The aim was to investigate the extent and longitudinal determinants of post-traumatic growth (PTG) in cancer survivors.
MethodsA sample of 1316 cancer survivors with various cancer types was examined using the EORTC QLQ-FA12 to assess fatigue, the EORTC QLQ-C30 pain items to assess pain and the Patient Health Questionnaire (PHQ-4) to assess emotional distress two years after diagnosis (t0). Additionally, patients rated how well they felt informed about fatigue at t0. PTG was assessed with the 21-item PTG-Inventory four years after diagnosis (t1) comprising the five subdimensions appreciation of life, relation to others, personal strengths, new possibilities and spiritual change.
ResultsRegarding the extent of PTG, most positive developments were experienced in the PTG subdimension appreciation of life whereas the subdimension spiritual change was the least pronounced domain. Fatigue, pain and emotional distress were longitudinal but non-linear predictors of long-term PTG. Additionally, poor informedness about fatigue was associated with less PTG.
ConclusionsPTG can be perceived even years after a traumatic cancer event and is longitudinally associated with common cancer side effects like fatigue, emotional distress and pain. Further research into the role of individuals' informedness contributing to PTG is needed.
The number of cancer survivors is increasing across Europe, requiring more intensive research into this cancer population (Kalager et al., 2021; Lagergren et al., 2019; Malvezzi et al., 2015). Cancer as a life-threating illness can have substantial negative impact on individuals’ lives, not only during the acute treatment but also in the aftermath. Frequently reported sequelae are among others fatigue, depression, sleep problems, cognitive impairment, distress and reduced quality of life (Lagergren et al., 2019; Wu & Harden, 2015).
Individuals with a cancer diagnosis are forced to adapt their personal life circumstances. This can be accompanied or challenged by the aforementioned negative consequences on the one hand, but also result in positive long-term effects on the other hand. The latter recently gained growing attention in research, attempting to better understand the whole process of coping with cancer. The positive psychological change experienced after the struggle of a challenging life event, e.g. cancer, was defined as post-traumatic growth (PTG). PTG can be experienced in different important life domains resulting in the development of an increased appreciation of life, deeper relationships to others, more personal strengths, new possibilities and a higher spiritual thrive (Tedeschi & Calhoun, 1996, 2004). The theoretical framework is based on the assumption that PTG arises out of an individuals’ integration of fundamental beliefs about oneself and the world into the new reality of life (Janoff-Bulman & McPherson Frantz, 1997; Tedeschi & Calhoun, 2004). Therefore, PTG can also be seen as a coping strategy (Tedeschi & Calhoun, 2004). It has further been proposed that cognitive processing of the trauma-causing event is crucial for its successful overcoming (Tedeschi & Calhoun, 1996). In line with this, study results suggested that cognitive processing, e.g. through deliberate rumination and positive refraiming are linked to higher PTG (Hill & Watkins, 2017; Taku et al., 2009). Although it seems difficult to identify specific factors that support or hinder these favorable processes, one component might be education, which has been shown to be positively linked to PTG (Chen et al., 2019). Moreover, PTG has been associated with socio-demographic characteristics in such that female sex and lower age are linked to higher levels of PTG (Boyle et al., 2017; Wu et al., 2019). Further studies indicated that psychosocial variables, e.g. more social support, optimism and a high amount of perceived stress of the cancer were associated with higher PTG (Sharp et al., 2018; Wu et al., 2019).
However, there are also some inconsistent findings regarding influencing factors of PTG. For instance, some studies found a positive association of PTG with emotional distress (Cordova et al., 2007) while others reported negative or no relationships (Dekel et al., 2012; Mystakidou et al., 2008). Furthermore, also curvilinear associations between PTG and emotional distress have been observed (Lechner et al., 2006).
Besides, fatigue appears to be understudied with regard to PTG although it is one of the most common side effects during cancer and its treatment (Fabi et al., 2020; Ma et al., 2020). Fatigue describes a deliberating subjective feeling of constant physical, emotional and mental exhaustion. It is reported by approximately 30 % of survivors even 5 years after cancer treatment (Fabi et al., 2020; Schmidt et al., 2022, 2018). It is possible that fatigue could have a significant impact on PTG also years after treatment, because it might affect the severity of trauma experienced. Another frequently reported, burdensome and sometimes long-term side effect of cancer treatment is pain (Zielińska et al., 2021). To our knowledge no studies exist that analyze the influence of pain on PTG, although it is plausible that pain could have an adverse impact on an individual's wellbeing and coping.
