The exact causal mechanisms of depression remain unclear due to the complexity of the triggers, which has led to limitations in treating depression using modern drugs. High-intensity interval training (HIIT) is as effective as medication in treating depression without toxic side effects. Typically, HIIT requires less time commitment (i.e., shorter exercise duration) and exhibits pronounced benefits on depressive symptoms than other forms of physical exercise. This review summarizes the risk reduction and clinical effects of HIIT for depression and discusses the underlying mechanisms, providing a theoretical basis for utilizing HIIT in treating depression.
MethodsA database search was conducted in PubMed, Embase, Web of Science, and Scopus from inception up to October 2022. The methodological quality of the included literature was evaluated by the physiotherapy evidence database (PEDro) scale criteria. The review focused on evaluating the changes in depression risk or symptoms of HIIT interventions in healthy individuals, patients with depression, and patients with other disorders co-morbid with depression. Consequently, the mechanisms associated with depression related HIIT were summarized.
ResultsA total of 586 participants (52 % female; mean age: 43.58±8.93 years) from 22 studies were included. Implementing HIIT using different exercise types alleviates depressive symptoms in individuals with depression and in individuals with depression who have exhibited comorbidities and reduced depression scale scores in subjects immediately after acute exercise. In addition, the long-interval HIIT and short-interval HIIT in the treatment of patients with cardiovascular or psychiatric disorders may reduce depressive symptoms via complex exercise-related changes on several levels, including by effecting the following measures: releasing monoamines, reducing neuronal death, inducing neurogenesis, modulating the functional homeostasis of the HPA axis, and enhancing the level of inflammation in the body.
ConclusionHIIT is a relatively safe and effective antidepressant, which may involve multiple neurobiological mechanisms (release of monoamines, reducing neuronal death, inducing neurogenesis, modulating the functional homeostasis of the HPA axis, and enhancing the level of inflammation in the body), thereby reducing the risk or symptoms of depression in participants.
AbbreviationsHIIT high-intensity interval training long-interval high-intensity interval training short-interval high-intensity interval training World Health Organization selective serotonin reuptake inhibitors serotonin-norepinephrine reuptake inhibitors coronavirus disease 2019 rating of perceived exertion repeated sprint training sprint interval training randomized controlled trials physiotherapy evidence database 5-hydroxytryptamine norepinephrine dopamine monoamine oxidase inhibitors brain-derived neurotrophic factor hypothalamic-pituitary-adrenal corticotropin-releasing hormone adrenocorticotropic hormone alpha-methyl-para-tyrosine glucocorticoid receptor kynurenine low-density lipoprotein high-density lipoprotein interleukin tumor necrosis factor peroxisome proliferator-activated receptor-gamma coactivator-1alpha fibronectin type III domain-containing protein 5 regulatory T cells interleukin-1 receptor antagonist C–C motif chemokine ligand 2 C–X–C motif ligand 15 heart rate hypoxia inducible factor-1
Depression is a potentially fatal disorder that severely limits psychosocial functioning and is the leading cause of disability worldwide (Murray & Lopez, 1996; Sanacora et al., 2008). According to World Health Organization (WHO) report, depression is the second most disabling disorder globally and is expected to be the leading one by 2030 (Malhi & Mann, 2018). Depression is characterized by symptoms such as depressed mood, anhedonia, fatigue, irritability, inattention, sleep disruption, decreased appetite and cognition, and suicidal tendencies, which significantly reduce patients' quality of life and impose a significant burden on their families and society (Yang et al., 2015). Epidemiological studies have found that the onset of depression is characterized by unpredictability, a high recurrence rate, and comorbidity with multiple diseases (Krishnan & Nestler, 2008; Malhi & Mann, 2018; Penninx et al., 2011). The duration of depressive episodes, the number of episodes in a lifetime, and the pattern of onset are uncertain. In addition, nearly 80 % of patients experience at least one illness relapse after treatment. Furthermore, depression complicates the treatment and prognosis of many chronic diseases. These factors underscore the need to identify effective treatment strategies for depression.
Significant progress has been made in understanding the pathophysiology of depression. However, the exact causal mechanisms of the disorder remain unclear due to the complexity of its predisposing factors (e.g., environmental factors, genetic factors, and stress). Several hypotheses have been proposed for its pathogenesis, such as the monoamine hypothesis (Segal et al., 1974), neuroplasticity and neurogenesis hypothesis (Duman & Monteggia, 2006), neuroendocrine hypothesis (Goodyer et al., 2000; Harris et al., 2000; Knorr et al., 2010), and depression-inflammation hypothesis (Maes et al., 2012). Based on these hypotheses, some drugs have been developed for the treatment of depression, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). However, clinical studies have indicated that SSRIs have been effective in only 30 % of patients (Haenisch & Bönisch, 2011). These antidepressants also lead to significant side effects such as nausea, headache, and sexual dysfunction (Moret et al., 2009). Notably, multiple drugs are utilized concurrently in the treatment of depression. This treatment strategy not only increases the burden on the liver but also causes depression-like and anxiety-like symptoms in 80 % of patients (Haenisch & Bönisch, 2011).
In addition to medication, the current treatment for depression includes psychological interventions and physical therapy. Although psychological interventions can alleviate symptoms in patients with mild to moderate depression, they are less effective in treating patients with major depression (Weitz et al., 2015). However, studies have proposed that combining psychological intervention and pharmacotherapy is more effective in treating patients' symptoms (Cuijpers et al., 2014). Psychological intervention is considerably disadvantageous owing to its application by less-experienced therapists and its relatively higher costs (Guaiana et al., 2022; Lopes et al., 2020). The effect of this treatment is comparable to that of medication (Blumenthal et al., 2012). Moreover, it not only exerts a positive therapeutic effect, but also exhibits safer, healthier, and less costly characteristics than other methods (the toxic side effects of medication utilized in the treat poor students) (Kandola et al., 2019). Although the effectiveness of physical exercise training in treating depression has been proven (Blumenthal et al., 2012), patients receiving this treatment are often abandoned owing to the long duration of a single exercise session and the need for related exercise equipment and facilities. However, high-intensity interval training, as one of the top health and fitness trends worldwide, is currently gaining popularity among various populations due to characteristics such as setting an exercise duration with the same or more significant physical exercise-related improvements in weight control and reducing the total and visceral fat percentage than conventional aerobic training (Kercher et al., 2023).
Since the outbreak of the coronavirus (COVID-19) in 2019, the overload of healthcare resources and related prevention policies have impacted hospital visits in depressed patients. In addition, the tremendous mental stress and related home isolation policies have considerably modified individuals’ lifestyles, increasing their risk of undergoing depression, especially among healthcare workers (Pappa et al., 2020). Furthermore, more than 30 % of patients with COVID-19 also exhibit depressive disorders after treatment (Benedetti et al., 2021; Bo et al., 2021). In summary, HIIT may be a strategy for the prevention and treatment of depression. However, in most of the systematic reviews and meta-analyses, the studies mainly focused on the enhancement of aerobic exercise on subjects' depressive symptoms, and relatively few studies have been conducted to address whether HIIT supports the alleviation or treatment of depression risk or symptoms; however, the population characteristics of subjects were not categorized in these reports (Bridle et al., 2018; De Sousa et al., 2021; Gill et al., 2020; Heissel et al., 2023; A. Marques et al., 2020; Wang et al., 2022; Wegner et al., 2020; Wegner et al., 2014). Notably, these studies are more inclined to explore whether exercise is effective for the treatment of depression, whereas relatively few studies have explored the biological mechanisms associated with exercise to reduce depression. Therefore, this study summarizes the current state of evidence regarding the effects of HIIT on depression and further categorizes the subject populations according to their characteristics while rating, the intensity of the exercises included in the studies according to Garber reports; thus, it more optimally evaluates the effectiveness of HIIT in alleviating or treating the depression risk or symptoms (Garber et al., 2011). In addition, this study provides an overview of neurobiological mechanisms that may drive the aforementioned effects.
Materials and methodsRegistrationThis study protocol has been registered with PROSPERO under the registry number CRD42023465813.
Definition of high-intensity interval trainingHigh-intensity interval training is a form of exercise that involves repetitive high intensity activity or sprints with short rest or recovery intervals (Coates et al., 2023; Sharp et al., 2022). Although the physical exercise characteristics of HIIT are currently defined, the intensity of the exercise should be clarified. There is currently a large body of literature supporting the following notion: exercise intensities of 85 %–95 % of the maximum heart rate (peak HR) or 15–17 on the Borg rating of the perceived exertion (RPE) scale are most appropriate (Dun et al., 2019).
Data sources and retrieval strategiesTwo reviewers (Y.X.X and Y.J.L) independently retrieved articles on the HIIT-based treatment of depression from PubMed, Embase, Web of Science, and Scopus. The study included related papers that were published from the establishment of the database to October 2022. Search terms included depression, major depressive disorder, depressive disorder, high-intensity interval training, and high-intensity interval exercise. Literature was searched using a Boolean logic search method without special filters, with specific search formulas described in the Supplementary Material.
