In this research, the effect of La substitution by Pr cation in the A-site of ABO3 perovskites was carried out using the Pechini method for use in solid state fuel cells (SOFCs). The atomic compositions of Pr varied from x=0.35, 0.49, 0.53, 0.56, 0.63 and 0.7 in the La0.7−xPrxCa0.3MnO3 (LPCM) perovskites. The XRD patterns indicated that all the perovskites crystallized in the orthorhombic system with Pnma space group, but only in atomic compositions of 0.53 and 0.70 is possible to obtain pure phases; whereas the other compositions present narrow peaks of La and Pr oxides that are reduced with the amount of the cationic substitution. The Rietveld methodology and its analysis by the Goldsmith tolerance factor indicated that the stability of the samples was achieved through Jahn-Teller distortion in the b-axis. The Mn3+/Mn4+ atomic ratios determined by XPS measurements suggested that the substitution of La ions by Pr promotes the hole doped system instead of electron system. The electrical conductivity showed that the synthesized perovskites are mixed ionic and electronic conductors which positively influence their application in SOFCs. In particular, the La0.17Pr0.53Ca0.3MnO3 may be a good cathode candidate for these devices.
En esta investigación, el efecto de la sustitución del catión Pr por el La en el sito de A de las perovskitas ABO3 se llevó a cabo utilizando el método de Pechini para uso en celdas de combustible de estado sólido (SOFC). La composición atómica de Pr fue variada de x=0.35, 0.49, 0.53, 0.56, 0.63 y 0.7 en las perovkitas La0.7−xPrxCa0.3MnO3 (LPCM). Los patrones de DRX indicaron que todas las perovskitas cristalizaron en el sistema ortorrómbico con grupo espacial Pnma, pero únicamente de las composiciones atómicas de 0.53 y 0.70 es posible obtener fases puras; mientras que, otras composiciones presentan picos estrechos de óxidos de La y Pr que se reducen con la cantidad de sustitución catiónica. La metodología de Rietveld y el análisis realizado mediante el factor de tolerancia Goldsmith indicó que la estabilidad de las muestras fue lograda a través de la distorsión de Jahn-Teller en el eje-b. Los radios atómicos Mn3+/Mn4+ indican que la sustitución de La por iones Pr promueve el sistema dopado a través de huecos. La conductividad eléctrica demostró que las perovskitas sintetizadas son conductores mixtos iónicos y electrónicos que influyen positivamente en la aplicación en SOFC. Particularmente, La0.17Pr0.53Ca0.3MnO3 es un buen cátodo para este tipo de dispositivos.
Solid oxide fuel cells (SOFCs) are electrochemical energy conversion devices that directly convert chemical energy into electricity with high-energy conversion efficiency [1]. SOFCs are considered as low environmental impact devices with flexible fuel feeding, low carbon emissions and noise during the working process [2,3]. Typically, fuel cells require ceramic materials (cathode–electrolyte–anode) that present stability during the oxidation–reduction process (chemical, thermal and mechanical), adequate compatibility with electrolyte, hermeticity, porous structure for the adequate transport of gases to the cell active sites transportation to reaction sites and high electrical conductivity [4]. Unfortunately, the used materials still have significant drawbacks, such as high operating temperatures (800–1000°C), long time required to heat and cool devices during operating cycles, degradation of components, thermal stress, and diffusion between components [5,6]. To overcome these disadvantages, different perovskite materials have been proposed to reduce the operating temperature between 600 and 800°C, but also they reduce the catalytic activity and increase the applied bias on the cathode side, where cell performance plays a key role [5,7–9]. Stoichiometric and non-stoichiometric ABX3 perovskites have been widely reported as cathode materials for IT-SOFCs [10–13]. In site A, La, Sr, Ca, Pb, and Pr have been explored, while in B-site cations of Ti, Mn, Ni, Fe, Co and Zr have been reported. The X-site is commonly occupied by oxygen with two types of sites, one with coordination six and others with coordination eight or twelve [14–16]. Among these perovskites, manganites with the general formula of Ln1−xMxMnO3 (Ln=trivalent rare earth ions, alkaline rare earth element M=3d transition) have been studied with a significant impact on the performance of SOFCs, which can be modulate depending on the ratio of Mn valences (Mn3+/Mn4+). This proportion has a direct influence on the performance of electrical and magnetic properties [17,18]. For example, lanthanum strontium manganite (La1−xSrxMnO3, LSM)-based perovskites showed high electrical conductivities due to distortions in their structures; that is, they are sensitive to the stoichiometry of the perovskite, relating the MnO6 structure connectivity and the Jahn-Teller effect which in turn conditions its electron holes [19–21]. Therefore, perovskite-type manganites are considered the most versatile materials because they show high magnetoresistance, multiferroicity and have reasonable performance both as anode or cathode in IT-SOFCs using different fuels [21–27]. Although it occurs more severely at high temperatures (>750°C) and high cell polarization, even at intermediate temperatures, loss of stability in reducing atmospheres has been observed on the cathodic side of IT-SOFCs devices. This instability causes diffusion and degradation in the cathode–electrolyte interface, with the consequent formation of insulating phases such as La2Zr2O7 or SrZrO3, negatively affecting ion conductivity [28]. The total or partial replacement of Sr in the A-site by other lanthanide elements such as Ce, Pr, Nd, Sm, and Gd or with Ca, Co, Cu, and Cr can contribute to the reduction of these disadvantages. For example, a reduction in the mismatch of the thermal expansion coefficient at the electrode–electrolyte interface, a reduction in the cell polarization, and an improvement in overall electro-catalytic activity have been reported [29–32]. Recently, it has been determined that the electrical and catalytic properties of lanthanum manganite perovskites depend to a large extent on the structure and morphology, but it is necessary to obtain pure phases during the synthesis [33,34]. In this way, Rietveld's refinement has been one of the most useful techniques to quantitatively identify the phases and structural changes of this type of material [11,35–37]. Ongoing with the proposal of new materials for energy production that help to reduce cathodic polarization resistance and the operating temperatures, in this study, the analysis of different synthesis parameters was carried out to produce pure La0.7−xPrxCa0.3MnO3 perovskites by lanthanum substitution (x=0.35, 0.49, 0.53, 0.56, 0.63, and 0.7) through the Pechini sol–gel method. Rietveld refinement was carried out to evaluate the conditions under which a pure phase can be reached. Additionally, the influence of different Pr contents on the microstructure, phase composition, and electrical conductivity was determined. The results are discussed in terms of potential applications as a cathode electrode for SOFCs.
ExperimentalPerovskite preparationThe modified sol–gel (Pechini) method was used to prepare samples of La0.7−xPrxCa0.3MnO3 perovskites (x=0.35, 0.49, 0.53, 0.56, 0.63 and 0.7) [38]. Precursors, lantanum (III) nitrate hexahydrate La(NO3)3·6H2O (99% trace metals basis), praseodymium (III) nitrate hexahydrate Pr(NO3)3·6H2O (99% trace metals basis), calcium nitrate tetrahydrate Ca(NO3)2·4H2O (≥99.0%), and manganese (II) nitrate hydrate Mn(NO3)2·H2O (99.99% trace metals basis) were acquired from Sigma-Aldrich. Equimolar amounts of La, Pr, Ca, and MnO3 were weighed and dissolved in deionized water to give a concentration of 0.25M. The solution was then acidified with 2mL of nitric acid (HNO3) (ACS reagent, 70%). In a next step, citric acid (ACS reagent, ≥99.5%) was also added in a 1:4 (metal:citric acid) ratio and ethylene glycol (ReagentPlus®, ≥99%) in ratio 1:10. The final solution was stirred for 5min in an ultrasonic bath (40kHz, Branson Ultrasonics Corporation) and heated for 4h at 170°C on a hot plate to generate the gel. Once the dried precursor was obtained, samples were sintered in a muffle furnace at 1000°C for 4h in an air atmosphere. Then, to 500mg of the obtained powder, 0.1–0.2mL of polyvinyl alcohol (PVA) was added as binder and it was heated at 300°C in an oven for 30min to be ground in an agate mortar. Finally, the powders were compacted uniaxially at 10MPa/cm2.
