Retinitis pigmentosa (RP) is a group of inherited diseases that lead to degeneration of the retina and decreased vision. The World Health Organization reports around 1,300 million people affected by some type of visual impairment worldwide. The prevalence is 1 in every 4000 inhabitants and it is the first cause of blindness of genetic origin, frequent in men with a percentage of 60% and 40% in women. There is a lack of information on this pathology in the world, mainly on the existing treatments for this disease, so this bibliographic review aims to update the existing or under-study treatments and inform the limitations of each of these therapies. This review of scientific literature was carried out by consulting databases such as PubMed and Web of science, the search will be limited to articles from the years 2018–2022.
There are several types of therapy in studies: gene therapy, transcorneal electrical stimulation, use of neuroprotectors, optogenic therapy, stem cell transplants and oligonucleotide therapy, which will be discussed in this article, both their benefits and the existing barriers in each treatment experimental. In conclusion, each of these therapies promises a viable treatment in the future for selective groups of people with retinitis pigmentosa, however, some therapies have shown benefit at the beginning of the disease, losing their efficacy in the long term
La retinosis pigmentaria (RP) es un grupo de enfermedades de origen hereditario que produce la degeneración de la retina y una disminución de la visión. La Organización Mundial de la Salud reporta alrededor de 1.300 millones de personas afectadas por algún tipo de deficiencia visual a nivel mundial. La prevalencia es de 1 en cada 4000 habitantes y es la primera causa de ceguera de origen genético, frecuente en los varones con un porcentaje del 60% y el 40% en mujeres. Existe falta de información sobre esta patología en el mundo, principalmente sobre los tratamientos existentes para esta enfermedad, por lo que esta revisión bibliográfica tiene como objetivo actualizar los tratamientos existentes o en estudio e informar las limitaciones que tiene cada una de estas terapias. Se realizó esta revisión de literatura científica mediante la consulta en bases de datos como PubMed y Web of sciense, la búsqueda será limitada por artículos de los años 2018 al 2022.
Existen varios tipos de terapia en estudios: terapia génica, estimulación eléctrica transcorneal, uso de neuroprotectores, terapia optogénica, trasplantes de células madres y terapia con oligonucleótidos, de los que se hablará en este artículo, tanto sus beneficios y las barreras existentes en cada tratamiento experimental. En conclusión, cada una de estas terapias promete un tratamiento viable en el futuro para grupos selectivos de personas con retinosis pigmentaria, sin embargo, algunas terapias han demostrado beneficio al inicio de la enfermedad, perdiendo su eficacia a largo plazo.
Retinitis pigmentosa (RP) is a group of inherited diseases that cause degeneration of the retina and decreased vision.1 This disease presents in the second and third decade of life with progressive loss of bilateral peripheral visual field, followed by loss of central vision that progresses to total blindness.2
The World Health Organisation (WHO) reported that 1.3 billion people worldwide are affected by some form of visual impairment.3 The prevalence is 1 in 4000 people and the age of onset ranges from childhood to adulthood.4 It is the leading cause of blindness of genetic origin and is more frequent in men with a percentage of 60%, while 40% corresponds to women.5
RP belongs to the group of hereditary retinal diseases, as it can be inherited in an autosomal dominant, autosomal recessive, X-linked or mitochondrial manner.3 In recent years, new altered genes have been identified, which explains that this pathology is not a single disorder but a group of entities that are genetically distinct but have phenotypic similarities.4 RP has been found to affect more than 300 genes, which is why it is difficult to find its pathogenesis and an effective treatment for this disease.6
The age of onset is considered from the time the patient reports the first visual symptoms, which may be in childhood or adulthood.7,8 The first symptom is nyctalopia or loss of night vision and loss of peripheral visual field.2 This is followed by damage to the cones, which leads to loss of visual acuity and may lead to total blindness.9
Diagnosis of this condition is based on clinical presentation and fundus examination. However, RP is confirmed by electrophysiology and genetic testing. At the time of diagnosis, cone and rod reduction may be evident.10 In addition, it should be noted that patients lose 10–17% of cone function annually.11 Currently, wide-field fundus fluorescence is one of the most widely used tests to display the full extent of the pigment epithelium in the retina and retinal atrophy.12 Patients with RP often show a ring of hyperfluorescence in the macula that defines the area of viable central retina from the larger area of atrophied peripheral retina.13
This study aims to investigate treatment updates for patients with retinitis pigmentosa, discuss the clinical criteria for the diagnosis of retinitis pigmentosa, discuss the types of treatment and compare existing treatments for this condition.