A review of Casellas-Grau et al. (2017) highlighted reasons for the inconclusive study results in determining PTG: They argued that PTG has often been analyzed only in cross-sectional study designs. Menger et al. (2020) also pointed out that longitudinal studies are needed to better understand the time courses of PTG. Considered together, it seems that there is still no full picture of PTG determinants, which underlines the need to foster more research in cancer populations, especially in cancer survivors (Mayer et al., 2017). To address the aforementioned research gaps, we examined the extent and the determinants of PTG in survivors of different cancer types and treatments. For this, we analyzed longitudinal data, considering sociodemographic variables, psychosocial and also modifiable factors at t0 (two years after cancer diagnosis) to predict PTG at t1 (four years after cancer diagnosis). In accordance with the assumptions of Tedeschi and Calhoun (2004), we hypothesized that higher levels in fatigue, emotional distress and pain at t0 as indicators of higher distress might lead to higher PTG at t1.
We further assumed that information about cancer sequelae such as fatigue might be helpful in guiding a more rational type of cognitive processing as it could influence individual's general cancer literacy. A lack of information about cancer and its consequences has been shown to be associated with a decreased wellbeing, strengthening the assumption that it might play an important role in coping with the disease (Brown et al., 2016; Husson et al., 2011). So, we hypothesized that feeling well informed about fatigue would lead to higher levels of PTG and would influence the relationship between fatigue and PTG.
MethodsParticipants and proceduresAbout 2 years after cancer diagnosis (t0), 2508 patients who underwent different cancer treatments were enrolled in the FiX study (Fatigue in Germany – Examination of prevalence, severity, and date of screening and treatment). Patients were randomly sampled from the Epidemiological Cancer Registry of Baden-Württemberg, Germany. Individuals older than 18 years and diagnosed with a primary tumor of stomach, colon, rectum, liver, pancreas, lung, malignant melanoma, breast, endometrium, ovaries/cervix, prostate, kidney, ladder, non-Hodgkin lymphoma or leukemia were eligible for the study. Details of the study have been described previously (Schmidt et al., 2021). A follow-up survey (t1) was conducted approximately two years later (four years after diagnosis), and was completed by 1316 cancer free survivors.
The FiX study was conducted in accordance with the ethical standards of the Helsinki Declaration. The study was approved by the Ethic Committee of the Medical Faculty of the University of Heidelberg (S654/2016). All participants have given written informed consent.
InstrumentsPTG was assessed at t1 with the 21-item PTG inventory (PTGI) of Tedeschi and Calhoun (1996) to examine if changes in participants' life domains occurred as a result of cancer. This questionnaire consists of the 5 subscales appreciation of life, new possibilities, personal strengths, relation to others and spiritual change which can be summed up to a total PTG score. Since the subscales comprise different numbers of items, the values were transformed into a range of 0–100 % for better comparability. Higher percentages indicate higher perceived change respectively.
At t0, fatigue was assessed multidimensionally with the EORTC QLQ-FA12 (Weis et al., 2017). All 12 items were answered on a 4-point Likert scale. We calculated a total sum score of the 12 items (Kecke et al., 2017) and transformed it to a scale of 0 to 100. Higher values indicate higher levels of fatigue.
Emotional distress was assessed at t0 with the short version of the Patient Health Questionnaire (PHQ-4) (Löwe et al., 2010), consisting of two items measuring symptoms of anxiety and two items measuring symptoms of depression. Each item was answered on a 4-point Likert scale and then added up to a total score. Higher values indicate higher emotional distress.
Pain at t0 was measured according to the symptom scores based on the EORTC QLQ-C30 (Fayers, 2001). Higher scores represent higher symptom burden.
In addition to the t0-measurements, participants were asked whether they felt well informed about fatigue. Answers ranged from 1 = “not at all informed” to 4 = “very well informed”. Participants could also state if they did not know how to answer the question.
