (47) = 2.76, p < 0.005, while in the condition of deviant stimuli, ACC was significantly higher with the low reward (M = 0.86, S.E. = 0.09) than with the high reward (M = 0.84, S.E. = 0.01), t (47) = −2.0, p < 0.05. The other main effects and interaction effects were not significant (ps > 0.05).
For RTs, there was a main effect of reward magnitude, F (1, 46) = 12.17, p < 0.005, ηp2 = 0.21, adolescents were quicker to respond on high reward (M = 434.10, S.E. = 6.24) compare to low reward (M = 441.60, S.E. = 12.80) in both groups. There was a main effect of stimulus type, F (1, 46) = 12.17, p < 0.005, ηp2 = 0.21, adolescents were quicker to respond on standard (M = 414.35, S.E. = 6.36) compare to deviant (M = 461.35, S.E. = 7.15) in both groups. Furthermore, there was a significant interaction between stimulus type and reward magnitude, F (1, 46) = 7.01, p< 0.05, ηp2= 0.13. Follow-up t tests showed in the condition of standard stimuli adolescents responded significantly faster on high reward cue (M = 408.07, S.E. = 6.29) relative to low reward cue (M = 420.38, S.E. = 14.74), t (47) =−5.65, p < 0.001. The other main effects and interaction effects were not significant (ps> 0.05).
The interaction between reward magnitude and inhibitory controlWe found no support for our second hypothesis: Regarding ACC costs, there was a main effect of reward magnitude, F (1, 46) = 6.21, p< 0.05, ηp2 = 0.12. ACC cost was significantly higher in high reward cue (M = 0.13, S.E. = 0.01) than low reward (M = 0.10, S.E. = 0.01) in both groups. The main effect of group and the interaction effect were not significant (ps > 0.05). Similarly, regarding RT costs, there was a main effect of reward magnitude, F (1, 46) = 7.01, p< 0.05, ηp2 = 0.13. High reward RT cost (M = 51.90, S.E. = 4.21) was significantly higher than low reward (M = 42.09, S.E. = 4.95) in both groups. The main effect of group and the interaction effect were not significant (ps> 0.05), which suggested that adolescents with R-NSSI and without NSSI displayed comparable inhibitory control in the context of monetary reward. Refer to Supplemental materials Table S3 for additional behavioral results.
Study 3Study 3 was to examine differences in interaction between inhibitory control and punishment sensitivity in two groups of adolescents (R-NSSI vs. no-NSSI).
MethodsParticipantsThe final (sub)sample (N = 73) included 35 adolescents without NSSI (40 % female, Mage = 12.86) and 38 with R-NSSI (60.5 % female, Mage = 12.97).
Integrated monetary incentive delay and two-choice oddball taskThe assessment of the interaction between punishment sensitivity and inhibitory control was performed using new revised MID−Oddball task in addition to replacing reward cues and feedback with punishment cues and feedback.
ResultsManipulations of punishment magnitude and inhibitory controlFor ACC, there was a main effect of stimulus type, F (1, 71) = 168.93, p < 0.001, ηp2 = 0.70, ACC was significantly lower with the deviant stimuli (M = 0.87, S.E. = 0.01) than with the standard stimuli (M = 0.96, S.E. = 0.004) in both groups. The main effect of group obtained marginal significance, F (1, 71) = 3.13, p = 0.08, ηp2 = 0.04, with the no-NSSI group (M = 0.91, SE = 0.008) performing better than the R-NSSI group (M = 0.93, S.E. = 0.009). The other main effects and interaction effects were not significant (ps> 0.05).
For RTs, there was a main effect of stimulus type, F (1, 71) = 306.41, p < 0.001, ηp2 = 0.81, adolescents were quicker to respond on the condition of standard (M = 422.28, S.E. = 4.55) than deviant (M = 449.52, S.E. = 11.86) in both groups. There was a significant interaction between stimulus type and punishment magnitude, F (1, 71) = 8.25, p< 0.05, ηp2 = 0.10. Follow-up t tests showed in the condition of deviant stimuli adolescents responded significantly faster on low punishment cue (M = 473.96, S.E. = 4.90) relative to high punishment cue (M = 478.56, S.E. = 4.75), t (72) =−1.98, p < 0.05. Furthermore, the interaction among group, punishment magnitude, and stimulus type were significant, F (1, 71) = 306.41, p < 0.05, ηp2 = 0.07. For high punishment and deviant stimulus condition, R-NSSI group took longer to respond to target than no-NSSI group (t (71) =−2.19, p < 0.05). The other main effects and interaction effects were not significant (ps > 0.05).