MethodologyThis literature review is descriptive in approach, compiling scientific articles in English, French and Spanish from different databases such as PubMed and Web of Science. The Clinical Trials page was also used, which shows all the experimental studies existing to date. The search process was carried out using key words, in Spanish Descriptores en Ciencias de la Salud (DeCS) "Terapias en investigación", "Retinitis pigmentosa", "Tratamiento", in English MESH terminology was used, which were "Treatment", "Retinitis Pigmentosa", "Therapies investigational", such terms were combined with Boolean operators "AND", "OR", "NOT". We included literature and systemic reviews, field studies, published in English and Spanish during the last five years and clinical trials completed or promising viable future therapies, while excluding clinical trials that do not yet have results.
ResultsA total of 382 articles were obtained. Most of them were clinical phase studies, experimental trials, literature reviews and case reports. Due to repetition of content among some articles, non-specific files, incomplete or non-specific clinical trials and studies with unfavourable results, 45 were selected for discussion in the present review.
DiscussionRP is a progressive disease which debuts with visual disturbances.14 There are no specific signs or symptoms, as very mild symptoms are present at the onset of the disease, which is why RP can go unnoticed or is diagnosed in advanced stages.14 The disease can present as an isolated, sporadic, autosomal dominant, autosomal recessive, X-linked (Xr), rarely mitochondrial or with a digenic cause.15
The early manifestations of signs and symptoms of this disease vary according to heredity and genotype, but in most cases at the time of diagnosis the electroretinogram is already highly altered.15 Early in the disease, retinal functions are mildly impaired, meaning that the patient has good, sometimes complete visual acuity and discrete colour vision.15 To assess these, a detailed refraction and visual acuity test as well as a colour test (desaturated Lanthony panel D-15 test) are required. As a first diagnostic clue, a defect in colour vision along the blue-yellow axis is usually found in the retina.15
Retinitis pigmentosa does not have a treatment allowing complete recovery of vision, although there are currently several studies and clinical trials being developed to help people with this pathology.16 Current research is focused on discovering treatments that provide neuroprotection, which can slow or halt the progression of the disease and prolong functional vision.16 To test and observe the therapeutic efficacy of experimental treatments, it is important to assess the severity of the disease before and after treatment.16 Tests that can be used to monitor the course of the disease are clinical assessment and visual acuity, although Goldman visual field (GVF) and electroretinography (ERG) can also be used16 as well as fundus autofluorescence because it is based on the detection of fluorophores which are physiologically and pathologically present mainly in photoreceptors and retinal pigment epithelium (RPE) to map the metabolic profile of the fundus.17 It is a non-invasive imaging modality that is used in clinical practice to non-invasively map changes at the level of the retinal pigment epithelium/photoreceptor complex and alterations in macular pigment distribution, making it important for visualising the progression of RP.17
As retinitis pigmentosa is known to be caused by several genetic mutations, gene therapy treatments must be individualised for each specific genetic mutation.14 Previously, treatment for these patients was limited to supportive care, including vitamin A supplementation and protection from sunlight, among others. Clinical trials have been conducted to determine the benefit of vitamin A and vitamin E which failed to demonstrate beneficial effects in these patients.14 Thus, for most patients with RP, there is currently no cure and no effective treatment to delay or halt the progression of the disease.14
Retinal degeneration inhibitor drugsAfter reviewing several studies, the use of Voretigene neparvovec-rzyl (Table 1) has been shown to be useful for the treatment of RP with mutated RPE65, as it has shown favourable and visible results in patients with this specific mutated gene.18 As a consequence of these trial results, neparvovec-rzyl is now on the market.18 On the other hand, 9-cis-retinol acetate, which is in phase 3 trials, and 9-cis-β-Carotene, which is approved by the US FDA, also have promising results due to the fact that the union of 9-cis-retinol with opsin forms isorhodopsin, regenerating the 11-cis-retinal visual chromophore, taking into account that the absence of this chromophore causes severe dysfunction of cones and rods leading to retinal degeneration. Likewise, 9-cis-β-carotene is converted to 9-cis-retinol in the retina, and follows the same procedure as mentioned above.19 In this way it prevents visual defects and regenerates the visual pigment.19
Experimental retinitis pigmentosa therapies.