Data/statistical analysisData analyses were performed using IBM SPSS statistics version 29. First, descriptive analyses for the 5 PTG subscales were performed. Then, statistical preconditions to perform regression analyses were checked. Cancer entity and cancer treatment (chemotherapy, radiotherapy, immune therapy, endocrine therapy, operation) were expected to be associated with PTG, but except chemotherapy they did neither show a significant effect on PTG nor influenced the other variables in the model significantly. Except chemotherapy, these factors were therefore not included in the final model for reasons of parsimony.
Before inclusion in the final model, fatigue, emotional distress and pain at t0 were log-transformed in order to achieve a better model fit. The model consisted of sociodemographic variables like age and sex, chemotherapy and psychosocial factors like emotional distress, fatigue and pain two years after cancer diagnosis to predict PTG four years after cancer diagnosis. In an additional step, we investigated the categorial variable "information about fatigue" (t0) as a potential predictor by adding it to the model. Further, it was tested whether the amount of information about fatigue would influence the fatigue-PTG association. These exploratory analyses were conducted to test potential relationship patterns of fatigue (t0), information about fatigue (t0), and PTG (t1) using the PROCESS macro for SPSS (Hayes, 2017). Missing data were not imputed as it has been found useful to use only complete cases for regression analysis if there are less than 5 % of cases with missing values (Urban et al., 2016). In our calculations of the regression analyses, the percentage of missing values was 3.3 % (43 of 1316).
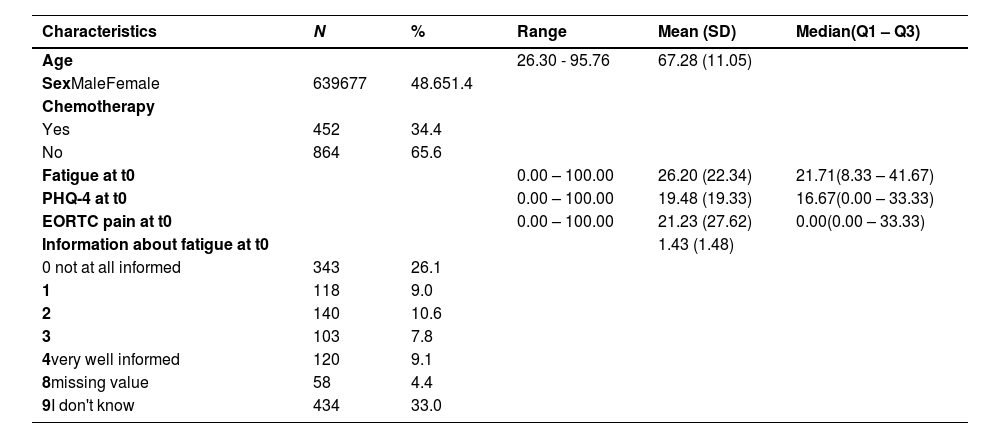
ResultsThe analyzed sample consisted of 1316 cancer survivors and was fairly balanced regarding sex (51.4 % female). The mean age at follow-up (t1) was 67.2 years (SD = 11.1). About one third (34.4 %) of the cancer free survivors had ever received chemotherapy. Further participant and variable characteristics are displayed in Table 1.
Patient and variable characteristics in the FiX-study.
Note. Q1-Q3 = Quartile 1 – Quartile 3; PHQ-4 = 4-item Patient Health Questionnaire; EORTC = European Organization for Research and Treatment of Cancer. t0= Baseline measure (two years after diagnosis).