Eligibility criteriaThe inclusion criteria for this systematic review adhered to the PICOS principles: (1) Population: Whether symptoms of depression were associated with study participants described as exhibiting mental illness; (2) Intervention: Whether the study utilized HIIT for the intervention and whether the specific intervention protocol was described. In addition, acute and chronic physical exercise studies were included. Evaluating acute and chronic physical exercise can enable researchers to more optimally understand the efficacy and durability of HIIT for depression. Expressly, acute physical exercise may reflect the short-term effects of HIIT on depression, and chronic physical exercise may reflect the durability of HIIT on depressive symptoms when performed over time (Wegner et al., 2014); (3) Control: Without any intervention (only for daily living). (4) Outcomes: Referring to Stillman et al.'s study, we assessed the effect of HIIT on depression using two levels of evidence. The first level is the cellular and molecular level, which discusses the mechanisms by which HIIT improves depression through the collection of human serum indicators (neurotrophic factors, cytokines), whereas the second level is the Behavioral and Socioemotional Mechanisms (which contains, for example, mood, motivation), which mainly contains Various scales (Stillman et al., 2016). (5) Study design: Randomized controlled trials (RCTs).
Study selection and data collectionAfter eliminating duplicated data using EndnoteX9, two reviewers (Y.X.X and Y.J.L) filtered the references by reading titles and abstracts. They, subsequently, read the full text of all the articles to select those that met the inclusion criteria, and disagreements that arose from this process were settled through group discussions or by a more experienced reviewer. All excluded studies and related reasons were recorded.
The following reference data were extracted from each study by two reviewers (C.Q.W and T.T.H): the first author, year of publication, participants, depression scale, sample size, age, interventions, intervention time, and outcome measures. Subsequently, the collected data were put into Excel sheets to be assessed by two reviewers. Disagreements were resolved through discussion or by Y.W (the more experienced reviewer).
Quality assessmentThe methodological quality of the included literature was evaluated by two reviewers using the physiotherapy evidence database (PEDro) scale criteria (Le et al., 2014). The scale exhibits a total of 11 items and a total score of 10, with higher scores representing higher-quality literature. A high methodological quality was indicated if the PEDro score of the included studies was ≥6.
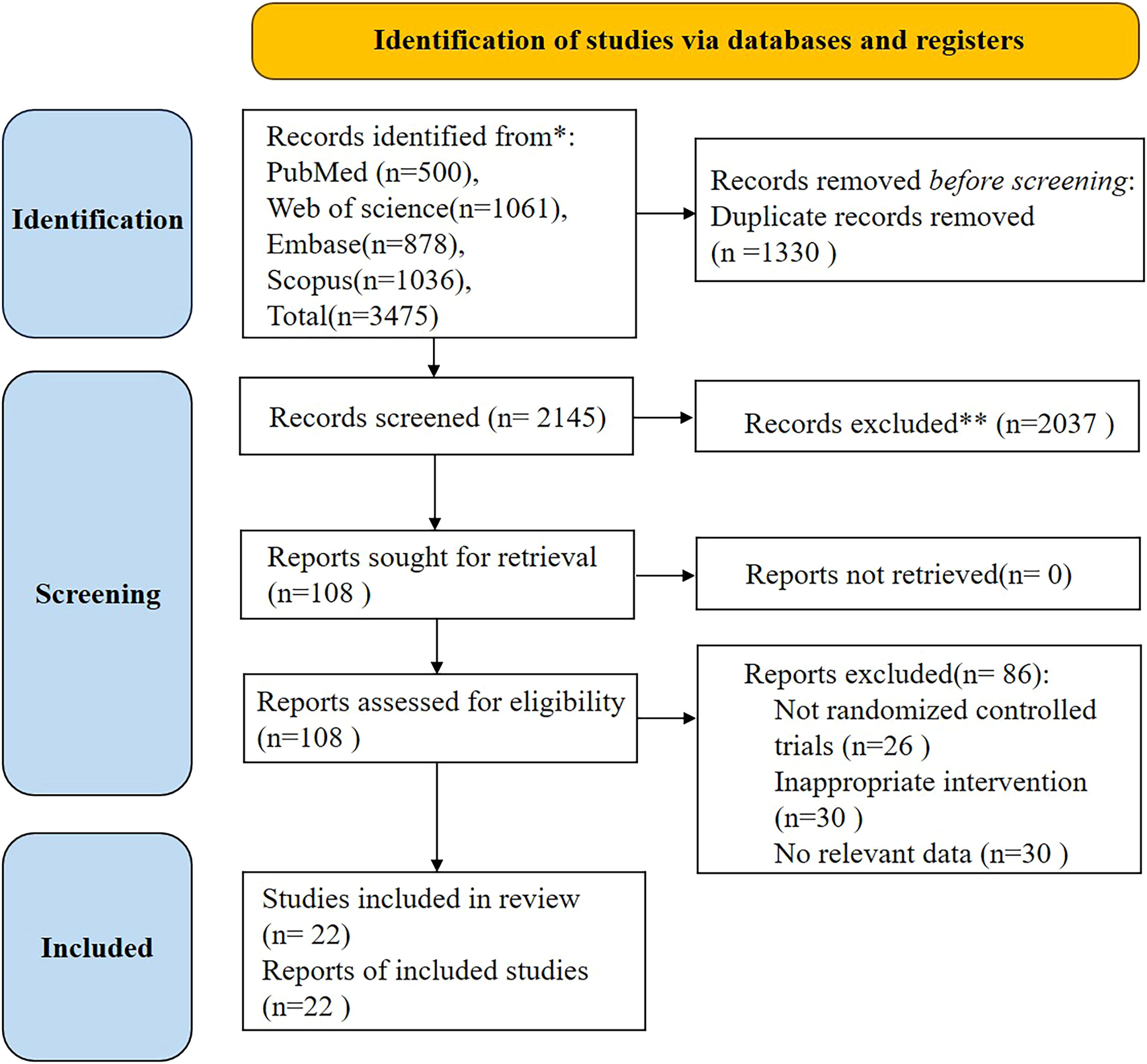
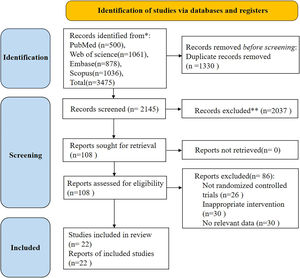
ResultsSearch results and characteristics of included studiesA total of 3475 article were obtained after searching through the database, and 2145 article remained after deduplication. The identified studies were reviewed at the abstract level, and 108 article were screened. A total of 22 texts were included after reading the full texts, including 8 in healthy individuals and 14 in other diseases complicated by depression (see Fig. 1). Of the 22 texts included, the total number of initial participants in the HIIT intervention was 586 (52 % female; mean age: 43.58 ± 8.93 years), and the number of participants who eventually completed the HIIT intervention was 523, with a 10.8 % dropout rate (see Table 1 and Table 2). Currently, HIIT characteristics have been more clearly defined; however, they can be further divided into four types based on the intensity and duration of the work interval intensity and duration, relief interval intensity and duration, exercise mode, number of repetitions, number of series, and recovery duration and intensity between series: (1) long-interval HIIT (HIITL) (intensity between 90 % and 100 % of maximal oxygen consumption [VO2max] with more than 60 seconds of effort); (2) short-interval HIIT (HIITS) (intensity between 100 % % and 120% VO2max with less than one minute of effort); (3) repeated sprint training (RST) (sprints lasting 3 to 7 s interspersed with a recovery period of generally less than 60 seconds); and (4) sprint interval training (SIT) (30 seconds of all-out effort interspersed with 120-240 seconds of passive recovery) (Buchheit & Laursen, 2013). The basic characteristics of the 22 included literature are illustrated in Table 1 and Table 2.