| Author | Year | Title | Sample | Type of study | Results |
|---|---|---|---|---|---|
| Goureau O, Orieux G.26 | 2020 | New approach therapeutic for retinitis pigmentary Transplantation of stem cell-derived photoreceptors | – | Bibliographic Review | Transplantation of retinal slices derived from retinal organoids could lead to functional restoration. However, the presence of retinal cells other than the grafted photoreceptors, as well as the organisation of these photoreceptors, hinders their ability to reconnect in the host tissue and restore visual function. |
| Giacalone J, et al.29 | 2019 | Development of a molecularly stable gene therapy vector for the treatment of RPGR-associated X-linked retinitis pigmentosa. | – | Experimental study | RPGR is the most affected gene among people with retinitis pigmentosa, so a molecularly stable therapeutic construct was devised to clone RPGR ORF15 and avoid mutations. |
| Gauvain G, et al.25 | 2021 | Optogenetic therapy: high spatio-temporal resolution and pattern discrimination compatible with vision restoration in non-human primates | 18 primates (macaca fascicularis) | Experimental study | Increased efficacy was demonstrated for both the mutated AAV capsid, AAV2.7m8, and the ChR-tdT fusion protein in primates. Thus, intravitreal injection of AAV2.7m8-ChR-tdT is expected to effectively transduce the retina also in blind patients. |
| Sanchez A, et al.23 | 2018 | GSK-3 modulation provides cellular and functional neuroprotection in the rd10 mouse model of retinitis pigmentosa. | – | Experimental study | Application of the GSK-3 inhibitor VP3.15 showed a neuroprotective effect on photoreceptor cells and enhanced vision and decreased the expression of neuroinflammatory genes in the retina. |
| Isiegas C, et al.20 | 2018 | Intravitreal injection of proinsulin-loaded microspheres Photoreceptor cell death and vision loss in rd10 mouse model of retinitis pigmentosa | – | Experimental study | Retinal degeneration in the rd10 mouse was slowed by a single intravitreal injection of hPI-PLGA-MS. Recombinant human proinsulin elicited a rapid and effective neuroprotective effect. |
| Castro A, et al.30 | 2021 | Gene correction restores phagocytosis in retinal pigment epithelium derived from retinitis pigmentosa: human induced pluripotent stem cells | – | Experimental study | Differentiation of patient-derived, genetically corrected pluripotent stem cells from a patient with a p.Ser331Cysfs*5 mutation showing MERTK protein deficiency was demonstrated and re-established. Reversal of the lost phagocytic function of retinitis pigmentosa was also achieved. This is the first study in this field. |
| Russell S, et al.18 | 2018 | Voretigene neparvovec-rzyl for the treatment of retinal dystrophy associated with the biallelic RPE65 mutation retinal dystrophy | – | Experimental study | An improvement in light sensitivity and navigation ability was demonstrated in subjects with hereditary retinal dystrophies associated with the RPE65 mutation. This significant improvement occurred within 30 days of injection and has been durable for 4 years to date. |
| Hussain RM, Gregori NZ, et al.19 | 2018 | Pharmacotherapy of retinal diseases with modulators of the visual cycle | – | Literature review | 9-cis-retinyl acetate (zuretinol) and 9-cis-β-Carotene (dunaliella bardawil algae) bypass the inherited defects of RPE65 and LRAT in order to regenerate the 11-cis-retinal visual chromophore, as its absence causes severe cone and rod dysfunction leading to retinal degeneration. |
| Xue KM, Maclaren RE.27 | 2020 | Antisense oligonucleotide therapeutics in clinical trials for the treatment of inherited retinal diseases. | – | Phase II clinical trial | Phase I/II clinical trials have demonstrated a good safety profile and feasible pharmacokinetics of antisense oligonucleotides administered intravitreally. |
| Pierdomenico J, Scholz R, et al.31 | 2018 | Neuroprotective effects of FGF2 and Minocycline in two animal models of hereditary retinal degeneration. | – | Experimental study | IVI of CNTF, PEDF or FGF2 improved photoreceptor outer segment morphology, but only FGF2 rescued a significant number of photoreceptors. Minocycline treatment reduced microglial activation and migration and resulted in significant photoreceptor rescue. Thus, the combined treatment had better results. |