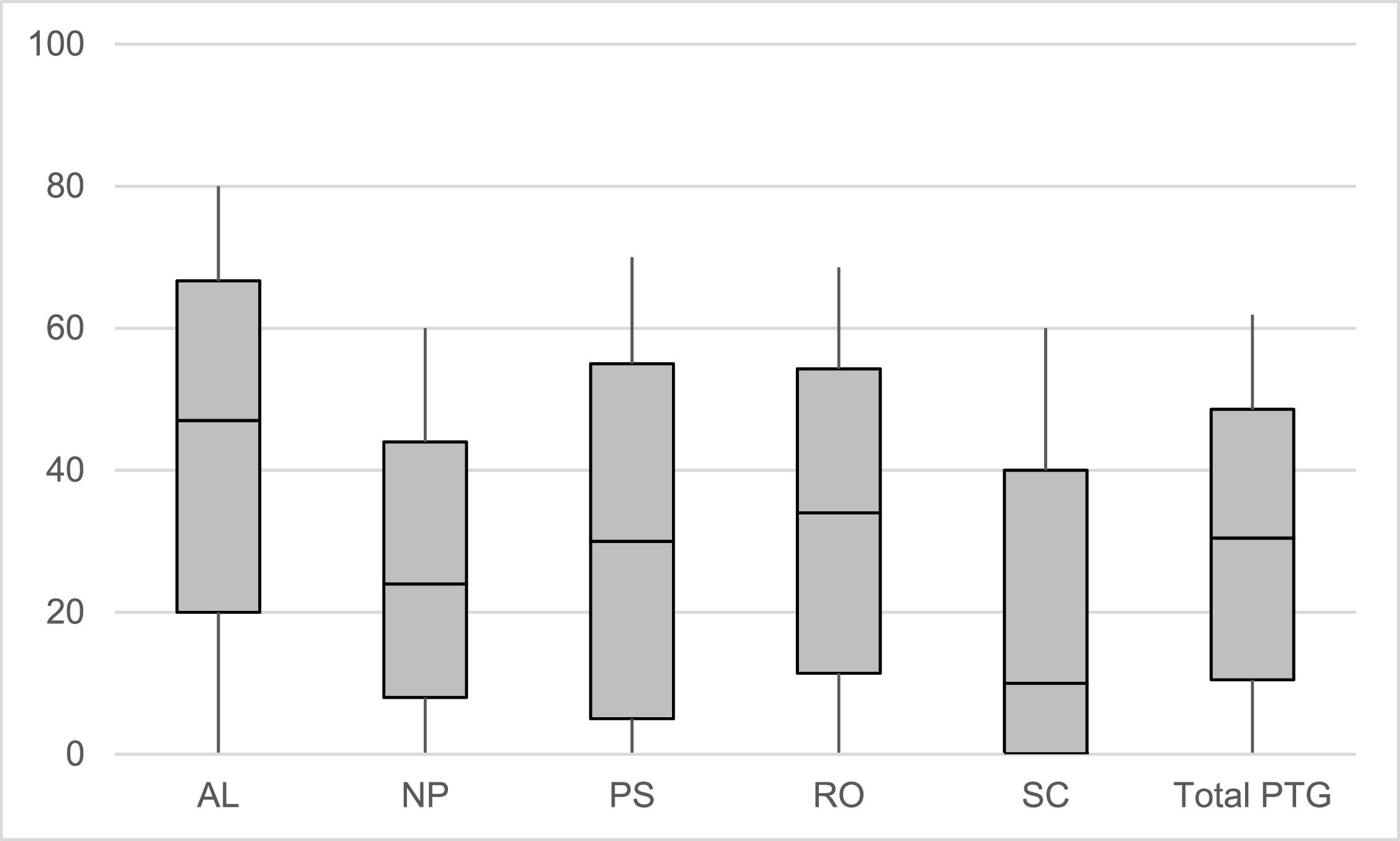
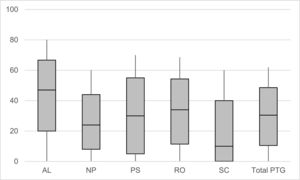
All PTG subscales showed good to very good internal consistencies (Cronbach's α = 0.85 to 0.92). Regarding the extent of PTG, most positive developments were experienced in appreciation of life (Median (Q1-Q3) = 47.0 % (20.0 %−66.7 %)), followed by an intensification of the relations to others (34.0 % (11.4 %−54.3 %)), personal strengths (30.0 % (5.0 %−55.0 %)) and new possibilities (24.0 % (8.0 %−44.0 %)). Least developments were reported in spiritual change (10.0 % (0.0 %−40.0 %)). The median of the total PTG score was 32.3 % (12.4 %−50.5 %). The non-parametric Friedman test indicated that the medians of all subgroups were significantly different from each other (overall χ2 = 1329.86, p < .001). Fig. 1 displays the extent and distribution of the prescribed PTG subdimensions. The characteristics of the original (not 0–100 % transformed) scales are presented in Supplement 1.
Extent of the five post-traumatic growth subdimensions and the total score.