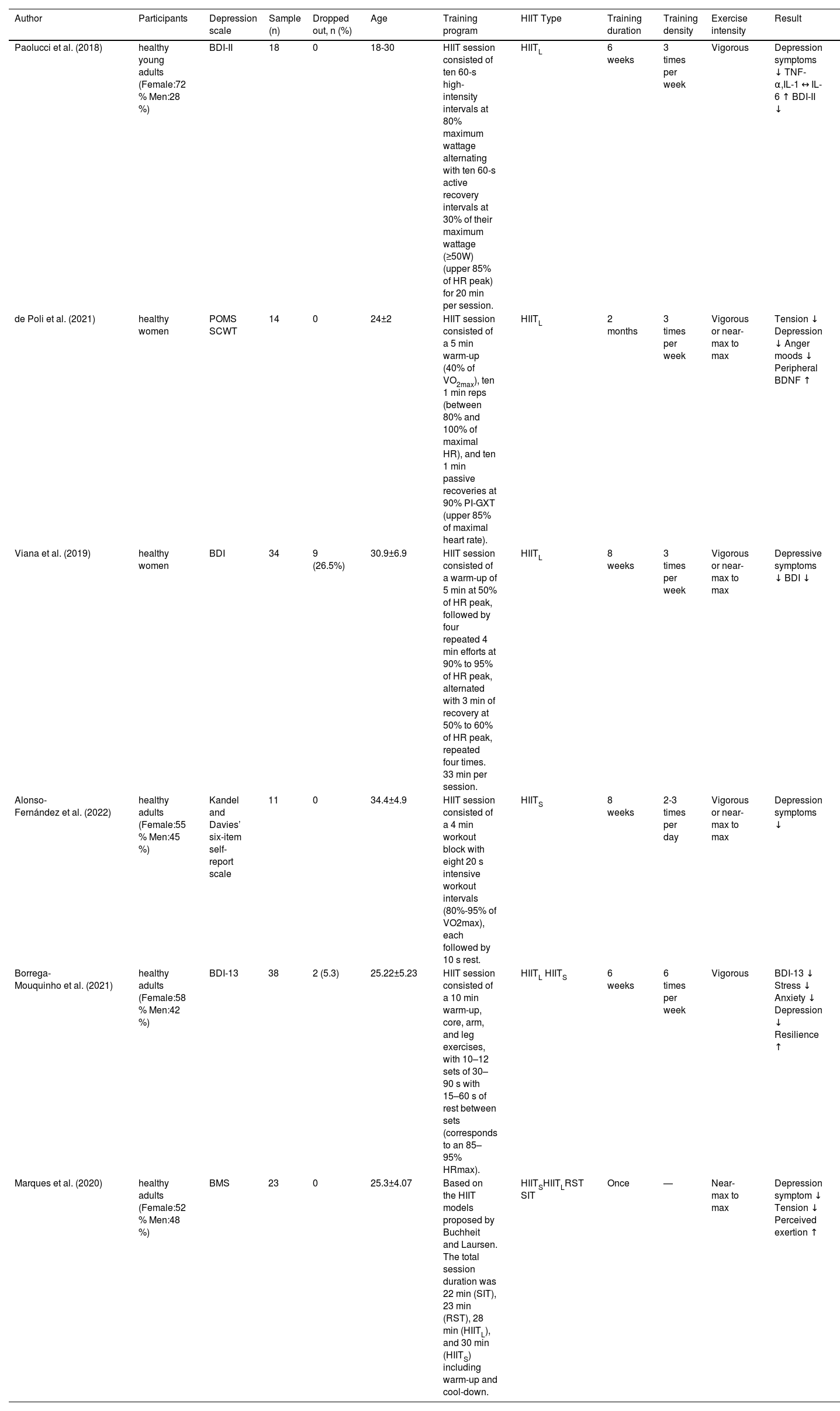
Exercise prescription and results of HIIT in healthy populations.
| Author | Participants | Depression scale | Sample (n) | Dropped out, n (%) | Age | Training program | HIIT Type | Training duration | Training density | Exercise intensity | Result | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paolucci et al. (2018) | healthy young adults (Female:72 % Men:28 %) | BDI-II | 18 | 0 | 18-30 | HIIT session consisted of ten 60-s high-intensity intervals at 80% maximum wattage alternating with ten 60-s active recovery intervals at 30% of their maximum wattage (≥50W) (upper 85% of HR peak) for 20 min per session. | HIITL | 6 weeks | 3 times per week | Vigorous | Depression symptoms ↓ TNF-α,IL-1 ↔ IL-6 ↑ BDI-II ↓ | |
| de Poli et al. (2021) | healthy women | POMS SCWT | 14 | 0 | 24±2 | HIIT session consisted of a 5 min warm-up (40% of VO2max), ten 1 min reps (between 80% and 100% of maximal HR), and ten 1 min passive recoveries at 90% PI-GXT (upper 85% of maximal heart rate). | HIITL | 2 months | 3 times per week | Vigorous or near-max to max | Tension ↓ Depression ↓ Anger moods ↓ Peripheral BDNF ↑ | |
| Viana et al. (2019) | healthy women | BDI | 34 | 9 (26.5%) | 30.9±6.9 | HIIT session consisted of a warm-up of 5 min at 50% of HR peak, followed by four repeated 4 min efforts at 90% to 95% of HR peak, alternated with 3 min of recovery at 50% to 60% of HR peak, repeated four times. 33 min per session. | HIITL | 8 weeks | 3 times per week | Vigorous or near-max to max | Depressive symptoms ↓ BDI ↓ | |
| Alonso-Fernández et al. (2022) | healthy adults (Female:55 % Men:45 %) | Kandel and Davies’ six-item self-report scale | 11 | 0 | 34.4±4.9 | HIIT session consisted of a 4 min workout block with eight 20 s intensive workout intervals (80%-95% of VO2max), each followed by 10 s rest. | HIITS | 8 weeks | 2-3 times per day | Vigorous or near-max to max | Depression symptoms ↓ | |
| Borrega-Mouquinho et al. (2021) | healthy adults (Female:58 % Men:42 %) | BDI-13 | 38 | 2 (5.3) | 25.22±5.23 | HIIT session consisted of a 10 min warm-up, core, arm, and leg exercises, with 10–12 sets of 30–90 s with 15–60 s of rest between sets (corresponds to an 85–95% HRmax). | HIITL HIITS | 6 weeks | 6 times per week | Vigorous | BDI-13 ↓ Stress ↓ Anxiety ↓ Depression ↓ Resilience ↑ | |
| Marques et al. (2020) | healthy adults (Female:52 % Men:48 %) | BMS | 23 | 0 | 25.3±4.07 | Based on the HIIT models proposed by Buchheit and Laursen. The total session duration was 22 min (SIT), 23 min (RST), 28 min (HIITL), and 30 min (HIITS) including warm-up and cool-down. | HIITSHIITLRST SIT | Once | — | Near-max to max | Depression symptom ↓ Tension ↓ Perceived exertion ↑ | |
BDI Beck Depression Inventory, BDI-II Beck Depression Inventory II, BDI-13 13-item Beck Depression Inventory, BMS Brunel Mood Scale, SCWT Stroop color word test, POMS profile of mood states questionnaire, CES-D Center for Epidemiologic Studies Depression Scale
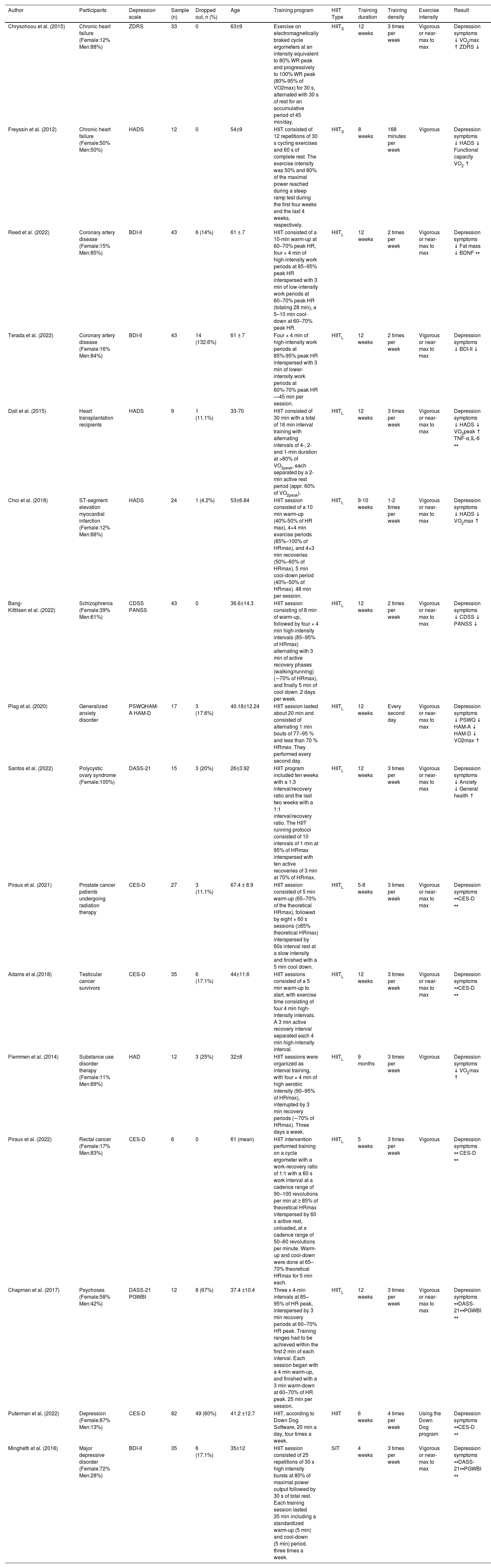
HIIT exercise prescriptions and outcomes in patients with depression and other disorders co-morbid with depression.