The interaction between punishment magnitude and inhibitory controlIn line with our third hypothesis: For RT cost, there was a significant interaction between group and punishment magnitude, F (1, 71) = 4.92, p< 0.05, ηp2 = 0.07. For high punishment RT cost, R-NSSI group (M = 63.93, S.E. = 5.07) was significantly higher than no-NSSI group (M = 50.47, S.E. = 4.34), t (71) =−2.00, p < 0.05, which suggested that adolescents with R-NSSI exhibited deficient punishment inhibitory control compared to adolescents without NSSI. The other main effects were not significant (ps> 0.05). Refer to Supplemental materials Table S4 for additional behavioral results.
DiscussionThis study examined the characteristics of reward/punishment sensitivity, and the process of interaction between reward/punishment sensitivity and inhibitory control relative to R-NSSI through three novel laboratory studies among two groups (no NSSI vs. R-NSSI). Compared to the no-NSSI group, the R-NSSI group displayed heightened reward/punishment sensitivity. More importantly, the results demonstrated that relative to the no-NSSI group, the R-NSSI group had lower levels of inhibitory control with punishment stimulus, but these distinctions were not found in contexts involving reward contexts.
Study 1 identified that R-NSSI group do differ in reward and punishment sensitivity from no-NSSI group, which adds new behavioral evidence regarding the fundamental features of monetary reward and punishment sensitivity in R-NSSI. In reward sensitivity, although RT and ACC showed no significant differences between groups, the R-NSSI group had a higher RT cost than the no-NSSI group. This suggests that adolescents with R-NSSI require more time and effort to achieve the same outcomes as their non-NSSI counterparts, indicating that reward stimuli have a greater motivational impact on the R-NSSI group. One of the functions of NSSI is induce a positive or desired state (Taylor et al., 2018), adolescence with high reward sensitivity tend to experience more positive affect or in anticipation of adding a positive stimulus by engaging in risky activities (Telzer, 2016). Thereby, NSSI may often be used by individuals with high reward sensitivity who want to feel better. Regarding the punishment stimulus, consistent with evidence from neurocognitive science in children and adolescence with NSSI (Tsypes et al., 2018; Pollak et al., 2022), this study suggesting that adolescents who engage in R-NSSI have higher avoid motivation to punishment or aversive events. When individuals are unable to adaptively regulate negative emotion but choose to suppress or avoid the feeling, then NSSI may be chosen because it usually serves the purpose of regulating distressing or aversive emotions, through avoidance or replacement of these states (Wu et al., 2021).
Study 2 further employed a novel behavioral task and found no significant difference in the interaction between reward sensitivity and inhibitory control in both R-NSSI and no-NSSI groups. This result aligns with another study that also utilized a behavioral task (Burk, 2020). Although a previous study found that interactions between reward sensitivity and inhibitory control were associated with the presence of NSSI, such studies predominantly utilized self-report measures (Jenkins et al., 2013). In contrast, the current study employed an objective behavioral task for measurement. These results suggest that discrepancies in assessment methods might be one reason for the inconsistencies in findings. Moreover, NSSI is a quite heterogeneous behavior, prior studies have focused on NSSI and have not directly distinguished adolescents who repeat self-injury. Additionally, previous studies have indicated that incentives such as reward-related signals can enhance inhibitory control. Specifically, the reward-related signal captures more attention, which in turn enhances signal monitoring and subsequently improves inhibitory control (Botvinick & Braver, 2015; Wang et al., 2019). Therefore, it is plausible that adolescents with R-NSSI could capture signals more quickly and initiate the inhibition response earlier, ultimately exhibiting inhibitory control abilities comparable to those of the no-NSSI group in reward contexts. However, given that behavioral tasks cannot reveal the more nuanced temporal processes of interaction between reward sensitivity and inhibition, future research could employ time-related potential techniques based on the behavioral task of the current study to further support this explanation.