CharacterizationThe crystalline structure of the synthesized perovskites was determined by X-ray diffraction (XRD) using a Bruker D8 Advance diffractometer equipped with a Lynxeye detector, a Kα radiation source (λ=1.5405Å) at a scan rate of 0.01°, step time of 738.4s at 35kV and 25mA; samples were analyzed in a 2θ range from 20° to 80°. An estimate of the crystallite size was carried out using Scherrer's equation [39]. Quantitative phase analysis based on refinement of the Rietveld structure of perovskites was carried out using the X’Pert High Score Plus V3.0e software package from PANalytical using a background polynomial of eight coefficients and 1/X term. The models were obtained from the database of the same program. The parameters for the phases were scale factors, unit cell, atomic coordination, and profile variables for both perovskites and oxides. It is important to mention that in the case of oxides, the atomic coordination was not refined. A pseudo-Voight function was chosen to fit the shape of the peak for the refinements in the HighScore Plus software.
Scanning electron microscopy was used to observe the microstructure of the synthesized perovskites with the SEM JEOL-6701F equipment, and the elemental analyses were also estimated by energy dispersive X-ray spectroscopy (EDS) before and after the sintering process. The surface elemental composition of the samples was analyzed with an X-ray photoelectron spectrometer with monocromatized Al Kα radiation (1486.6eV). The base pressure of the instrument was 10−8Torr. Survey scans were obtained in the 1400 to 0eV energy range at 1.0eV per step with a pass energy of 100eV. In addition, high-resolution XPS scans were completed at 0.2eV energy steps and a pass energy of 50eV (constant pass energy mode). The full-width at half-maximum (FWHM) measurement for the Cu 2p3/2 line in the metallic state at these settings is 1.6eV. These detailed scans were recorded for La3d, C1s, Pr3d, Mn2p, Ca2p, and O1s for the coated samples. The analyzed area of the XPS measurements was ∼800mm2. All binding energy (BE) measurements were corrected for charging effects with reference to the C1s peak at 285.5eV. This reference gave BE values with an accuracy of 0.2eV. The collected data were analyzed using the Shirley background subtraction method and performed with a Gaussian-Lorentzian profile. For quantitative analysis, signal intensities were measured using the integrated area under the detected peak. To assess the atomic surface ratios, the sensitivity factors provided by the SDP v4.0 software were used and the cross-section for X-ray excitation was calculated by the Scofield method [40]. Electrical properties were carried using the four-point probe method on sintered pellets. The pellets diameter was 15.57mm with a thickness of 1.06mm after the sintering process at 1000°C. Measurements were carried out with a Keithley Model 2410C by applying a DC voltage from 0.01 to 0.08V (Vapp) with steps of 0.01 and room temperature based on the proportional–integral derivative method. During the analysis, it was initially assumed that the tip of the probe was quite small and the resistivity could be computed as a bulk sample. Thereafter, these results were confirmed using the Van Der Pauw in Hall effect methodology using an ECOPIA HMS 3000 apparatus with a Hall field of 0.0550T orthogonal to the measurements.