| Author | Participants | Depression scale | Sample (n) | Dropped out, n (%) | Age | Training program | HIIT Type | Training duration | Training density | Exercise intensity | Result | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chrysohoou et al. (2015) | Chronic heart failure (Female:12% Men:88%) | ZDRS | 33 | 0 | 63±9 | Exercise on electromagnetically braked cycle ergometers at an intensity equivalent to 80% WR peak and progressively to 100% WR peak (80%-95% of VO2max) for 30 s, alternated with 30 s of rest for an accumulative period of 45 min/day. | HIITS | 12 weeks | 3 times per week | Vigorous or near-max to max | Depression symptoms ↓ VO2max ↑ ZDRS ↓ | |
| Freyssin et al. (2012) | Chronic heart failure (Female:50% Men:50%) | HADS | 12 | 0 | 54±9 | HIIT consisted of 12 repetitions of 30 s cycling exercises and 60 s of complete rest. The exercise intensity was 50% and 80% of the maximal power reached during a steep ramp test during the first four weeks and the last 4 weeks, respectively. | HIITS | 8 weeks | 168 minutes per week | Vigorous | Depression symptoms ↓ HADS ↓ Functional capacity VO2 ↑ | |
| Reed et al. (2022) | Coronary artery disease (Female:15% Men:85%) | BDI-II | 43 | 6 (14%) | 61 ± 7 | HIIT consisted of a 10-min warm-up at 60–70% peak HR, four × 4 min of high-intensity work periods at 85–95% peak HR interspersed with 3 min of low-intensity work periods at 60–70% peak HR (totaling 28 min), a 5–10 min cool-down at 60–70% peak HR. | HIITL | 12 weeks | 2 times per week | Vigorous or near-max to max | Depression symptoms ↓ Fat mass ↓ BDNF ↔ | |
| Terada et al. (2022) | Coronary artery disease (Female:16% Men:84%) | BDI-II | 43 | 14 (132.6%) | 61 ± 7 | Four × 4 min of high-intensity work periods at 85%-95% peak HR interspersed with 3 min of lower-intensity work periods at 60%-70% peak HR—45 min per session. | HIITL | 12 weeks | 2 times per week | Vigorous or near-max to max | Depression symptoms ↓ BDI-II ↓ | |
| Dall et al. (2015) | Heart transplantation recipients | HADS | 9 | 1 (11.1%) | 33-70 | HIIT consisted of 30 min with a total of 16 min interval training with alternating intervals of 4-, 2- and 1-min duration at >80% of VO2peak, each separated by a 2-min active rest period (appr. 60% of VO2peak). | HIITL | 12 weeks | 3 times per week | Vigorous or near-max to max | Depression symptoms ↓ HADS ↓ VO2peak ↑ TNF-α,IL-6 ↔ | |
| Choi et al. (2018) | ST-segment elevation myocardial infarction (Female:12% Men:88%) | HADS | 24 | 1 (4.2%) | 53±6.84 | HIIT session consisted of a 10 min warm-up (40%-50% of HR max), 4×4 min exercise periods (85%–100% of HRmax), and 4×3 min recoveries (50%–60% of HRmax), 5 min cool-down period (40%–50% of HRmax). 48 min per session. | HIITL | 9-10 weeks | 1-2 times per week | Vigorous or near-max to max | Depression symptoms ↓ HADS ↓ VO2max ↑ | |
| Bang-Kittilsen et al. (2022) | Schizophrenia (Female:39% Men:61%) | CDSS PANSS | 43 | 0 | 36.6±14.3 | HIIT session consisting of 8 min of warm-up, followed by four × 4 min high-intensity intervals (85–95% of HRmax) alternating with 3 min of active recovery phases (walking/running) (∼70% of HRmax), and finally 5 min of cool down. 2 days per week. | HIITL | 12 weeks | 2 times per week | Vigorous or near-max to max | Depression symptoms ↓ CDSS ↓ PANSS ↓ | |
| Plag et al. (2020) | Generalized anxiety disorder | PSWQHAM-A HAM-D | 17 | 3 (17.6%) | 40.18±12.24 | HIIT session lasted about 20 min and consisted of alternating 1 min bouts of 77–95 % and less than 70 % HRmax. They performed every second day. | HIITL | 12 weeks | Every second day | Vigorous or near-max to max | Depression symptoms ↓ PSWQ ↓ HAM-A ↓ HAM-D ↓ VO2max ↑ | |
| Santos et al. (2022) | Polycystic ovary syndrome (Female:100%) | DASS-21 | 15 | 3 (20%) | 26±3.92 | HIIT program included ten weeks with a 1:3 interval/recovery ratio and the last two weeks with a 1:1 interval/recovery ratio. The HIIT running protocol consisted of 10 intervals of 1 min at 95% of HRmax interspersed with ten active recoveries of 3 min at 70% of HRmax. | HIITL | 12 weeks | 3 times per week | Vigorous or near-max to max | Depression symptoms ↓ Anxiety ↓ General health ↑ | |
| Piraux et al. (2021) | Prostate cancer patients undergoing radiation therapy | CES-D | 27 | 3 (11.1%) | 67.4 ± 8.9 | HIIT session consisted of 5 min warm-up (65–70% of the theoretical HRmax), followed by eight × 60 s sessions (≥85% theoretical HRmax) interspersed by 60s interval rest at a slow intensity and finished with a 5 min cool down. | HIITL | 5-8 weeks | 3 times per week | Vigorous or near-max to max | Depression symptoms ↔CES-D ↔ | |
| Adams et al.(2018) | Testicular cancer survivors | CES-D | 35 | 6 (17.1%) | 44±11.6 | HIIT sessions consisted of a 5 min warm-up to start, with exercise time consisting of four 4 min high-intensity intervals. A 3 min active recovery interval separated each 4 min high-intensity interval. | HIITL | 12 weeks | 3 times per week | Vigorous or near-max to max | Depression symptoms ↔CES-D ↔ | |
| Flemmen et al. (2014) | Substance use disorder therapy (Female:11% Men:89%) | HAD | 12 | 3 (25%) | 32±8 | HIIT sessions were organized as interval training, with four × 4 min of high aerobic intensity (90–95% of HRmax), interrupted by 3 min recovery periods (∼70% of HRmax). Three days a week. | HIITL | 9 months | 3 times per week | Vigorous | Depression symptoms ↓ VO2max ↑ | |
| Piraux et al. (2022) | Rectal cancer (Female:17% Men:83%) | CES-D | 6 | 0 | 61 (mean) | HIIT intervention performed training on a cycle ergometer with a work-recovery ratio of 1:1 with a 60 s work interval at a cadence range of 90–100 revolutions per min at ≥ 85% of theoretical HRmax interspersed by 60 s active rest, unloaded, at a cadence range of 50–60 revolutions per minute. Warm-up and cool-down were done at 65–70% theoretical HRmax for 5 min each. | HIITL | 5 weeks | 3 times per week | Vigorous | Depression symptoms ↔ CES-D ↔ | |
| Chapman et al. (2017) | Psychoses (Female:58% Men:42%) | DASS-21 PGWBI | 12 | 8 (67%) | 37.4 ±10.4 | Three x 4-min intervals at 85–95% of HR peak, interspersed by 3 min recovery periods at 60–70% HR peak. Training ranges had to be achieved within the first 2 min of each interval. Each session began with a 4 min warm-up, and finished with a 3 min warm-down at 60–70% of HR peak. 25 min per session. | HIITL | 12 weeks | 3 times per week | Vigorous or near-max to max | Depression symptoms ↔DASS-21↔PGWBI ↔ | |
| Puterman et al. (2022) | Depression (Female:87% Men:13%) | CES-D | 82 | 49 (60%) | 41.2 ±12.7 | HIIT, according to Down Dog Software, 20 min a day, four times a week. | HIIT | 6 weeks | 4 times per week | Using the Down Dog program | Depression symptoms ↔CES-D ↔ | |
| Minghetti et al. (2018) | Major depressive disorder (Female:72% Men:28%) | BDI-II | 35 | 6 (17.1%) | 35±12 | HIIT session consisted of 25 repetitions of 30 s high intensity bursts at 80% of maximal power output followed by 30 s of total rest. Each training session lasted 35 min including a standardized warm-up (5 min) and cool-down (5 min) period. three times a week. | SIT | 4 weeks | 3 times per week | Vigorous or near-max to max | Depression symptoms ↔DASS-21↔PGWBI ↔ | |
BDI-II Beck Depression Inventory II, CDSS Calgary Depression Scale for Schizophrenia, CES-D Center for Epidemiologic Studies Depression Scale, DASS-21 Depression, Anxiety, and Stress Scale, HADS hospital anxiety and depression scale, HAD anxiety and depression, HAM-A/HAM-D Hamilton Inventories for Anxiety and Depression, ZDRS Zung's Depression Rating Scale, PANAS Positive and Negative Affect Schedule, PGWBI Psychological General Wellbeing Index. ↔ represents no statistical significance
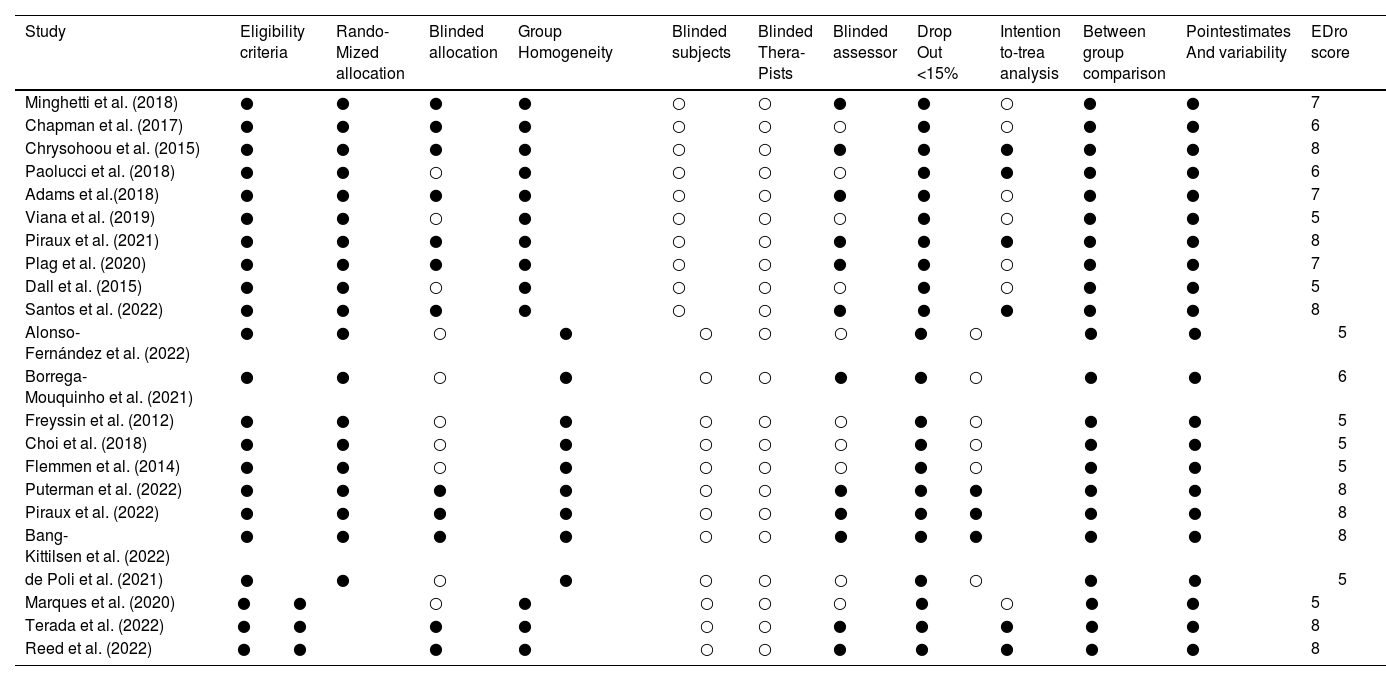