Consistent with Hypothesis 3, Study 3 found R-NSSI group exhibit deficient inhibitory control compared to no-NSSI group in the punishment contexts. Specifically, adolescents with R-NSSI took longer times to inhibit in response to cues depicting high punishment. This pattern also found in other risky behaviors such as obsessive-compulsive disorder, cocaine dependent individuals (Hester et al., 2013; Morein-Zamir et al., 2013). The result stresses abnormal inhibitory control processing, particularly during punishment, in R-NSSI, and suggest that symptoms of externalizing psychopathology including R-NSSI may be associated with difficulties in flexibly adapting behavior under punishment conditions. There is evidence shown that individual with reduced inhibitory control in the context of punishment may have the impaired learning from errors and punishment, which further result in deficient self-regulation and causing individual engage in maladaptive behaviors (substance abuse, procrastination, etc.) repeatedly (Wypych & Potenza, 2021; Przetacka et al., 2022). Another possible explanation for the observed effects is negative reinforcement. Punishment is the source of negative affect, and provide negative evaluative information. Consequently, individuals may engage in NSSI to regulate aversive emotions induced by costly punishment. Indeed, affect regulation following NSSI has been posited as a mechanism underlying reinforcement of these behaviors. In a vicious cycle, repeated negative reinforcement trials may further decrease the ability to control negative emotions, which in turn increase the frequency of NSSI.
There are important considerations to take into account when interpreting results. First, because of the cross-sectional design of the study, no conclusions can be drawn with regard to predictors of R-NSSI, future research is needed to examine the temporal relationship between both reward and punishment sensitivity, inhibitory control and engagement in R-NSSI; specifically, the predictive validity of higher reward or punishment sensitivity, and poor punishment inhibitory control in the development and maintenance of R-NSSI among adolescents. Second, the current study primarily utilized monetary stimuli as the incentive, beyond this, other incentives such as social stimuli may also play an important role in R-NSSI. Future studies could include other incentive cues to further validate the findings. Third, this study included a non-clinical sample of community adolescents, which limits the ability to generalize findings to clinical populations, especially in terms of explaining the comorbidity associated with R-NSSI. Future research could extend to clinical samples.
The current study makes a unique contribution to the literature by considering both reward and punishment processes in relation to R-NSSI, and offers unique insights into the interplay between reward/punishment sensitivity and inhibitory control in R-NSSI. Based on Study 1, heightened sensitivity to both rewards and punishments may represent risk factors for R-NSSI among adolescents, which is beneficial for both assessment and intervention strategies. It is advisable to integrate reward/punishment sensitivity into the assessment of transdiagnostic processes, as this may elucidate factors contributing to the persistence of R-NSSI. For intervention, clinicians could incorporate cognitive-behavioral techniques that specifically target the maladaptive thought patterns related to reward and punishment sensitivity. This approach can help adolescents reframe their perceptions of negative and positive stimuli, leading to more adaptive coping mechanisms. Studies 2 and 3 distinguish the reward and punishment reinforcement contexts in inhibitory control among adolescents. The divergence between reward and punishment in the cognitive control process reveals that adolescents with R-NSSI may have greater difficulties in processing punishment but not reward. This means it is necessary to help adolescents with R-NSSI access or learn appropriate regulation strategies or other more positive alternative behaviors to handle punishment in their daily lives. Furthermore, previous research suggests that reward-related stimuli facilitate cognitive control across development (Strang & Pollak, 2014); future interventions could fully utilize reward stimuli and create a positive environment to enhance the inhibitory control of individuals with NSSI.
Ethics approvalThe authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Funding, This research was supported by National Natural Science Foundation of China (31900772) and National Natural Science Foundation of China (32171069)
Accessibility of data, The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Please contact liuxia@bnu.edu.cn.
The authors thank students and their parents, the principal, and the teachers who contributed to this study.