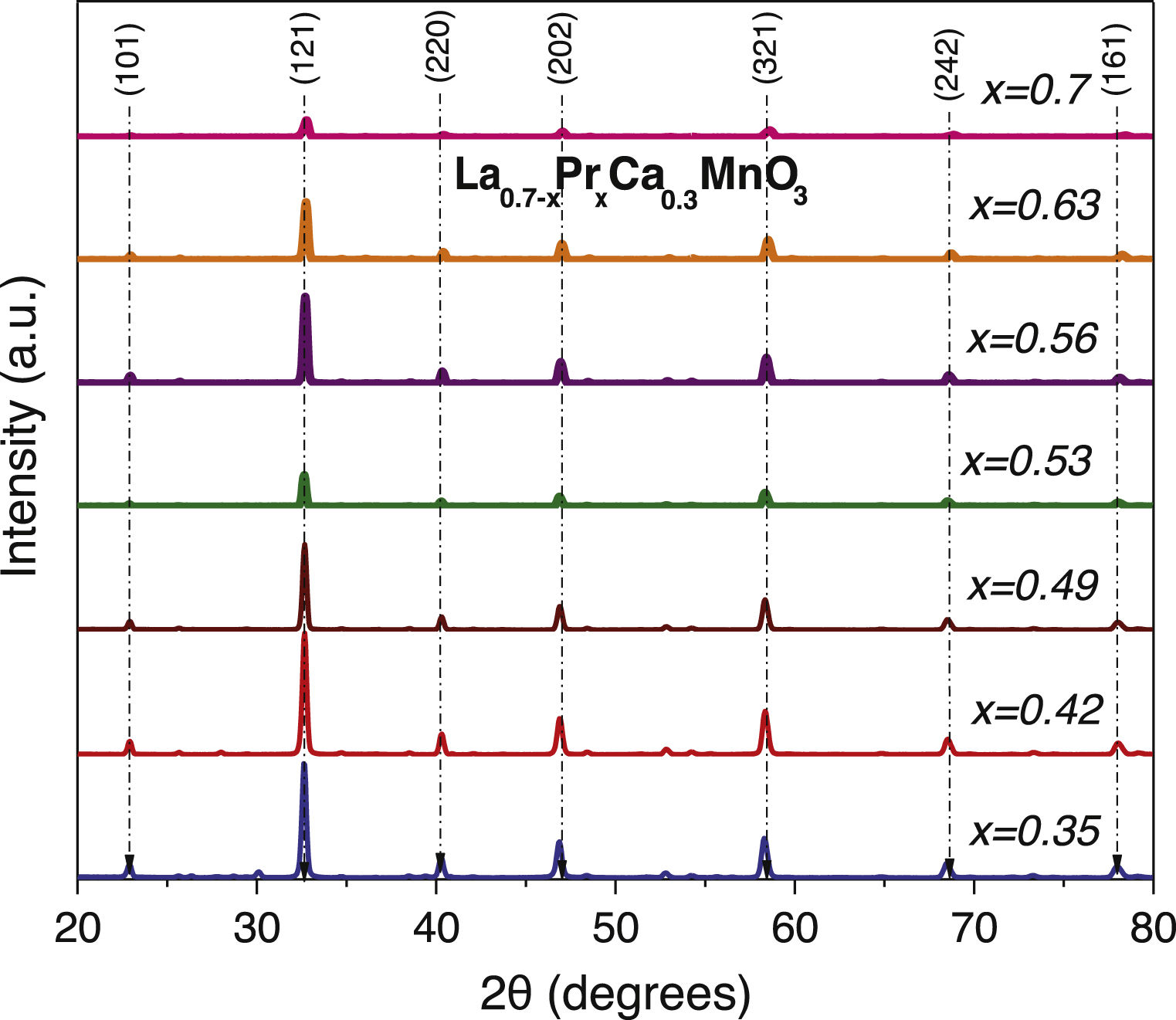
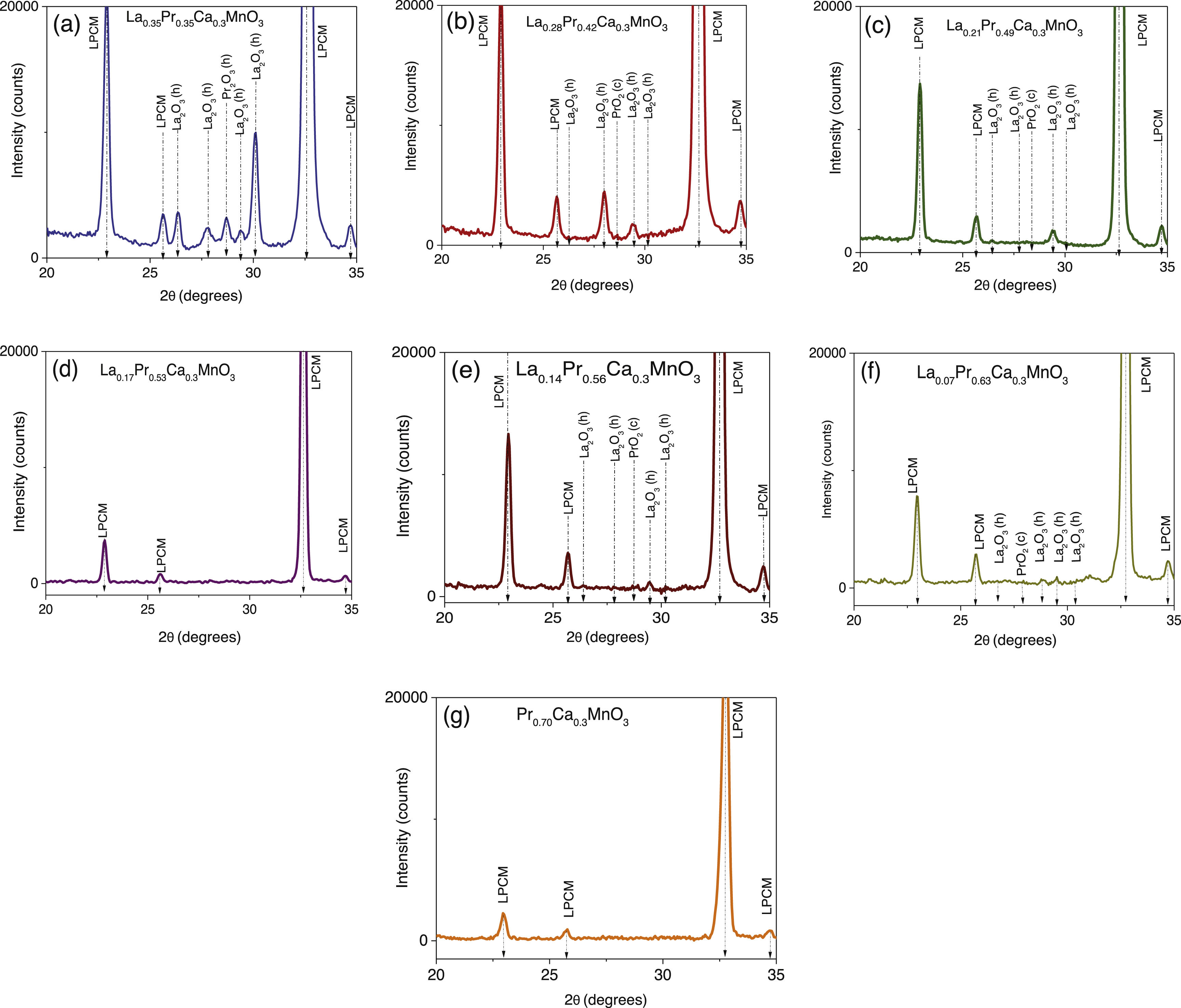
Results and discussionStructural analysisX-ray diffraction patterns were carried out in order to understand the effect of partial replacement of the A-site, Pr instead of La, and the structural deviation from the ideal cubic structure in ABO3 perovskites was determined. Fig. 1 shows the XRD spectra of the as-prepared perovskites by the sol–gel based Pechini method after the sintering process at 1000°C. As can be observed, the main intensity peaks matched well with the orthorhombic crystal system and Pnma space group (ICDD 98-009-2948 chart) which display the typical sharp and intense reflections of high crystallinity perovskites [41]. However, XRD spectra also show narrow signals in some compositions that could indicate the appearance of another phase. To confirm this assumption, a closer view of these patterns in the 20–35° range was carried out. Fig. 2a–g shows compositions in which other oxide compounds were identified; i.e. when La substitutions were between 0.35 and 0.49. These peaks matched with PrO2 (ICDD 06-0410 chart), and La2O3 (ICDD 83-1348 chart a=b=0.373nm, c=0.6129nm) which crystallize in the cubic and hexagonal phases [42–44]. It is evident that the intensity of the secondary phases (impurities) tends to decrease with the substitution of the La ion. However, under the experimental conditions of the Pechini method, only pure perovskites with two atomic compositions of Pr (x=0.53 and 0.70) can be obtained, which coincide with an orthorhombic crystal system and a Pnma space group. The formation of secondary phases negatively influences the ionic conductivity of SOFC operation due to decreased interparticle densification affecting the triple phase boundary.
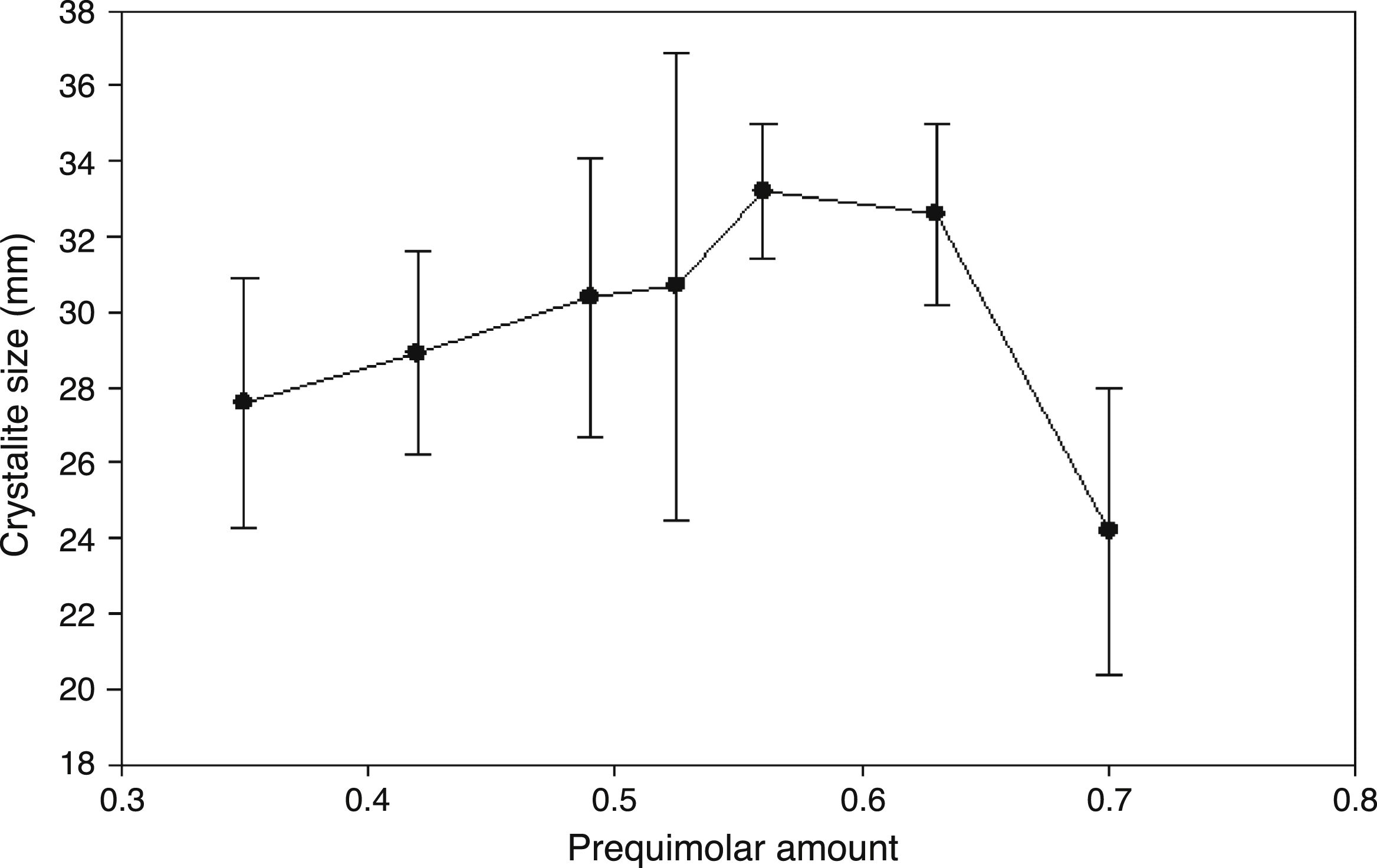
The Pechini method has been previously reported to synthesize different perovskites using citric acid and low temperatures in comparison with solid state reaction, but it reported considerable quantities of undesirable impurities (secondary phases) that require further treatment to reduce them [38]. In addition, the obtaining of Pr0.7Sr0.3Mn0.9Cr0.1O3 has been reported in the absence of impurities when a calcined process above 850°C is applied [45]. Then, although sintering temperature plays an important role in reducing secondary phases, it can be conditioned by structural incorporation in the A-site (lattice) for other types of rare earths in combination with the synthesis method. The crystallite size of the synthesized perovskites was estimated by the Scherrer's equation dnm=(kλ)/(β cos θ). In this formula, dnm is the crystallite size in nm, λ is the wavelength of the X-ray, β is the full-width at half-maximum, k is the shape factor, and θ is the Bragg's angle of diffraction. The estimation of the crystallite size was realized as an average of the three main intensity planes; (121), (202) and (321) and the results with their corresponding standard deviation are shown in Fig. 3. The addition of Pr in the ABO3 perovskite shows a gradual increase in the crystallite size from 27.6nm (x=0.35) to 33.2nm (x=0.56) and began to reduce with a partial substitution of x=0.63 (32.6) up to obtain a small crystallite size of 24.2nm with a total lanthanum substitution in the A-site, Pr0.7Ca0.3MnO3.