| Anonymous28 | 2021 | An open-label extension study to assess the safety and tolerability of QR-421a in subjects with retinitis pigmentosa (HELIA) | 21 patients | Phase II clinical trial | Evaluates the safety, tolerability and efficacy of QR-421a (ultevursen) in 21 patients with RP due to mutations in exon 13 of the USH2A gene.In these patients, a dose of 180 ug of QR-421 was administered in the contralateral eye and after 6 months a further 60 ug will be administered.This study was not completed. |
| Anonymous32 | 2018 | Study to evaluate the safety and tolerability of QR-421a in subjects with RP due to mutations in exon 13 of the USH2A gene (Stellar). | 20 patients | Phase II clinical trial | QR-421a administered as a single intravitreal injection is safe and well tolerated. Benefit was seen in relevant outcome measures appropriate for participants' disease severity, including retinal sensitivity, visual field and retinal structure. |
| Seungbum Kang, Henri Lorach33 | 2019 | Laser therapy of the retina preserves photoreceptors in a rodent model of MERTK-related retinitis pigmentosa. | 30 rats | Experimental study | Photocoagulation resulted in long-term preservation of photoreceptors (morphologically and functionally) and the magnitude of the benefit depended on the density of the laser pattern. Eyes treated with a dot spacing of 1.5 showed the best morphological and functional preservation during the 6 months follow-up. Eyes treated with SRT showed short-term morphological preservation, but no functional benefit. |
| Anonymous22 | 2022 | Dose escalation study to assess the safety/tolerability and efficacy of EA-2353 in subjects with retinitis pigmentosa. | 14 patients | Phase I/IIa clinical trial | The results will be published in 2025. |
| Henry Ho-Lung Chan, Hang-I Lam, et al.24 | 2019 | Delaying cone degeneration in retinitis pigmentosa by 12-month treatment with Lycium barbarum supplementation. | 42 patients | Phase II clinical trial | The treatment group showed less reduction in visual acuity compared to the placebo group after the intervention at both 6 months and 12 months. |
GSK-3, glycogen synthase kinase 3 enzyme; hPI-PLGA-MS, biocompatible and biodegradable poly-lactic acid-glycolic acid microspheres; AAV, adeno-associated virus; IVI, intravitreal injection; CNTF, ciliary neurotrophic factor; PEDF, pigment epithelium-derived factor; FGF2, basic fibroblast growth factor; QR-421, Ultevursen; SRT, retinal pigment epithelium targeted therapy.
There are also intravitreal injections (Table 1), which, like the one mentioned above, are studies still in the experimental phase.20 This injection contains biodegradable microspheres of poly (lactic co-glycolic) acid loaded with proinsulin20 which has been shown to significantly decrease cell death during neuronal development, i.e., preserving the structure and function of cones and rods, as well as their contacts with postsynaptic neurons, which has been shown to delay retinal degeneration in mice. These results indicate that this treatment could be used in the future as a neuroprotectant.20
Stem cell replacementThis treatment involves the transplantation of induced stem or progenitor cells into the retina, which can regenerate the layer of functioning retinocytes.21 There is the study of the molecule EA-2353, which seems to be the best experimental treatment in progress so far, as the application of this drug includes all people with retinitis pigmentosa, i.e. it does not exclude patients with different affected genes.21 The function of this molecule is to selectively activate endogenous retinal stem and progenitor cells, which can differentiate into photoreceptors and preserve or restore vision.21 Clinical trials are underway with 14 patients in 4 cohorts: low dose, medium dose, high dose and maximum tolerated dose.22 It will be administered intravitreally in the eye with the worst vision of the patient according to the best corrected visual acuity.22 The results will be visible in 2025.