The methodological quality assessment of the included studies is depicted in Table 3. Scores of the included studies ranged from 5 to 8, with 14 documents exhibiting a physiotherapy evidence database (PEDro) score of 6 or more. All studies described inclusion criteria and underwent random assignment, and 12 studies underwent allocation concealment; all studies were comparable at baseline; all studies were not blinded to patients and therapists, and 12 studies were blinded to assessors; all studies exhibited a <15 % % clinical sample dropout rate; 9 studies utilized intentional analysis; and all included literature underwent between-group, point-measure, and difference-in-value statistics.
Physiotherapy evidence database (PEDro) score of the included studies
| Study | Eligibility criteria | Rando-Mized allocation | Blinded allocation | Group Homogeneity | Blinded subjects | Blinded Thera-Pists | Blinded assessor | Drop Out <15% | Intention to-trea analysis | Between group comparison | Pointestimates And variability | EDro score | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minghetti et al. (2018) | ● | ● | ● | ● | ○ | ○ | ● | ● | ○ | ● | ● | 7 | ||||||||||||||
| Chapman et al. (2017) | ● | ● | ● | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 6 | ||||||||||||||
| Chrysohoou et al. (2015) | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | 8 | ||||||||||||||
| Paolucci et al. (2018) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ● | ● | ● | 6 | ||||||||||||||
| Adams et al.(2018) | ● | ● | ● | ● | ○ | ○ | ● | ● | ○ | ● | ● | 7 | ||||||||||||||
| Viana et al. (2019) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 5 | ||||||||||||||
| Piraux et al. (2021) | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | 8 | ||||||||||||||
| Plag et al. (2020) | ● | ● | ● | ● | ○ | ○ | ● | ● | ○ | ● | ● | 7 | ||||||||||||||
| Dall et al. (2015) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 5 | ||||||||||||||
| Santos et al. (2022) | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | 8 | ||||||||||||||
| Alonso-Fernández et al. (2022) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 5 | ||||||||||||||
| Borrega-Mouquinho et al. (2021) | ● | ● | ○ | ● | ○ | ○ | ● | ● | ○ | ● | ● | 6 | ||||||||||||||
| Freyssin et al. (2012) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 5 | ||||||||||||||
| Choi et al. (2018) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 5 | ||||||||||||||
| Flemmen et al. (2014) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 5 | ||||||||||||||
| Puterman et al. (2022) | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | 8 | ||||||||||||||
| Piraux et al. (2022) | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | 8 | ||||||||||||||
| Bang-Kittilsen et al. (2022) | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | 8 | ||||||||||||||
| de Poli et al. (2021) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 5 | ||||||||||||||
| Marques et al. (2020) | ● | ● | ○ | ● | ○ | ○ | ○ | ● | ○ | ● | ● | 5 | ||||||||||||||
| Terada et al. (2022) | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | 8 | ||||||||||||||
| Reed et al. (2022) | ● | ● | ● | ● | ○ | ○ | ● | ● | ● | ● | ● | 8 | ||||||||||||||
●adds a point on the score, ○ adds no point on the score. The item “eligibility criteria” is not included in the final score
The results of the reported studies indicate that the HIIT training modalities adopted for the healthy population were focused on two types: HIITS and HIITL. The healthy subjects treated with HIIT exhibited a significant decrease in the depression scale scores and the level of organism validation in this population. This observation indicates that HIIT can effectively reduce the risk of depression in this population (Table 1). Generally, HIIT of any type can reduce the risk of depression and symptoms in this population. Marques et al. (M. Marques et al., 2020) reported that depression scale scores were reduced in subjects right after acute exercise, indicating that HIIT exhibits a satisfactory effect in modulating depression.
HIIT reduces depressive symptoms in depressed patients and in patients with other comorbid depressionThe previous section has summarized that utilizing different types of HIIT exercise prescriptions effectively reduces the risk of depression in healthy populations. However, the treatment of depressive symptoms in patients with depression and in individuals with other disorders co-morbid with depression should be further investigated. A summary of the reported findings indicated that depressive symptoms were relieved in most patients treated with HIIT. However, depressive symptoms were not significantly improved in patients with cancer (Table 2). Notably, HIIT dramatically decreased depressive symptoms in patients with cardiovascular disease co-morbid with depression.
DiscussionHIIT exercise prescription for healthy individuals to reduce the risk of depressionThe literature analysis included in Table 1 revealed that there are four types of HIIT for healthy populations and that using one or a combination of two HIIT exercise forms effectively reduces the risk of depression in healthy patients. Specifically, the utilization of HIIT intervention in the healthy population led to a significant decrease in the risk of depression (depression, anxiety, stress, and tension) and to a significant increase in the level of perceived effort and BDNF in the subjects. Meanwhile, the research on the causes of the dropped-out population could not be attributed to the occurrence of adverse events with respect to the literature contained in the supplementary data (Table 1). This observation indicates that HIIT is a relatively effective and safe method for reducing the risk of depression in healthy populations. However, physical exercise prescriptions must often be adapted to the subject's scenario. However, the population of subjects utilizing the RST and SIT interventions was healthy, and the reported outcomes were immediately after the acute intervention. Therefore, further research is still needed to determine whether these two interventions can reduce depressive symptoms in depressed patients. Based on the preceding information, it can be concluded that HIIT exerts an excellent short-term effect in enhancing depression in healthy subjects; however, the long-term effect should be further explored.
In regard to exercise intensity, most of the literature utilizes maximum heart rate and maximum oxygen uptake as the basis for classification, with 80–95 % of maximum heart rate and maximum oxygen uptake being the commonly utilized exercise intensity parameters. It is considered that testing maximal oxygen uptake during exercise monitoring is more demanding in regard to equipment and venue. By contrast, the rapid development of smart wearable devices has made it more convenient for monitoring changes in personal heart rate indicators, which is more conducive to the daily exercise monitoring of the athletic population. Therefore, we recommend that the maximum heart rate be utilized as the basis for exercise intensity when formulating HIIT exercise prescriptions, thus making it easier for subjects to perform real-time exercise monitoring. It is worth noting that the study compared the immediate effects of HIITS, HIITL, RST, and SIT on depressed mood (Marques et al., 2020). The results indicated that although all four methods enhanced the depressed mood, there was no statistical difference between the groups. In addition, the study's results noted that SIT was the most preferred form of exercise among the subjects with high motivation to participate, indicating that SIT could be preferred as an intervention for improving depressed mood in healthy individuals.