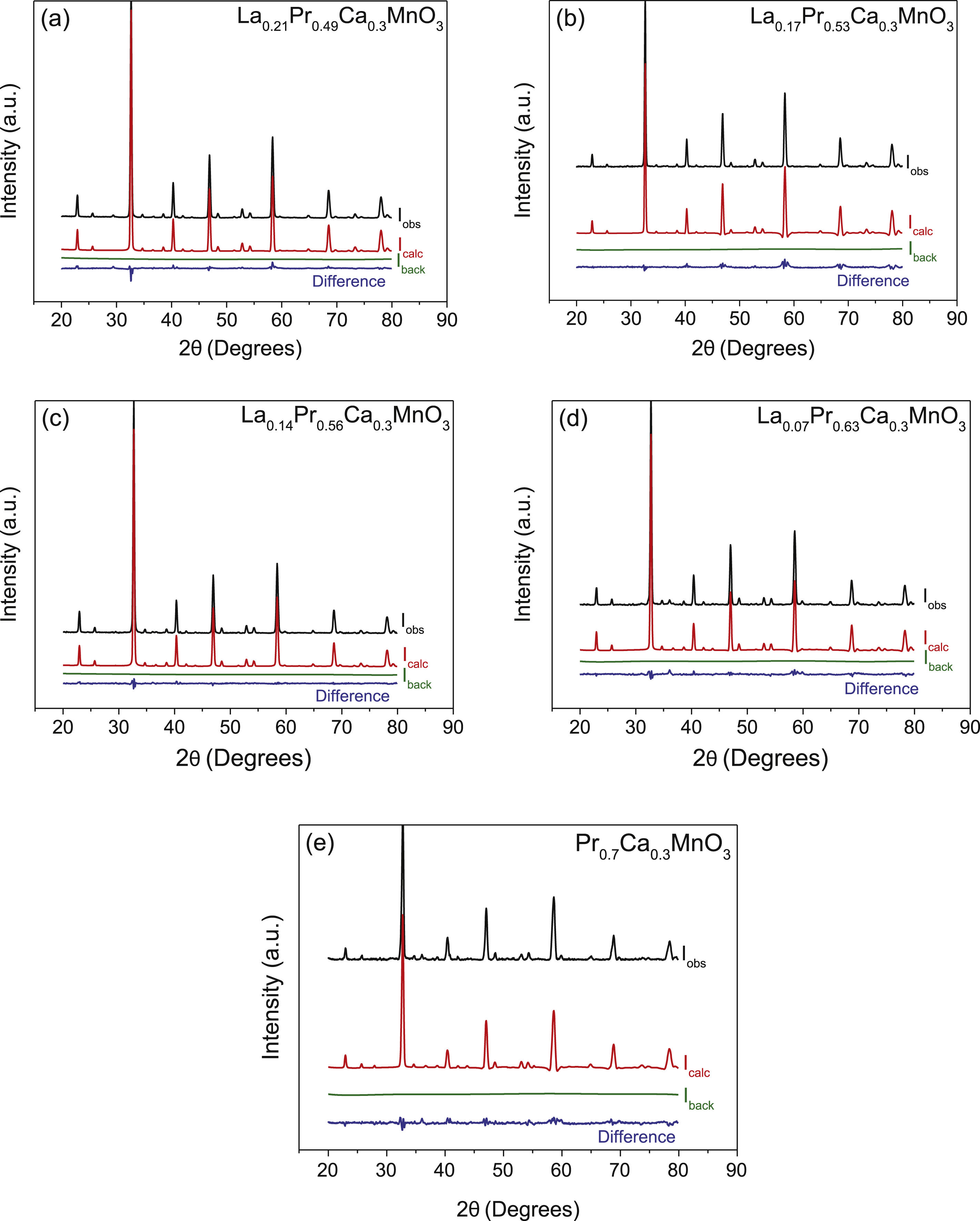
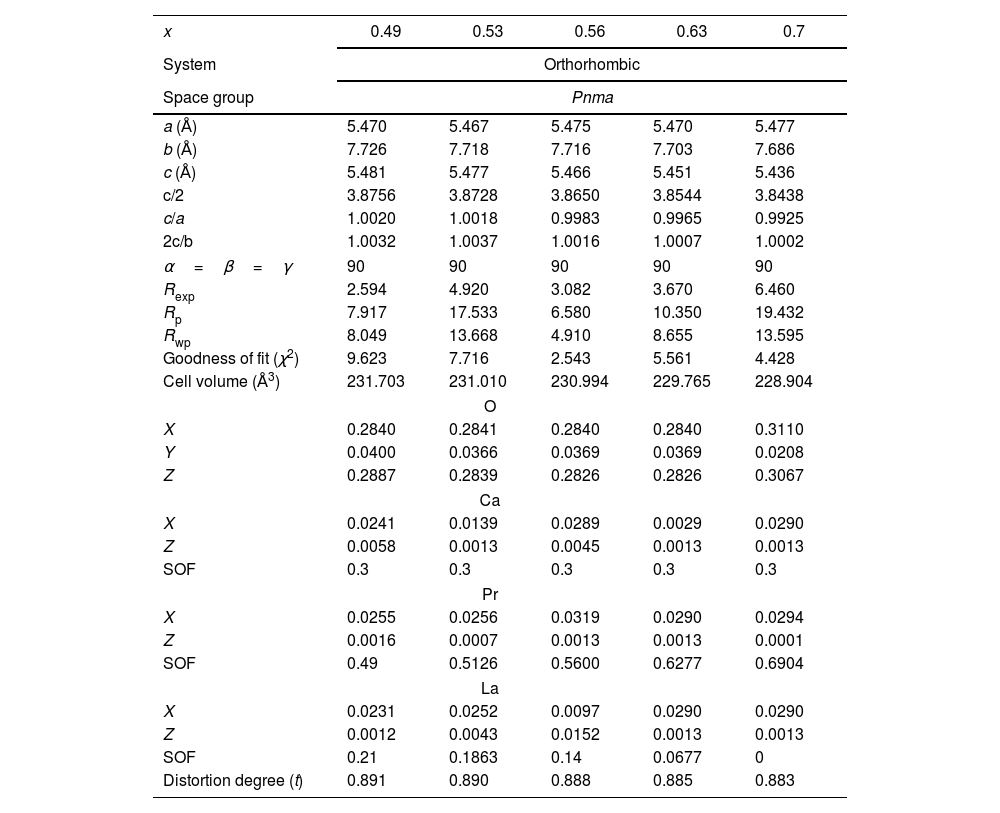
To confirm the existence of pure phases, a refinement of the structure was realized on the XRD spectra of the samples by the Rietveld method. Fig. 4a–e shows the refinement of the perovskites with an atomic substitution between 0.49 and 0.70. The analysis was initially carried out considering the atomic position of La2O3 and/or PrO2 as secondary phases and LPCM as the main structure. However, the Rietveld refinement confirms that except for a composition of x=0.53 and 0.70, the other compositions fit well only when a secondary phase is considered in the analysis. Thus, although samples with a Pr amount of 0.35, 0.49, 0.56 and 0.63 present a main orthorhombic structure, small lattice reflections of other symmetry inevitably also appear; whereas samples with a composition of 0.53 and 0.7 matched well with a pure phase of an ABO3 perovskite that belong to Pnma space group. The calculated parameters showed adequate values ensuring an adequate fitting between experimental–theoretical data, Table 1. The goodness of fit was obtained under (χ2≤10) validating the analysis with the used software. Some differences in the unit cell parameters, atomic coordination and the site occupancy were observed for oxygen (O1), calcium (Ca), praseodymium (Pr), and lanthanum (La), depending on La substitution, but not clear trend was observed in this parameter. However, the cell volume tends to decrease as the amount of Pr is increased from 231.703 to 228.904Å3, which is in agreement with the crystallite size and the obtaining of a pure phase. In comparison to other perovskites with lower amounts of Pr, such as those reported by Luque et al. (La0.325Pr0.30Ca0.375MnO3) [46], the unit cell parameters are higher than those observed in the analysis, but the Rwp was lower, suggesting a better fit. The stability of the perovskite was analyzed in terms of the Goldsmith tolerance factor t=(rA+ro)/(2rB+ro). This factor indicates stability in the ceramic materials when it is found in the following range 0.77≤t≤1.0 and depends on the ionic radio of the ions (cation and anions) [47]. The obtained perovskites presented a distortion degree close to 0.890, confirming that the samples present a stable structure. The orthorhombic distortion is characterized by a deformation that occurs with the growth of the formation of the net, and meet the relation c/2≤a≤b. This deformation is carried out through Jahn-Teller distortion with a clear elongation in the b-axis. It can be also observed that b and c lattice parameter decreased with the amount of Pr and in consequence c/a and 2c/b ratios are close to 1, confirming the final symmetry determined during the Rietveld analysis. These results represented a variation in the site occupancy that is reflected in a correlation between the site occupancy and the atomic quantity of these elements. Previously, it has been reported similar differences in the lattice parameters, for example synthesized perovskites based on Ca1−xGdxMnO3[48] and Gd1−xSrxMnO3 (GSMO) (0.2≤x≤0.5) [49] had differences between a and c parameters, suggesting that Jahn-Teller distortion take places by the presence of doping them with Mn3+ ion in the octahedral environment. In other works, the volume of the unit cell also decreased with an increase in the x content, mainly due to the distortion by the deficit of cations A, B and oxygen, which is in agreement with our synthesized perovskites.