Neuroprotection for the retinaAnother therapy that appears to be the next viable therapy is modulation of the enzyme glycogen synthase kinase 3 (GSK-3) (Table 1).23 This enzyme is a serine/threonine kinase, which is mainly expressed in the CNS and plays a key role in regulating the balance between proinflammatory and anti-inflammatory cellular responses. In this study, a competitive inhibitor of GSK-3, iminothiadiazole family VP3.15, was added, which was shown to provide protection in photoreceptor cells and decrease the expression of neuroinflammatory genes in the retina; however, this study is still in the experimental phase and has only been carried out in rats.23
Lycium barbarum, used as a neuroprotectant in 42 patients with RP, was also found, administered at a daily oral dose of 2 packets/day, each containing 5 g net weight, to each patient for 12 months.24 For the analysis, the best eye of each subject at the first visit was selected.24 The results of this study showed that a 12-month treatment of RP patients with Lycium barbarum L. was able to preserve visual acuity and macular structure. Its neuroprotective effect is thought to delay or minimise the deterioration of central visual function (Table 1).24
Optogenetic therapiesOptogenetic therapies are also being tested (Table 1), using adeno-associated virus mutated in fusion with the microbial opsin ChR-tdt, which caused increased light sensitivity in primates with expression in more cells and higher sensitivity in retinal ganglion cells.25 This demonstrates effective transduction of the ganglion cell layer in the perifoveal retinal ring in primates with retinal degeneration. The results of this study will enable clinical trials for the application of this treatment in the future.25
Photoreceptor transplantationThis treatment consists of determining the culture conditions that would allow human embryonic stem cells and induced pluripotent stem cells to adopt a neuroectodermal identity followed by the identity of the ocular territory before moving on to a neural retina or the retinal pigment epithelium lineage; the last stage was aimed at differentiating the multipotent retinal progenitors that make up the neural retina into photoreceptors.26
Stem cell-derived photoreceptor transplantation (Table 1) can restore photoreceptor function, however, there are limitations due to the presence of retinal cells other than the grafted photoreceptors and their organisation, which hinders reconnection in the host tissue and thus visual restoration.26
Antisense oligonucleotidesAnother type of study utilises antisense oligonucleotides (Table 1), which are designed to specifically reduce the level of a pathogenic messenger RNA, alter its splicing pattern or block translation.27 This substance could block the translation of disease-causing genes, thus slowing down the progression of the disease.27 This treatment would complement viral vector-mediated gene augmentation, which is often limited by the size of the transgene. This study has demonstrated safety, feasible durability and early efficacy. It should be remembered that this therapy would benefit only patients with altered RHO, i.e., approximately 25% of patients with retinitis pigmentosa.27