HIIT exercise prescription for patients with depression and patients with other disorders co-morbid with depressionIn a study analyzing the effects of SIT on patients with simple depression, a 4-week SIT intervention was found to improve depression. Other studies conducted HIIT interventions in patients with cardiovascular disease, psychiatric disease, and metabolic disease with comorbid depression, all exhibiting positive effects of exercise interventions. By summarizing the characteristics of exercise prescriptions, it can be observed that the intervention period is generally 12 weeks, the duration of each intervention is 20–30 min, and the intensity of exercise is 80–95 % of the maximum heart rate and maximum oxygen uptake. Particularly, in treating depressive symptoms in patients with psychiatric disorders, HIIT interventions significantly reduced depressive symptoms in only 12 days (Plag et al., 2020), indicating that HIIT exhibits an apparent advantage in regard to improving depressive symptoms in this group of patients. However, the number of included studies and heterogeneity, the summarization of optimal intervention parameters for different disorders was not possible.
Furthermore, the following finding merits research attention: all the cancer literature reported that HIIT did not improve the depressive state of patients in this population. The conventional wisdom is that HIIT is safe and feasible for prolonged periods because it is enjoyable and consistent, facilitating the consolidation and sustainability of the exercise effect (Malik et al., 2018; Stork et al., 2018). However, not all HIIT is enjoyable and pleasurable, and Saanijoki et al (Saanijoki et al., 2015). indicated that HIIT (4–6 sets of 30 s of all-out cycling + 4 min of recovery) led to severe pain, tension, and other negative emotions in middle-aged subjects compared to 6 days of regular aerobic exercise. Similarly, cancer patients should also bear psychological burdens and negative emotions for a long time and are more likely to experience fatigue due to reduced resistance. Therefore, prolonged, high-intensity HIIT exercise may exacerbate peripheral and central fatigue and, in turn, reduce the pleasure and satisfaction of exercise and, finally, directly affect its clinical effects (Oliveira et al., 2013; Rooijackers et al., 2017). However, it is undeniable that the status and quality life of the cancer patients have improved after receiving HIIT treatment. Therefore, considering the unique characteristics of this disease group, the exercise form with increasing intensity can be gradually tested in clinical practice. In addition, during the exercise process, strict exercise supervision should be conducted to observe the immediate changes in the patient's Electrocardiography (ECG) and in other indicators to ensure the patient's exercise safety.
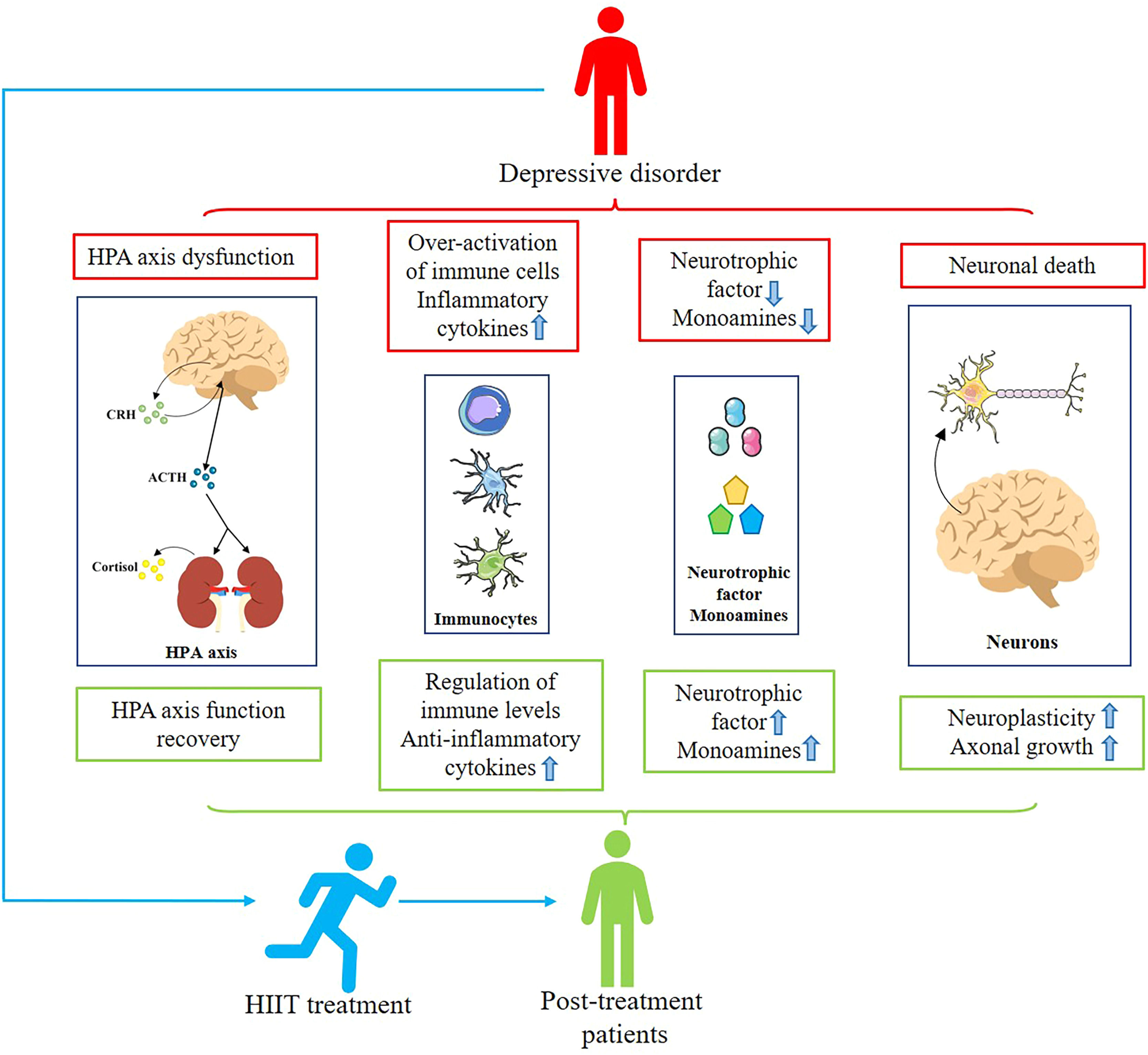
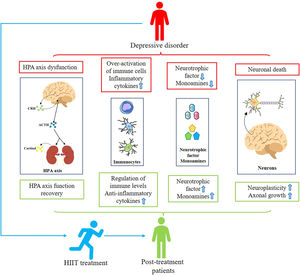
Mechanisms involved in treating depression with HIITCurrent research into the treatment of depression using physical exercise focuses primarily on aerobic exercise. However, since HIIT is not the same as aerobic exercise characteristics, some differences exist in the mechanisms involved in treating depression. Subsequently, we aim to briefly discuss the biological mechanisms associated with HIIT for depression through reported basic and clinical studies and in conjunction with the results of this systematic review (Fig. 2).
Mechanisms associated with HIIT for depression. Patients with depression suffer from HPA axis disturbances, increased inflammation levels, insufficient secretion of monoamines and neurotrophic factors, and neuronal death. Patients treated with HIIT showed restoration of HPA axis homeostasis, decreased inflammation levels, increased secretion of monoamines and neurotrophic factors, and decreased neuronal remodeling and death, alleviating depressive symptoms.
BDNF is one of the essential neurotrophic factors that regulate brain development, neuroplasticity, and axonal growth and exerts an irreplaceable role in the development of neurological disorders such as depression (Castrén & Monteggia, 2021; Zagrebelsky & Korte, 2014). Moreover, BDNF is susceptible to motor regulation. Studies have indicated that both the form and intensity of exercise can affect BDNF levels (Szuhany et al., 2015; TaheriChadorneshin et al., 2017). BDNF levels increased significantly after treatment with HIIT in healthy populations in the included articles. However, subjects with coronary artery disease did not exhibit significant changes in their BDNF levels after undergoing HIIT treatment. These two different results may be occasioned by the difference in the timing of the tests undertaken in these two studies. HIIT has a higher intensity than aerobic exercise and, thus, exhibits a more pronounced effect on BDNF levels. Current research reveals that the exercise-induced increase in BDNF levels may be related to the accumulation of lactate, which can only increase the release of BDNF through the activation of Sirtuin-1 deacetylase in exercising muscles (de Poli et al., 2021; El Hayek et al., 2019). The following observation is noteworthy: it has been revealed that by consistently detecting exercise-induced changes in lactate levels, it was found that lactate levels returned to baseline after a 30 min break from exercise, potentially leading to the increase in BDNF levels, which did not occur in the Reed et al's study (Reed et al., 2022; Valiente-Pallejà et al., 2020).