Summary of structural and refinement parameters of La0.7−xPrxCa0.3MnO3 perovskites.
| x | 0.49 | 0.53 | 0.56 | 0.63 | 0.7 |
|---|---|---|---|---|---|
| System | Orthorhombic | ||||
| Space group | Pnma | ||||
| a (Å) | 5.470 | 5.467 | 5.475 | 5.470 | 5.477 |
| b (Å) | 7.726 | 7.718 | 7.716 | 7.703 | 7.686 |
| c (Å) | 5.481 | 5.477 | 5.466 | 5.451 | 5.436 |
| c/2 | 3.8756 | 3.8728 | 3.8650 | 3.8544 | 3.8438 |
| c/a | 1.0020 | 1.0018 | 0.9983 | 0.9965 | 0.9925 |
| 2c/b | 1.0032 | 1.0037 | 1.0016 | 1.0007 | 1.0002 |
| α=β=γ | 90 | 90 | 90 | 90 | 90 |
| Rexp | 2.594 | 4.920 | 3.082 | 3.670 | 6.460 |
| Rp | 7.917 | 17.533 | 6.580 | 10.350 | 19.432 |
| Rwp | 8.049 | 13.668 | 4.910 | 8.655 | 13.595 |
| Goodness of fit (χ2) | 9.623 | 7.716 | 2.543 | 5.561 | 4.428 |
| Cell volume (Å3) | 231.703 | 231.010 | 230.994 | 229.765 | 228.904 |
| O | |||||
| X | 0.2840 | 0.2841 | 0.2840 | 0.2840 | 0.3110 |
| Y | 0.0400 | 0.0366 | 0.0369 | 0.0369 | 0.0208 |
| Z | 0.2887 | 0.2839 | 0.2826 | 0.2826 | 0.3067 |
| Ca | |||||
| X | 0.0241 | 0.0139 | 0.0289 | 0.0029 | 0.0290 |
| Z | 0.0058 | 0.0013 | 0.0045 | 0.0013 | 0.0013 |
| SOF | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Pr | |||||
| X | 0.0255 | 0.0256 | 0.0319 | 0.0290 | 0.0294 |
| Z | 0.0016 | 0.0007 | 0.0013 | 0.0013 | 0.0001 |
| SOF | 0.49 | 0.5126 | 0.5600 | 0.6277 | 0.6904 |
| La | |||||
| X | 0.0231 | 0.0252 | 0.0097 | 0.0290 | 0.0290 |
| Z | 0.0012 | 0.0043 | 0.0152 | 0.0013 | 0.0013 |
| SOF | 0.21 | 0.1863 | 0.14 | 0.0677 | 0 |
| Distortion degree (t) | 0.891 | 0.890 | 0.888 | 0.885 | 0.883 |
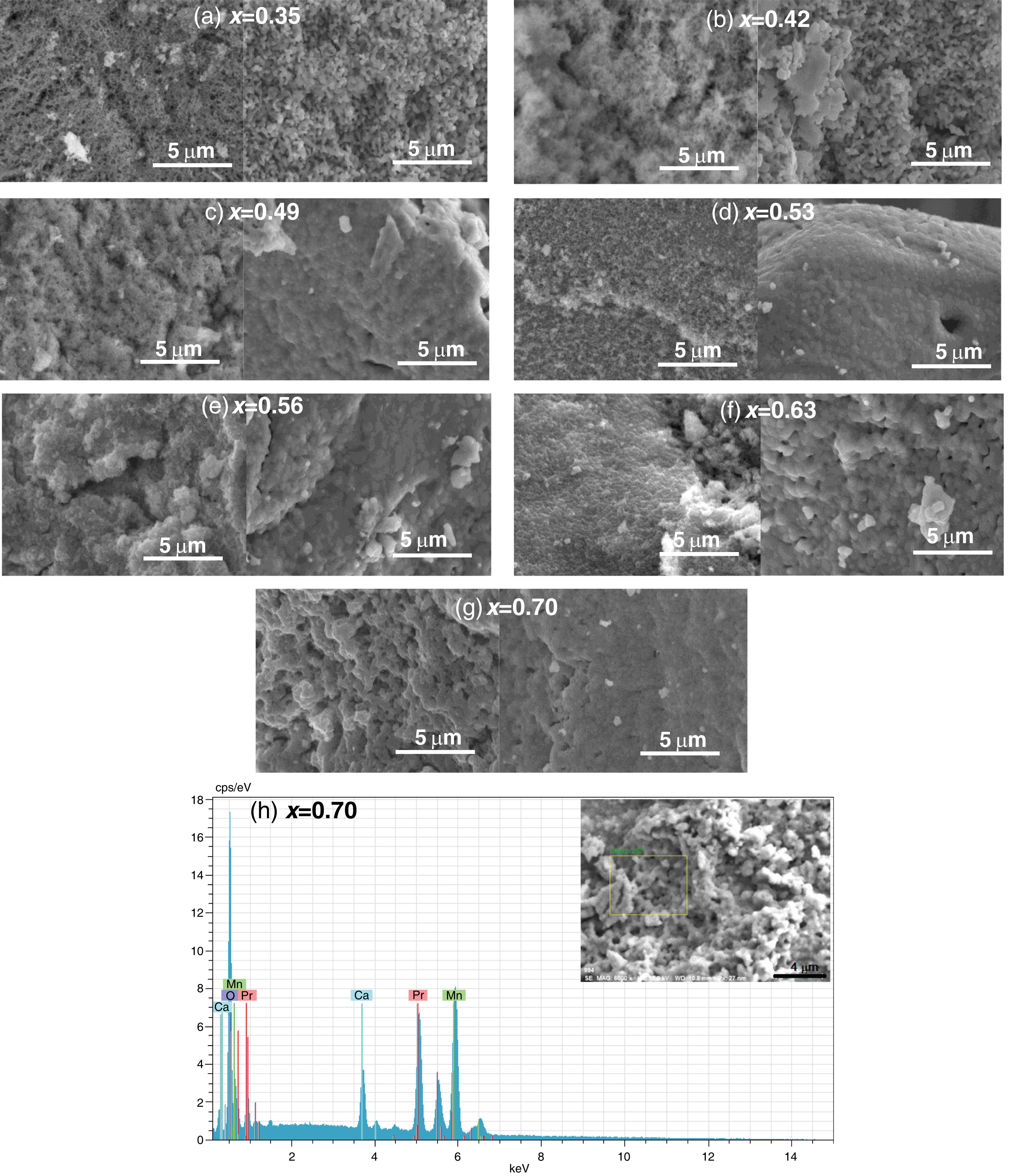
To evaluate the effect of the sintering process on the porosity and either agglomeration or uniform grain formation, SEM measurements were performed on La0.7−xPrxCa0.3MnO3 samples before and after the sintering process was performed at 1000°C (Fig. 5a–g). The morphological aspects of the samples, before the sintering process, display irregular micro-semispherical grains with high spacing between them (porosity); while the calcined specimens at 1000°C showed a densification with the typical cauliflower-like morphology [50], significantly reducing the porosity. This type of morphology has been previously observed in different perovskites such as SrFeO∼2.85 where the increase in size and decrease in porosity were mainly attributed to the increase in calcination temperature [38]. In particular, the densification and grain size variation in the as-obtained ABO3 perovskites are also influenced by the Pr content, which can be correlated with the differences in ionic radii between La and Pr. A similar behavior has been previously observed for strontium-doped lanthanum manganite perovskites [50]. The porosity volume percentage in the samples was estimated, finding the following variation: 15% (0.35), 14% (0.42), 15% (0.49), 13% (0.53), 12% (0.56), 10% (0.63) and 8% (0.70). Previously, the influence of the sintering temperature in different perovskites for stoichiometric compositions has been studied, where it has been shown that an adequate treatment to achieve the densification of green pellets is above 1000°C, therefore the remained porosity observed in this study can be avoided with temperatures around 1500°C [51]. In our research group, thermogravimetric and thermal expansion