Another clinical trial, aimed at patients with a mutation in exon 13 in the USH2A gene, included 21 patients who were administered 180 ug of QR-421 in the contralateral eye, and a repeat dose of 60 ug every 6 months.28 Adverse effects in this study were conjunctival hemorrhage, eye pain and conjunctival hyperemia. This study was not completed.28 The participants in the HELIA study had participated in the previous Stellar study, in which three different groups were administered single doses of 50, 100 and 200 ug, in which improvements in retinal sensitivity, visual field and retinal structure were demonstrated in the first 3 months and analysed with full threshold test, dark-adapted chromatic perimetry and optical coherence tomography of the photoreceptor ellipsoid area respectively.28 However, treatment data and response have not been reported so far.28
Laser therapyOn the other hand, we also have the Mer receptor tyrosine kinase (MERTK) gene mutation, which accounts for approximately 1–2.5% of RP cases.34 This gene is responsible for phagocytosis of detached photoreceptor outer segments (POS) by the retinal pigment epithelium (RPE).35 Thus, mutations in this gene impair phagocytosis and cause the accumulation of detached POS and subsequent formation of subretinal debris which, over time, results in the gradual loss of photoreceptors.35
It is believed that, although mutations in the MERTK gene impair phagocytosis, there is still minimal phagocytic activity of RPE cells.33 Therefore, deletion of part of the photoreceptor cells would result in less daily POS shedding.33 The RPE cells with lower phagocytic capacity could then sustain the lower POS recycling load to prevent its accumulation, thus delaying photoreceptor degeneration.33
Photocoagulation was used which resulted in long-term preservation of photoreceptors, with morphologically and functionally visible results, and the magnitude of the benefit depended on the density of the laser pattern.36 Eyes treated with a spot size of 1.5 showed better morphological and functional preservation during the 6 months follow-up.33 Eyes treated with selective RPE treatment showed short-term morphological preservation, but no functional benefit.33 Eyes treated with non-retinal damaging treatment showed no preservation benefit from the treatment.33 It must be emphasised that there must be an optimal laser treatment density that causes minimal acute damage but maximises long-term photoreceptor preservation because, if too many photoreceptors are removed, the amount of daily POS detachment would be less, but overall retinal function would be compromised.36 On the other hand, if not enough photoreceptor cells are removed, the treatment would not work.36