Furthermore, in clinical and animal studies, HIIT was found to increase the expression of peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α), thereby increasing the expression of fibronectin type III domain-containing protein 5 (FNDC5); thus, the further release of BDNF was promoted (Agudelo et al., 2014; Little et al., 2011; Wrann et al., 2013). The released BDNF subsequently reverses neuronal death due to depression via the BDNF-TrkB-PI3k-Akt and BDNF-TrkB-MAPK/ERK signaling pathways and also increasing neurogenesis and synaptic activity, thus treating depressive symptoms (Coyle & Duman, 2003; Fries et al., 2022).
Monoamines exhibit a significant effect on the development of depression. A study found that 5-HT and dopamine (DA) concentrations increased, albeit insignificantly, in HIIT-treated subjects compared to subjects who did not undergo HIIT. However, norepinephrine (NE) concentrations were significantly increased in the HIIT-treated subjects. Notably, current reports on NE are somewhat controversial in regard to its biological role in the development of depression, and it is unclear whether noradrenaline reuptake inhibitors (NRIs) are developed based on the monoamine hypothesis of inadequate NE release at synaptic sites. These drugs increase NE concentrations at synaptic sites, thereby alleviating depressive symptoms.
Contrastingly, alpha-methyl-para-tyrosine (AMPT), an inhibitor of NE synthesis, reverses the antidepressant effects of NRIs. This finding indicates that NE exerts a role in the treatment of depression (Anand & Charney, 2000). Conversely, some studies have also revealed that the release of NE is associated with perceived stress and that NE promotes the release of CRH and further activates the HPA axis to produce glucocorticoids. The release of glucocorticoids may account for the perceived stress reported in some HIIT-treated subjects (Paolucci et al., 2018; Seki et al., 2018; Tanaka et al., 2000). However, the depletion of NE in the average population does not lead to depressive symptoms. By contrast, the specific biological effects of increased NE levels on the pathological process of depression in HIIT-treated subjects merits investigation.
The specific biological mechanism of the NRIs was explored, and it was found that the modification of the drug may increase BDNF levels while elevating NE concentrations at synaptic sites, thus reversing the neuronal death in cortical areas occasioned by depression (Seki et al., 2018). Notably, the same rise in both substances was detected in HIIT-treated subjects (Seki et al., 2018), which may potentially indicate that HIIT can treat depression; however, this research topic merits further exploration.
HIIT modulates HPA axis homeostasis in depressionThe HPA axis is an essential endocrine stress axis, where corticosterone release under psychosocial and physical threat affects the metabolic, cardiovascular, and central nervous systems (Tortosa-Martínez et al., 2018). The secretion of glucocorticoids (corticosterone) occasioned excessive activation of the HPA axis in depression is considered to be one of the pathogenic factors, and excessive corticosterone release leads to abnormalities in the feedback mechanisms of the HPA axis. However, it is inaccurate to consider exercise-mediated increases in corticosterone levels as an adverse reaction (Hackney & Walz, 2013). In addition to regulating the HPA axis, corticosterone in can regulate glucose homeostasis and immune status (Fujio et al., 2016; Güemes et al., 2016; Straub & Cutolo, 2016; Verberne et al., 2014). Although an increase in corticosterone levels has been observed in HIIT-treated subjects (de Araujo et al., 2016; White, 2011), recent studies on corticosterone have revealed that its levels are dynamic and closely related to circadian rhythms, time of day, and environment. However, current studies on exercise-mediated changes in corticosterone levels have focused on a single time point, misleading the evaluation of corticosterone changes. A recent study found that corticosterone levels increased in subjects after short-term HIIT treatment but decreased significantly with increasing treatment duration relative to baseline levels during the treatment period (de Araujo et al., 2016). Furthermore, the exercise-mediated increase in corticosterone concentrations was not mild enough to significantly reduce glucocorticoid receptor levels and, thus, did not lead to depressive symptoms (Droste et al., 2007). Instead, it modulated the HPA axis and alleviated the hyperactivation of the HPA axis.
Another possible reason why HIIT modulates HPA axis homeostasis is as follows: an increased expression of the GR occurred, and its mRNA was detected in animal models of depression and patients with prostatic artery hypertension after HIIT treatment (Correia et al., 2022; Liu et al., 2022). Increased GR levels reduce glucocorticoid damage to hippocampal tissue, decrease hippocampal atrophy, enhance cognitive function, and alleviate the susceptibility to depression. The increase in GR could reduce glucocorticoid damage to hippocampal tissue, reduce hippocampal atrophy, enhance cognitive function and reduce the susceptibility to depression. It also restores the negative feedback function of the HPA axis that is impaired by depression, thus treating depressive symptoms.
HIIT modulates inflammatory levels in depressionA growing body of evidence indicates that inflammation exerts a contributing role in the pathogenesis of depression (Felger & Treadway, 2017; Köhler et al., 2017). However, the inflammatory response is not always harmful, and an appropriate inflammatory response facilitates the body's clearance of potentially toxic substances while aiding in repairing damaged tissues (Medzhitov, 2008). However, when the inflammatory response is uncontrolled, over-activated immune cells and their release of pro-inflammatory cytokines and chemokines can damage normal tissue cells, leading to pathological changes (Medzhitov, 2008). Pathological studies have revealed that depressed patients exhibit an uncontrolled inflammatory response (chronic inflammation), which is manifested by the excessive activation of immune cells and massive release of pro-inflammatory cytokines in the periphery and brain, leading to neuronal damage, hippocampal tissue atrophy, and HPA axis dysfunction (Bauer & Teixeira, 2019; Schachter et al., 2018). Along with the rapid development of exercise immunology, it was found that exercise can regulate the immune response level. In the literature included in this systematic review there were 2 studies that reported a downward trend in the inflammatory cytokine TNF-α in subjects after HIIT treatment however there was no statistical difference (Dall et al., 2015; Paolucci et al., 2018). The reduction of TNF-α and IL-1 can regulate the dysfunction of the HPA axis in depression and reduce the release of ACTH, CRH, and corticosterone (Postal & Appenzeller, 2015). In addition, HIIT can increase the number of regulatory T (Treg) cells to further regulate the inflammatory state, which reduces the number and function of neurotoxic microglia in the brain, regulates the inflammatory state in the brain, reduces neuronal death, and, thus, treats depressive symptoms (Frank et al., 2007; Jia et al., 2021; Wang et al., 2012). Therefore, future research could focus more on the changes of relevant inflammatory cytokines in the organism after HIIT exercise and on analyzing the mechanisms associated with the reduction of depressive symptoms.
Changes in the concentration of IL-6, a classical pro-inflammatory cytokine, reflect not only the organism's inflammatory state but also are one of the crucial indicators of the degree of depression (Mac Giollabhui et al., 2021). A study found, a significant increase in IL-6 levels in the peripheral circulation of subjects treated with HIIT. However, this observation does not indicate that HIIT treatment promotes depression. Exercise-mediated IL-6 is primarily produced by skeletal muscles and possesses biological functions that are opposite to monocyte- and adipose tissue-derived IL-6 (Poole et al., 2011). Exercise-mediated elevation of IL-6 inhibits the production of TNF-α, leading to an elevation in the levels of IL-10 and interleukin-1 receptor antagonist (IL-1RA), which may have contributed to the decrease in circulating TNF-α levels after HIIT (Gleeson et al., 2011; Starkie et al., 2003; Steensberg et al., 2003). Overall, the increase in IL-6 in the context of exercise exhibits anti-inflammatory properties and exerts a positive role in treating depression. Notably, with reduced inflammation levels, HIIT can switch the KYN pathway in depression from a neurotoxic to a neuroprotective branch, thereby treating depressive symptoms (Javelle et al., 2021; Joisten et al., 2021).
HIIT regulates maximal oxygen uptake in depressionThe brain is one of the organs with a high demand for oxygen. Under hypoxic conditions there is an increase in the release of reactive oxides due to abnormalities in mitochondrial metabolism, which affects the brain (Bhatt et al., 2020; Kang et al., 2021). It has been found that oxidative stress exerts a crucial role in the pathogenesis of depression, and that the maximal oxygen uptake capacity is reduced in depressed patients compared to healthy individuals (Krogh et al., 2010). Several studies included in this systematic review reported a significant increase in the following factors: the maximal oxygen uptake capacity and the relief of depressive symptoms in subjects treated with HIIT (Choi et al., 2018; Chrysohoou et al., 2015; Dall et al., 2015; Flemmen et al., 2014; Freyssin et al., 2012; Plag et al., 2020). This observation may be rationalized as follows: increased maximal oxygen uptake capacity enhances cardiorespiratory fitness, which in turn promotes cerebrovascular function, neuronal growth and connectivity, and gut flora metabolism (Bang-Kittilsen et al., 2022; Chaddock-Heyman et al., 2013; Zhang et al., 2022). Additionally, higher maximal oxygen uptake was associated with greater brain volume and protein integrity (Chaddock-Heyman et al., 2013). Therefore, the patient's overall state and mental health was enhanced.