coefficient measurements of La0.7−xPrxCa0.3MnO3 perovskites were previously reported in the temperature range of 25–1000°C for a different synthesis method, indicating that sintered pellets at 1000°C have sufficient densification and chemical compatibility with yttria stabilized zirconia (YSZ) to be used as a cathode electrode for SOFCs. It was also determined that the amount of Pr in the perovskite favors the similarity with the thermal expansion coefficient between the cathode and the electrolyte (YSZ) [34]. According to these works, the porosity of the samples obtained by the Pechini method can be formed during the synthesis process (powders), as a result of a rapid release of gases during the initial drying process. Subsequently, with the calcination process, the grains become larger with a cauliflower-like shape increasing the degree of densification due to the rupture of the pore wall [52]. Then, considering an ionic diffusion pathway, the particles were melted and fused depending on both calcination temperature and Pr content. In this way, granular growth occurs in a three-dimensional aligned manner to form large agglomerates.
SEM images of the La0.7−xPrxCa0.3MnO3 perovskites before and after the sintering process at 1000°C, (a) 0.35, (b) 0.42, (c) 0.49, (d) 0.53, (e) 0.56, (f) 0.63 and (g) 0.70. (h) Selected EDS spectra of the perovskites with a composition x=0.70. Images within the graph display the SEM images indicating the selected area for EDS analysis.
The elemental composition of the samples was analyzed by energy dispersive spectroscopy (EDS). Fig. 5h shows a representative EDS spectra for La0.7−xPrxCa0.3MnO3 perovskites that crystallized in a pure phase (x=0.7). The area selected for analysis confirmed the presence of Pr, Ca, Mn and O without contaminants; which can also be observed for other compositions in the supplementary material (Figs. S1–S3). To quantitatively determine the composition of the samples, XPS analyses were performed and are discussed below.
Chemical compositionThe chemical composition of the as-prepared samples XPS measurements was analyzed in pellets after the sintering process at 1000°C. The oxygen vacancy concentration, the oxidation states of the metal ions, Pr4+/Pr3+ and Mn3+/Mn4+ ratios that condition the distortion in the orthorhombic structure, and the electrochemical performance was also analyzed through XPS fitting.
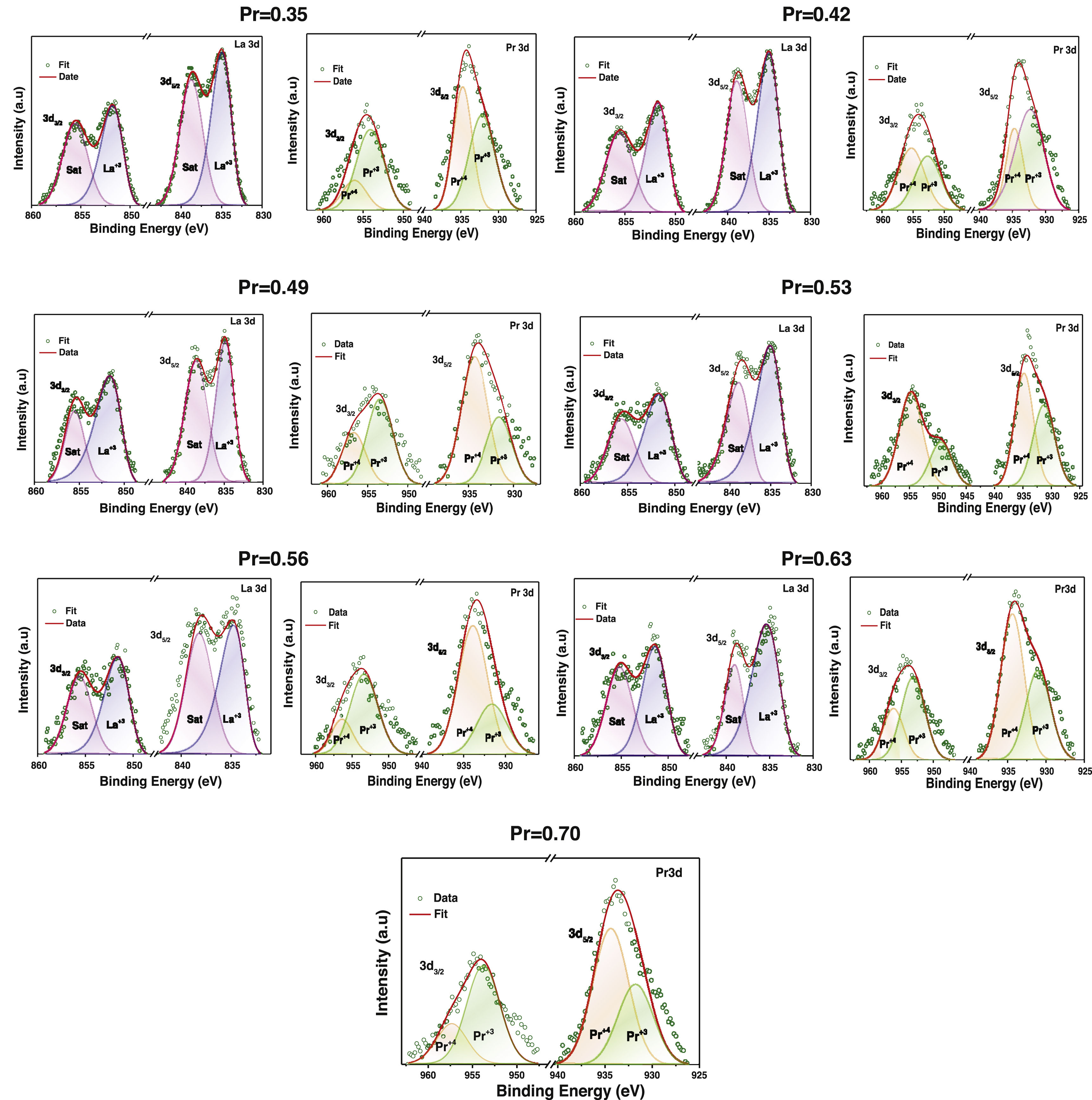
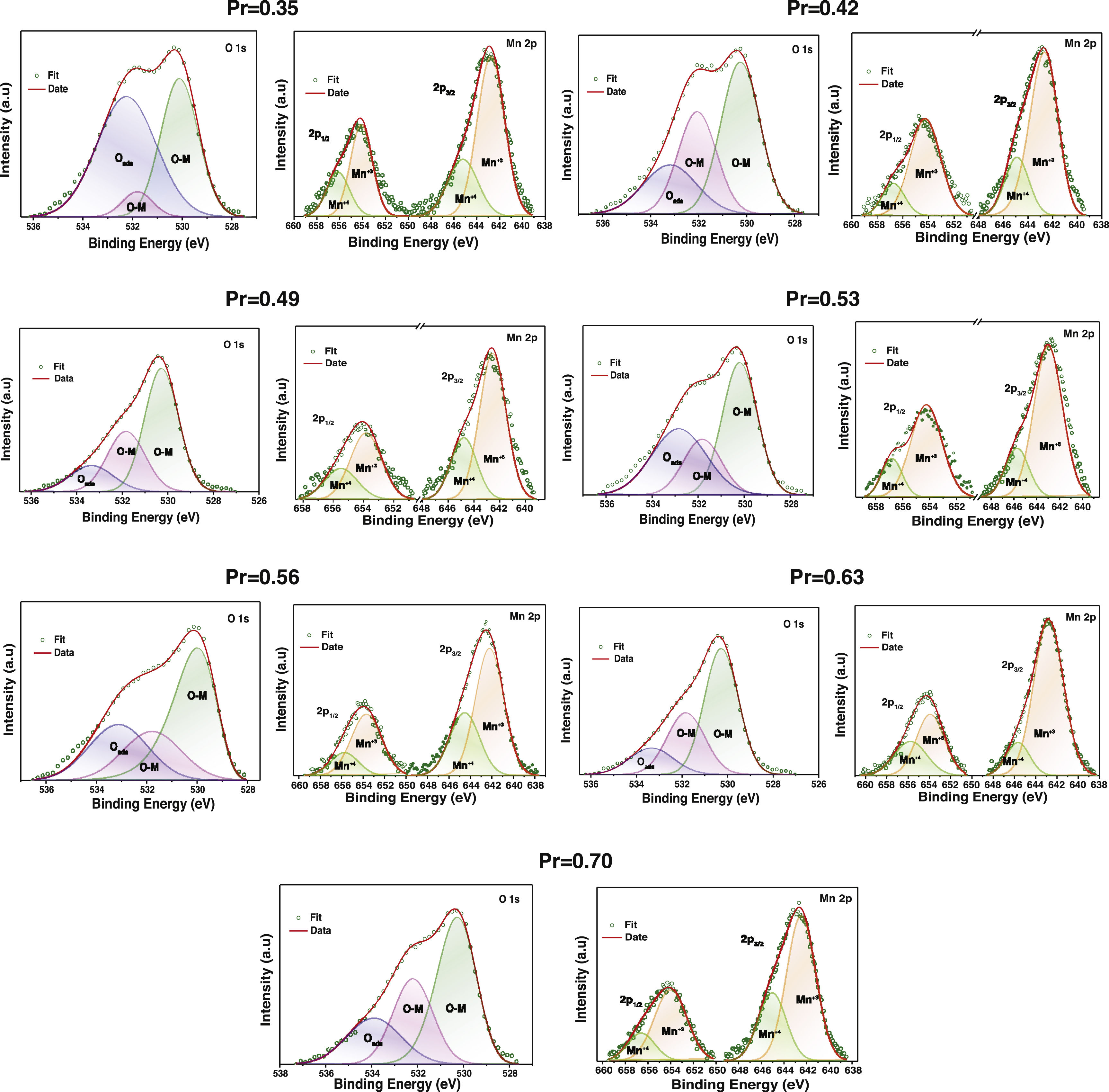
Figs. 6 and 7 show experimental XPS spectra as a function of the La substitution with its corresponding adjustment to determine the atomic percentage corresponding to its different electronic states. The lanthanum deconvolution presents traditional peaks in 3d core level spectra (3d3/2 and 3d5/2). Small displacements were observed in the binding energies with the amount of Pr as a consequence of the variation of the peaks shape. It is known that La and Pr are hygroscopic in nature and the formation of oxide–hydroxide compounds and the changes in the composition can explain the observed variations [53]; however, the La doublets with their corresponding satellite maintain a known difference of ∼17eV. On the other hand, praseodymium presents two oxidation states Pr3+ and Pr4+ in the 3d3/2 and 3d5/2 core levels (Fig. 6). The Pr3d3/2 core level (∼954.52eV) presented the highest ratio of Pr3+, whereas an inverse trend was obtained in the Pr3d5/2 core level (∼934.14eV). The energy separation between both peaks of ∼20.5eV is in agreement with previous observations [54]. The Pr3+/Pr4+ ratio tends to decrease with the addition of Pr, which is more evident between x=0.35 (1.64) and x=0.53 (0.59) and from there it begins to increase between 0.56 (1.06) and 0.70 (1.17). Then, a rise in the quantity of Pr4+ species will cause an increase in electrical performance [55]. The Mn2p core level showed two asymmetric peaks (2p1/2 @∼654.22eV and 2p3/2 @∼642.89eV) that fit well with two oxidation states of manganese (Mn3+ and Mn4+) (Fig. 7). The high peaks during deconvolution correlated with Mn3+ ions and the other couple to the Mn4+ ions, confirming the coexistence of the two oxidizing states [56].
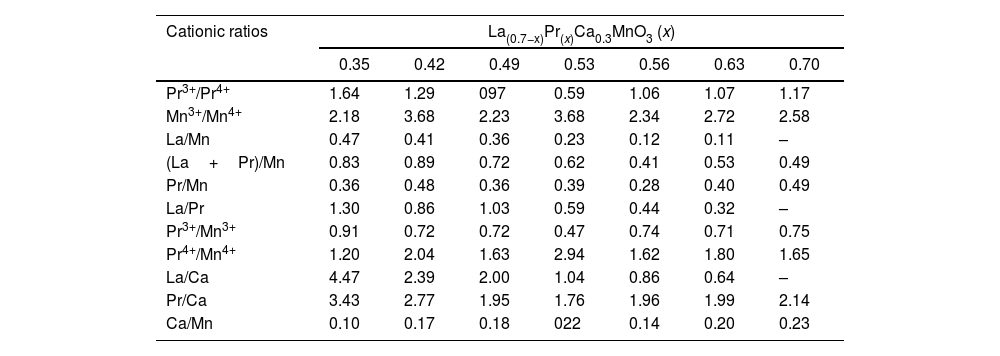
The variation of the binding energies depending on the Pr amount can be observed in Table 2. The Mn3+/Mn4+ atomic ratios varied from 2.18 (x=0.35) to 3.68 (x=0.53), indicating that La substitution by Pr ions promotes the hole doped system instead of the electron system; this ratio increases with substitution of La. Thus, the appearance of Mn4+ species is mainly due to manganese oxidation and/or charge compensation in the perovskite base structure (LaMnO3±δ) during lanthanum substitution, causing oxygen loss. In general, La/Mn, La/Ca and (La+Pr)/Mn ratios are reduced with the amount of Pr; although some specific compositions show a contrary tendency. Other cationic ratios such as Pr3+/Mn3+, Pr4+/Mn4+ Ca/Mn displayed values related to the lanthanum substitution. Then, the discrepancy has been previously observed in different perovskites and attributed to Mn enrichment during La substitution by a migration of lanthanum to the surface.
Cationic ratios for the La0.7−xPrxCa0.3MnO3 perovskites.
| Cationic ratios | La(0.7−x)Pr(x)Ca0.3MnO3 (x) | ||||||
|---|---|---|---|---|---|---|---|
| 0.35 | 0.42 | 0.49 | 0.53 | 0.56 | 0.63 | 0.70 | |
| Pr3+/Pr4+ | 1.64 | 1.29 | 097 | 0.59 | 1.06 | 1.07 | 1.17 |
| Mn3+/Mn4+ | 2.18 | 3.68 | 2.23 | 3.68 | 2.34 | 2.72 | 2.58 |
| La/Mn | 0.47 | 0.41 | 0.36 | 0.23 | 0.12 | 0.11 | – |
| (La+Pr)/Mn | 0.83 | 0.89 | 0.72 | 0.62 | 0.41 | 0.53 | 0.49 |
| Pr/Mn | 0.36 | 0.48 | 0.36 | 0.39 | 0.28 | 0.40 | 0.49 |
| La/Pr | 1.30 | 0.86 | 1.03 | 0.59 | 0.44 | 0.32 | – |
| Pr3+/Mn3+ | 0.91 | 0.72 | 0.72 | 0.47 | 0.74 | 0.71 | 0.75 |
| Pr4+/Mn4+ | 1.20 | 2.04 | 1.63 | 2.94 | 1.62 | 1.80 | 1.65 |
| La/Ca | 4.47 | 2.39 | 2.00 | 1.04 | 0.86 | 0.64 | – |
| Pr/Ca | 3.43 | 2.77 | 1.95 | 1.76 | 1.96 | 1.99 | 2.14 |
| Ca/Mn | 0.10 | 0.17 | 0.18 | 022 | 0.14 | 0.20 | 0.23 |
O1s spectra which were deconvoluted into three peaks referring to chemisorbed oxygen (Oads), ∼532.2eV, and lattice oxygen (O–M), ∼531.7 and ∼530.1 [57]. Adsorbed oxygen species are known to promote the reactivity at the surface of electrode materials and the redox process. Therefore, the fact that the proportion of the O–M species is higher than the Oads and that this varies positively with the Pr substitution is consistent with the compute Mn3+/Mn4+ ratio [58]. The surface composition is in good agreement with the theoretical calculation (see supplementary material).