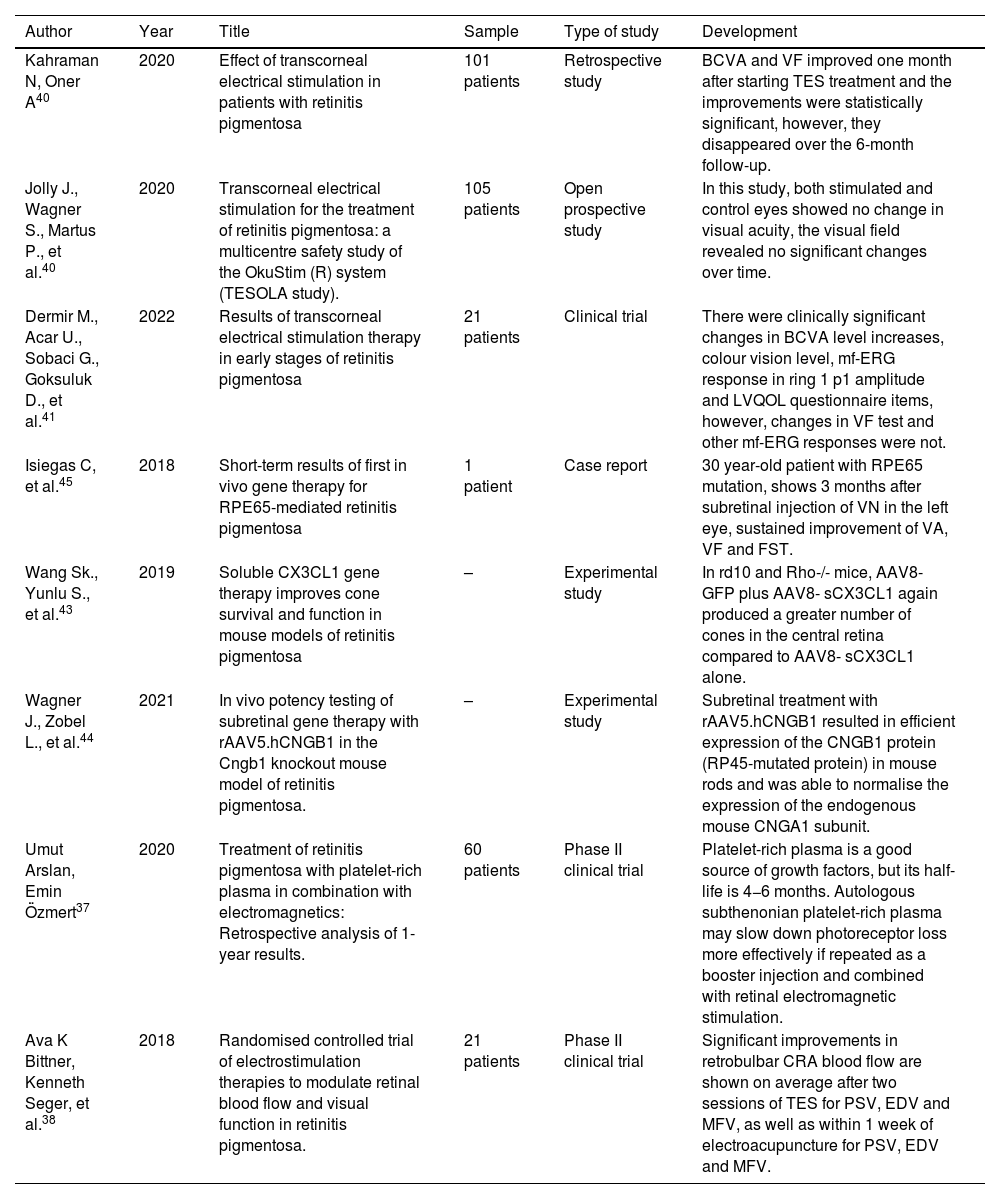
Transcorneal stimulation vs. gene therapyIn one study, transcorneal stimulation with the application of autologous platelet-rich plasma was used.37 To obtain this plasma, 20 ml of blood was collected from the antecubital veins, transferred into 2 sterile 10 ml citrated tubes, and the plasma was separated with a refrigerated centrifuge at 4.0 C° for 8 min at 2500 rpm centrifugation.37 The lower 1/3 of the upper plasma was drawn into a sterile 2.5 ml syringe as a growth factor rich section. The 1.5 ml autologous platelet-rich plasma (PRP) solution was then injected into the subtenonian space under topical anaesthesia.37
On the other hand, electromagnetic stimulation aids in increasing retinal blood flow.38 This study involves stimulating the retina and visual pathways with an electromagnetic field strength of 2000 milligauss, a frequency of 42 Hz and a duration of 30 min.37 This stimulation is performed prior to the application of platelet-rich plasma.37 Another study on electrical stimulation to modulate blood flow and visual stimulation provided evidence of increased blood flow within retinal vessels that may be associated or collateral to improvements in visual function following electroacupuncture or TES.38 In the same study, three patients with retinitis pigmentosa who received 3–6 cycles of TES treatment were documented to assess the duration of the effect of this therapy.39 Repeated improvements in central visual function could be seen between four and seven weeks after treatment, with regression to baseline values between treatment cycles, but no significant decreases in vision beyond baseline values.39 It was also found that this therapy helped to reduce macular lesions (geographic atrophic lesion) in the patients.39 This study did not demonstrate vision loss during the 4 years of follow-up, but as long as treatment is routinely performed.39
The results of transcorneal electrical stimulation as well as gene therapy involve several limitations and disadvantages since,according to studies, patients who are treated with transcorneal electrical stimulation improve after a few weeks of treatment, although these effects disappear as the months pass if they do not receive the stimulation routinely (Table 2).40–42 Similar results are found in transcorneal electrical stimulation associated with the administration of autologous platelet-rich plasma, where it was shown that rich plasma is a good source of growth factor, the drawback being its half-life is 4–6 months, so this therapy could be useful if plasma injections were repeated every 6 months and combined with retinal electromagnetic stimulation (Table 2).37
Results between transcorneal electrical stimulation and gene therapy.