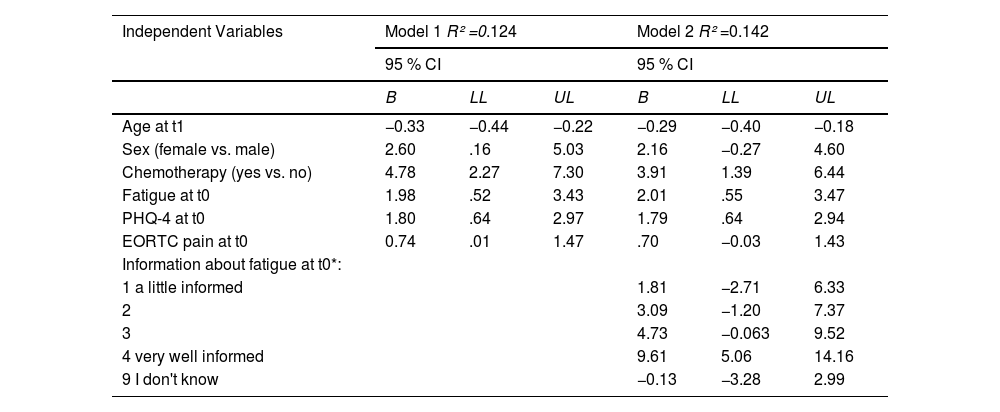
The results of the linear regression analyses are shown in Table 2. Model 1 revealed that age, sex and prior chemotherapy were significant predictors of total PTG, indicating that lower age (B = −0.33, 95% Confidence Interval (CI) [−0.44; −0.22]), female sex (B = 2.60, 95% CI [.16; 5.03]) and receiving chemotherapy (B = 4.78, 95% CI [2.27; 7.30]) were associated with higher PTG. Further, fatigue (B = 1.98, 95% CI [.52; 3.43]), emotional distress (B = 1.80, 95% CI [.64; 2.97]) and pain (B = 0.74, 95% CI [.01; 1.47]) were positive longitudinal determinants of PTG, suggesting that higher levels in these variables contributed to higher PTG. All variables together explained 12.4 % of variance in PTG.
Exploration of determinants of post-traumatic growth with hierarchical multiple regression analysis.
Note. Models included all listed variables simultaneously. B = unstandardized coefficients, CI = confidence interval, LL = lower limit, UL = upper limit, PHQ-4 = 4-item Patient Health Questionnaire. Fatigue, PHQ-4 and EORTC pain variables were log-transformed before analysis. t0 = two years after cancer diagnosis, t1 = four years after cancer diagnosis.
*reference category of "information about fatigue" was 0 = not at all informed.
In Model 2 we added the item information about fatigue (t0) in our analysis, which explained an additional significant albeit small amount of variance in PTG (increase in explained variance by 1.8 %). Survivors who felt poorly informed about their fatigue reported significantly less positive changes in PTG than patients who felt well informed (Table 2).
Results of the exploratory mediation analysis are depicted in Supplement 2. The coefficients revealed that information about fatigue may partially mediate the relationship between fatigue at t0 and PTG at t1. Moderation analysis did not show an effect of information about fatigue on the relationship between fatigue and PTG.
DiscussionWe found most positive developments of PTG in the domain appreciation of life whereas spiritual change was significantly the least pronounced domain. Previous literature reported consistently least changes in spiritual change, suggesting that this subdimension appears to be less important at least in Western countries (Holtmaat et al., 2017; Lelorain et al., 2010; Sharp et al., 2018; Thornton & Perez, 2006). Concerning the extent of PTG in the subdimensions, our sample showed mostly comparable mean scores to other studies. In direct comparison to the study of Lelorain et al. (2010), who also examined PTG in long-term disease-free survivors, our results revealed lower mean scores in PTG. However, it is noteworthy that PTG seems to be perceived even four years after cancer diagnosis.
Consistent with previous findings in the literature, we found lower age and female sex associated with higher levels of PTG (Boyle et al., 2017; Husson et al., 2011; Wu et al., 2019). It can be assumed that the disruption or trauma due to cancer diagnosis and its treatment affect life plans more in younger than in older individuals leading to higher PTG levels (Blank & Bellizzi, 2008; Manne et al., 2004). Females report more PTG than males, but reasons for that remain still speculative. However, different response patterns in patient-reported outcomes between females and males have been observed previously, indicating that women often report higher symptom burden (Hertler et al., 2020).