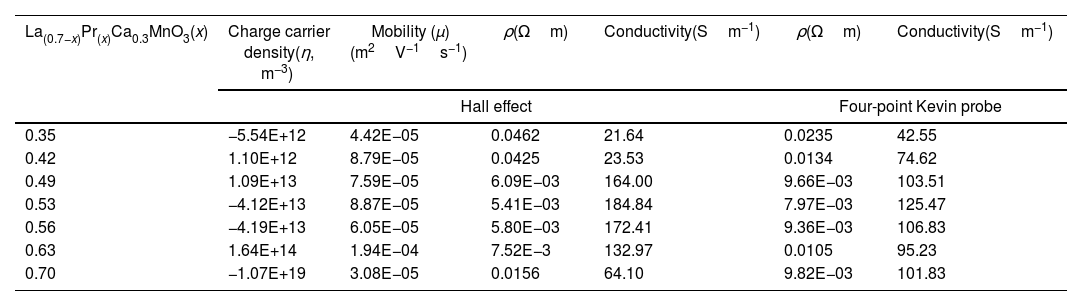
Electrical measurementsThe electrical conductivity of the electrode materials is influenced by the crystal structure; therefore, to explain changes in these properties with the Pr composition and to evaluate whether the synthesized materials can be considered as suitable materials for solid state fuel cells (SOFCs), the four-point probe method was evaluated after the sintering process at room temperature. The methodology contemplates an equidistant spacing between points, c.a. 0.04 inches (0.1016cm), so the resistance value was calculated by linear interpolation and multiplied by the factor 2πS=0.638372cm. The thickness of the pellets used was 1.06mm with a diameter of 15.57mm. The resistivity and conductivity values as a function of lanthanum substitution can be observed in Table 3. The obtained measurements showed an increase in conductivity as the Pr content increased between 0.35 and 0.53, reaching a maximum value of 125.47Sm−1, and began to decrease with a greater Pr amount, between x=0.56 (106.83Sm−1), and x=0.63 (95.23Sm−1). Finally, when a total substitution of La was made, the electrical conductivity of the electrode material increased again up to reach a value of 101.83Sm−1. The variations can be related to the differences in the observed phases and the (La+Pr)/Mn ratio. The highest electrical conductivity is lower than that reported for other types of electrode materials such as La1−xCaxFe0.9Mn0.1O3−δ (100Scm−1) [59] or La0.9Sr0.1M0.9Mg0.1O2.9 (M=Al, Ga, Sc, In) with values of 95Scm−1 or 180Scm−1[59], although the values obtained in this work (42.55–125.47Sm−1) may present a mixed behavior of ionic–electronic conductivity with the temperature as found for the perovskites of La0.9Sr0.1Al0.9Mg0.1O2.9[60] and La0.9Sr0.1Sc0.9Mg0.1O2.9 (50–130Sm−1) [61]. To confirm this assumption, an analysis of the charge carriers as a function of the Pr amount was performed by the Hall effect method combined with a resistivity measurement at room temperature. It is important to note that silver dye was used during the measurements to ensure contact in the system with a cloverleaf configuration.
Electrical measurements of La0.7−xPrxCa0.3MnO3 perovskites at room temperature.
| La(0.7−x)Pr(x)Ca0.3MnO3(x) | Charge carrier density(η, m−3) | Mobility (μ)(m2V−1s−1) | ρ(Ωm) | Conductivity(Sm−1) | ρ(Ωm) | Conductivity(Sm−1) |
|---|---|---|---|---|---|---|
| Hall effect | Four-point Kevin probe | |||||
| 0.35 | −5.54E+12 | 4.42E−05 | 0.0462 | 21.64 | 0.0235 | 42.55 |
| 0.42 | 1.10E+12 | 8.79E−05 | 0.0425 | 23.53 | 0.0134 | 74.62 |
| 0.49 | 1.09E+13 | 7.59E−05 | 6.09E−03 | 164.00 | 9.66E−03 | 103.51 |
| 0.53 | −4.12E+13 | 8.87E−05 | 5.41E−03 | 184.84 | 7.97E−03 | 125.47 |
| 0.56 | −4.19E+13 | 6.05E−05 | 5.80E−03 | 172.41 | 9.36E−03 | 106.83 |
| 0.63 | 1.64E+14 | 1.94E−04 | 7.52E−3 | 132.97 | 0.0105 | 95.23 |
| 0.70 | −1.07E+19 | 3.08E−05 | 0.0156 | 64.10 | 9.82E−03 | 101.83 |
The Hall effect is used to determine the charge carrier density (η), depending on the sign, the electrode can be ascribed as n or p; that is, acceptor of electrons (η>0) or increase the electrons for conduction, donor of electrons (η<0). The Hall effect results indicate that electrical conductivity can be generated in both p and n-type carriers (Table 3); that is, they can conduct ionically and electronically in a mixed manner. On the other hand, the electrical characterization using the Van der Pauw technique showed a similar trend to that obtained by the four-point method, and the differences between them may be related to the characteristics of each technique. Thus, the samples that presented the highest electrical conductivity are samples with an atomic composition of 0.53 (184.84Sm−1) and acceptor character. The correlation with the electrical properties suggests that a combination of the Pr amount and the pure phase obtained in the perovskite synergistic influences the electrical performance. It is also clear that higher Pr amounts favored electron transport, which can positively be used in the performance of cathode for SOFCs.
ConclusionsThis paper reports the production of La0.7−xPrxCa0.3MnO3 perovskites by lanthanum substitution through the simple Pechini sol–gel method. Several compositions were analyzed (x=0.35, 0.49, 0.53, 0.56, 0.63 and 0.7) to determine the effect of Pr amount on the structural, morphological, and electrical parameters as a potential application in SOFCs. All the synthesized La0.7−xPrxCa0.3MnO3 perovskites match the orthorhombic crystal system and the Pnma space group. The substitution of lanthanum by atomic percentage of Pr caused the formation of small peaks corresponding to secondary phases of Pr and La oxides (x=0.35, 0.49, 0.56 and 0.63), while the pure phases were obtained with a partial substitution of Pr at 0.53 and complete substitution to obtain Pr0.7Ca0.3MnO3 perovskites, which was confirmed by Rietveld refinement. Depending on the size of the crystallites, the cell volume tends to decrease with the atomic percentage of Pr. The addition of Pr in the perovskite is characterized by an orthorhombic Jahn-Teller distortion with a clear elongation in the b-axis. The Mn3+/Mn4+ atomic ratios ranged from 2.28 (x=0.35) to 3.69 (x=0.53), promoting the hole doped system instead of the electron system. Although the electrical conductivity at room temperature is low compared to previous work, the substitution of Pr by this method promotes mixed ionic–electronic conduction. The sample that showed the highest electrical conductivity is the one with an atomic weight of x=0.53 (184.84Sm−1) with an acceptor character, which can potentially be used as a cathode in SOFCs for energy production.
The authors are thankful for the financial support provided by Instituto Politécnico Nacional through the SIP projects: 2023-0839, 2023-0842, 2023-0348, 2023-1159, 2023-1159 and COFAA. This work has also been funded by CONACYT through project CB-2015-252181 and SNII.