| Author | Year | Title | Sample | Type of study | Development |
|---|---|---|---|---|---|
| Kahraman N, Oner A40 | 2020 | Effect of transcorneal electrical stimulation in patients with retinitis pigmentosa | 101 patients | Retrospective study | BCVA and VF improved one month after starting TES treatment and the improvements were statistically significant, however, they disappeared over the 6-month follow-up. |
| Jolly J., Wagner S., Martus P., et al.40 | 2020 | Transcorneal electrical stimulation for the treatment of retinitis pigmentosa: a multicentre safety study of the OkuStim (R) system (TESOLA study). | 105 patients | Open prospective study | In this study, both stimulated and control eyes showed no change in visual acuity, the visual field revealed no significant changes over time. |
| Dermir M., Acar U., Sobaci G., Goksuluk D., et al.41 | 2022 | Results of transcorneal electrical stimulation therapy in early stages of retinitis pigmentosa | 21 patients | Clinical trial | There were clinically significant changes in BCVA level increases, colour vision level, mf-ERG response in ring 1 p1 amplitude and LVQOL questionnaire items, however, changes in VF test and other mf-ERG responses were not. |
| Isiegas C, et al.45 | 2018 | Short-term results of first in vivo gene therapy for RPE65-mediated retinitis pigmentosa | 1 patient | Case report | 30 year-old patient with RPE65 mutation, shows 3 months after subretinal injection of VN in the left eye, sustained improvement of VA, VF and FST. |
| Wang Sk., Yunlu S., et al.43 | 2019 | Soluble CX3CL1 gene therapy improves cone survival and function in mouse models of retinitis pigmentosa | – | Experimental study | In rd10 and Rho-/- mice, AAV8-GFP plus AAV8- sCX3CL1 again produced a greater number of cones in the central retina compared to AAV8- sCX3CL1 alone. |
| Wagner J., Zobel L., et al.44 | 2021 | In vivo potency testing of subretinal gene therapy with rAAV5.hCNGB1 in the Cngb1 knockout mouse model of retinitis pigmentosa. | – | Experimental study | Subretinal treatment with rAAV5.hCNGB1 resulted in efficient expression of the CNGB1 protein (RP45-mutated protein) in mouse rods and was able to normalise the expression of the endogenous mouse CNGA1 subunit. |
| Umut Arslan, Emin Özmert37 | 2020 | Treatment of retinitis pigmentosa with platelet-rich plasma in combination with electromagnetics: Retrospective analysis of 1-year results. | 60 patients | Phase II clinical trial | Platelet-rich plasma is a good source of growth factors, but its half-life is 4−6 months. Autologous subthenonian platelet-rich plasma may slow down photoreceptor loss more effectively if repeated as a booster injection and combined with retinal electromagnetic stimulation. |
| Ava K Bittner, Kenneth Seger, et al.38 | 2018 | Randomised controlled trial of electrostimulation therapies to modulate retinal blood flow and visual function in retinitis pigmentosa. | 21 patients | Phase II clinical trial | Significant improvements in retrobulbar CRA blood flow are shown on average after two sessions of TES for PSV, EDV and MFV, as well as within 1 week of electroacupuncture for PSV, EDV and MFV. |
BCVA, Mean visual acuity; VF, Visual field; TES, Transcorneal electrical stimulation; BCVA, Best corrected visual acuity; mf-ERG, multifocal electroretinography; LVQOL, Low vision quality of life; VN, Voretigene neparvovec-rzyl; VA, Visual acuity; FST, Full field stimulus threshold test; Rho−/−, Rhodopsinnull; RP45, Retinitis pigmentosa type 45; TES, transcorneal electrical stimulation; CRA, colour Doppler of the central retinal artery; PSV, mean peak systolic velocity; EDV, end-diastolic velocity; MFV, mean flow velocity.
Gene therapy, on the other hand, has good long-term results, but most of these therapies are for patients with specific altered genes, such as RPE65, which corresponds to less than 10% of patients with this pathology, so these therapies are limited (Table 2).20,43,44
ConclusionsAfter this review, it can be concluded that experimental treatments for retinitis pigmentosa may be useful in the future for people with this pathology, although each of them have certain limitations. It must also be remembered that these therapies are still under study, so the administration of these treatments and the use of the therapies will have to wait for some time before they are certified and can be administered. All treatments that are still in experimental studies promise to be viable and useful in the future, but it must be kept in mind that gene therapies are more limited because they are made for patients with specific genetic alterations. With respect to other therapies, they do not discriminate patients based on their genetic alteration, although their results have only been good at the beginning of treatment, losing their efficacy in the long term. There is one therapy that promises to be viable for patients with RP regardless of which gene is affected, i.e., the application of the EA-2353 molecule but, like all the trials mentioned in this review, it is still in clinical trials.
FundingSelf-financing
Conflict of interestNo conflicts of interest were declared by the authors.
I thank God and my parents for not leaving me alone at this stage of my life and for supporting me from a distance with their words and advice.