Recent studies have reported a potential target for the treatment of depression, hypoxia inducible factor-1 (HIF-1), by mediating mitochondrial metabolism in response to the phenomenon of oxidative stress occasioned by insufficient oxygen levels in tissues (Kang et al., 2021). Intermittent hypoxic acclimatization training with hyperbaric oxygen therapy could increase HIF-1 expression and alleviate depressive symptoms in subjects (Kang et al., 2021; Zhang et al., 2022). It is noteworthy that HT treatment also increased HIF-1 expression in the body, which may also contribute to the alleviation of depressive symptoms via HIIT (Holloway et al., 2015; Tryfonos et al., 2021).
Overall, the elevation of maximal oxygen uptake following HIIT treatment may be a causative factor for a complex mechanism that acts to alleviate depression. However, the exact mechanism of this phenomenon remains relatively understudied, and further investigation on this phenomenon is required at a later stage to refine the specific biological mechanisms.
Other methods in which HIIT treats depressionObesity is a multifactorial metabolic disease, and epidemiological studies have found that more than a quarter of adults worldwide are obese, and that nearly two-thirds of adults are overweight. The accumulation of fat is strongly associated with increased all-cause mortality and the development of related diseases (Batrakoulis & Fatouros, 2022). Recently, studies have revealed that there is also a high degree of co-morbidity between obesity and depression. Chronic high-fat dietary intervention in mice was found to significantly attenuate the hyperexcitability of AgRP neurons in response to starvation and depression-like stimuli, and this dysfunction of AgRP neurons can lead to severe psychiatric disorders (Xia et al., 2021). It is worth noting that irregular eating habits, lack of physical activity, and a sedentary lifestyle are among the major causes of obesity (Alonso-Fernández et al., 2022; López-Moreno et al., 2020). In the Batrakoulis study, it was revealed that HIIT treatment for obese individuals led to a significant increase in psychological well-being during training and that this increase could be sustained even after the exercise was over (Batrakoulis et al., 2020). In addition, HIIT is an effective weight control program that induces considerable cardiorespiratory, neuromuscular, and metabolic adaptations in obese individuals. A meta-analysis indicated that interval exercise can effectively reduce the body's total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides levels and can reduce the release of fatty acids into the circulation, thereby regulating the body's fat metabolism and blood lipid levels and alleviating the risk of cardiovascular disease and depression in subjects (Batrakoulis et al., 2022). Infiltration of M1-type macrophages with pro-inflammatory functions into adipose tissue in the peripheral circulation leads to increased pro-inflammatory adipokines (TNF, IL-6, IL-18, C–C motif chemokine ligand 2 [CCL2], and C–X–C motif ligand 15 [CXCL15]), thus increasing inflammation and promoting the development of depression (Gleeson et al., 2011). HIIT regulates macrophage phenotype switching from pro-inflammatory type M1 to anti-inflammatory type M2 by inhibiting the activity of the TLR4/MyD88/NF-κB signaling pathway (Luo et al., 2021). In addition, HIIT reduces the expression of intercellular adhesion molecule 1 in tissues and decreases the infiltration of macrophages into adipose tissues in the peripheral circulation, thereby reducing the release of adipose pro-inflammatory cytokines and chemokines (Bošanská et al., 2010; Chow et al., 2005; Fredrickson et al., 2021; Kawanishi et al., 2010). Overall, HIIT can regulate the inflammatory response in depression. Meanwhile, HIIT has exhibited some advantages in enhancing the mental health of obese individuals and reducing the risk of cardiovascular disease in subjects.
Lipocalin is a hormone secreted almost exclusively by adipocytes, and circulating levels of lipocalin are negatively correlated with visceral fat accumulation and the degree of depression (Leo et al., 2006; Matsuzawa, 2010; Yamauchi & Kadowaki, 2013). An increase in lipocalin levels was observed in subjects after HIIT treatment (Shing et al., 2013). In depression, lipocalin may produce antidepressant effects by promoting hippocampal neurogenesis and neural stem cell proliferation and by enhancing 5-HT neuronal activity and transmission (Li et al., 2021), which may also account for the use of HIIT in treating depression.
Challenges of HIIT in clinical careAlthough most of the current research indicates that HIIT exhibits immense potential for promoting physical health, there are potential shortcomings in the recently published literature on HIIT. However, recent publications reveal the potential shortcomings in the current research on HIIT, which make it challenging to apply HIIT in clinical settings (Ekkekakis & Biddle, 2023; Ekkekakis, Swinton, et al., 2023; Ekkekakis, Vallance, et al., 2023). In the results of this systematic review, it was found that both the healthy population and the population with other disorders co-morbid with depression exhibited a certain rate of dropout after HIIT treatment. It is noteworthy that the dropout rate was significantly higher in the population with other comorbidities of depression (dropout rate: 23 %) compared to the healthy population (dropout rate: 8 %) (Supplementary Table 1). The study noted that when choosing the intensity of exercise, individuals preferred moderate-intensity exercise (Ekkekakis & Biddle, 2023). However, higher exercise intensities result in lower compliance and higher dropout rates, which calls into question the sustainability of HIIT (Ekkekakis & Biddle, 2023). In addition, it was noted that unsupervised HIIT subjects would train below the assigned intensity (Ekkekakis & Biddle, 2023; King et al., 1991). The aforementioned factors could all contribute to the actual therapeutic effect of HIIT.
Furthermore, it was found that the adverse effects that occurred during HIIT treatment were mostly concentrated in the population with other disorders complicating depression (Supplementary Table 1). Therefore, researchers should carefully assess the patient's own condition before determining whether HIIT can be utilized for treatment. It is important to note that when prescribing exercise for sedentary and physically inactive populations, it is prudent to use a lower intensity and a slower exercise regimen (Ekkekakis, Vallance, et al., 2023). Vigorous physical exertion can increase the incidence of adverse effects (e.g., cardiovascular disease, stroke, sports injuries) (Burley et al., 2016; Calverley et al., 2020; Lucas et al., 2015). Overall, how to better apply HIIT in clinical treatment and how to better meet the needs of different populations needs to be further explored.
Strengths and limitationsA total of 586 subjects were included in this study, which can more optimally reflect the role of HIIT in reducing the risk or symptoms of depression. Meanwhile, in targeting the group of patients with depression complicated by other diseases, the therapeutic effect of HIIT on depression risk or symptoms in different diseased populations can be more optimally evaluated by further differentiating the diseases. In addition, the current study summarizes and discusses the biological mechanisms related to reducing depression wind or symptoms via HIIT by combining basic research.
It is worth noting that the current study also has exhibited some limitations, in which the evaluation of the role of HIIT in reducing depression symptoms or risk was conducted through the changes in the relevant depression scale scores utilized in the included studies. On one hand, due to the depression assessment scales utilized in the included studies, the age of the subjects, the gender ratio, and the outcome indicators were not entirely consistent. On the other hand, the statistical methods in the included studies may exhibits multiple tests, greater flexibility in analyzing methods, and a lack of continuity testing, which may lead to some bias in the results (Ekkekakis, Swinton, et al., 2023). In addition, in the group of other disorders with comorbid depression, the limited sample size of the included literature has may also contributed to the creation of bias.
ConclusionIn conclusion, HIIT has exhibited potential in reducing the risk and symptoms of depression in subjects, and it may have enhanced the level of psychological well-being in addition to the level of physical well-being through multiple pathways (e.g., increasing monoamine release, improving the level of inflammation in the body, elevating maximal oxygen uptake). However, it is essential to develop an appropriate exercise prescription and duration of treatment when utilizing HIIT to treat depression because this method is closely related to the treatment outcome. The formulation of the exercise prescription should consider the patient's condition (e.g., age, presence of comorbidities, and use of other treatments), treatment period, and treatment intensity. In addition, the existence of a dose-response effects (Batrakoulis et al., 2019) in HIIT treatment merits further exploration by researchers to enhance the biological mechanisms of exercise-mediated depression treatment.
Availability of data and materialsNot applicable.
This work was supported partly by the National Natural Science Foundation of China (32161143021, 81271410), Henan Natural Science Foundation of China (182300410313), and Henan University Talent Program (SYLYC2022095).
Ethics approval and consent to participateNot applicable.
Consent for publicationNot applicable.
CRediT authorship contribution statementYuxiang Xu: Conceptualization, Writing – original draft. Yongjie Li: Conceptualization, Writing – original draft. Changqing Wang: Writing – original draft, Writing – review & editing. Tingting Han: Writing – original draft, Writing – review & editing, Supervision. Yue Wu: . Song Wang: Writing – original draft, Writing – review & editing, Supervision. Jianshe Wei: Writing – original draft, Writing – review & editing, Supervision.
Not applicable.