Except for chemotherapy, no other type of therapy had an effect on the extent of PTG. This could lead to the hypothesis that chemotherapy might have an additional distressing influence compared to not undergoing chemotherapy. Surprisingly, cancer entity did not show a significant effect on PTG either, indicating that PTG may occur regardless of cancer entity.
In line with our hypothesis, fatigue, emotional distress and pain seem to be relevant predictors of long-term PTG. This finding supports the assumptions of the PTG model by Tedeschi and Calhoun (1996) that distress is an important precondition to develop PTG. As we found the best model fit with log-transformed predictors, this could be interpreted as that their association with PTG is not linear. It seems that beyond certain points, higher symptom burden may no longer lead to a further increase in PTG. With regard to the existing inconclusive findings of the association of PTG and emotional distress, our results tend to support a positive, but non-linear relationship of these constructs. Consistent with that, Eisma et al. (2019) found high levels of distress limited the amount of experienced PTG in bereaved adults. Another study concluded that moderate levels of general stress are associated with highest PTG compared to low or high stress in cancer patients (Coroiu et al., 2016).
Our study is one of the first that investigated fatigue and pain as predictors of PTG in cancer survivors, showing significant log-linear associations. As fatigue is one of the most frequent sequelae of cancer (Fabi et al., 2020), future studies could especially aim for a better understanding of the mechanisms of fatigue leading to PTG. Given the relatively wide CIs of the estimates, it must be noted that our data does not yield sufficient precision for a clear prediction of PTG from the determinants considered. However, it provides insights into factors associated with PTG that may warrant further investigation.
Information about fatigue explained a small but significant additional amount of variance in the model. It might be argued that higher level of information about this frequent cancer side effect is important to help individuals make sense of their experiences. In particular, facts about the existence, prevalence and type of fatigue may be important initial information. Further information should include possible treatment options and advice on managing fatigue and could be provided through patient education programs (Du et al., 2015; Schmidt et al., 2022). It could also lead to a more rational cognitive processing about fatigue, reducing feelings of helplessness through facilitated self-empowerment and contributing to higher PTG (Brown et al., 2016; Schmidt et al., 2022). This may underline the importance that providing adequate information about cancer and its side effects is crucial for patients and their coping with the disease (Brown et al., 2016; Husson et al., 2011). Aiming for a better understanding of the role of information about fatigue in the process of fatigue at t0 and PTG at t1, we found a potential partially mediating effect of information about fatigue. Due to the exploratory analysis, we cannot point to any causal interpretations here. Nevertheless, our results seem to support the theory of Tedeschi and Calhoun (2004): after experiencing the traumatic cancer event and its potential side effects, higher levels of information about fatigue could influence cognitive processing positively and thus mediate the impact of fatigue on PTG. Further research should clarify the role of patient informedness in the development of PTG.
Limitations of our study have to be mentioned. First, PTG contains self-reported data that was collected retrospectively. It is therefore possible that positive recollection bias might have distorted the response behavior. Second, the explained variance of our models is relatively small. This is an indication that further important factors contributing to PTG have not been taken into account. Studies showed that e.g. social support and positive appraisal are important factors in the development of PTG (Lelorain et al., 2012; Shand et al., 2015). Third, PTG was only assessed once, which does not allow a longitudinal development of PTG to be illustrated. Further, several individuals did not answer the question about fatigue informedness at all, thus these results have to be interpreted with care.
Nevertheless, our longitudinal study covering 15 different cancer entities allows for a broader picture of the extent of PTG and its longitudinal determinants in cancer survivors. In sum, PTG can be perceived even years after a traumatic cancer event and is associated with important cancer side effects like fatigue, emotional distress and pain. Our results further indicated that individuals’ informedness also may play a role in the development of PTG, pointing to a modifiable factor during cancer treatment (Evans et al., 2022). This strengthens the existing recommendation to provide adequate education about manifestation and management of cancer side effects in clinical practice. Further research is warranted to explore the role of general cancer literacy in the development of PTG.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors thank all participants for completing the survey, the Cancer Registry of Baden-Württemberg for supporting the recruitment, Sabine Holzmeier for data management and support of study conduct, Paul Reinke and Hannah Klassen for study support.