To evaluate the presence of SARS-COV-2 specific IgA and IgG antibodies in tears of unvaccinated and anti-COVID-19 vaccinated subjects with previous history of SARS-COV-2 infection. To compare results in tears with those in saliva and serum and correlate with clinical data and vaccination regimens.
MethodsCross-sectional study including subjects with a previous history of SARS-CoV-2 infection, both unvaccinated and vaccinated against COVID-19. Three samples were collected: tears, saliva and serum. IgA and IgG antibodies against S-1 protein of SARS-CoV-2 were analyzed with a semi-quantitative ELISA.
Results30 subjects, mean age 36.4 ± 10, males 13/30 (43.3%) with history of mild SARS-CoV-2 infection were included. 13/30 (43.3%) subjects had received a 2-dose regimen and 13/30 (43.3%) a 3-dose regimen of anti-COVID-19 vaccine, 4/30 (13.3%) subjects were unvaccinated. All the participants with full anti-COVID-19 vaccination (2-or 3-doses) presented detectable anti-S1 specific IgA in all three biofluids, tears, saliva and serum. Among unvaccinated subjects, specific IgA was detected in 3/4 subjects in tears and saliva, whereas IgG was not detected. Considering IgA and IgG antibodies titers, no differences were observed between the 2- and 3-dose vaccination regimen.
ConclusionsSARS-CoV-2-specific IgA and IgG antibodies were detected in tears after mild COVID-19, highlighting the role of the ocular surface as a first line of defense against infection. Most naturally infected unvaccinated individuals exhibit long-term specific IgA in tears and saliva. Hybrid immunization (natural infection plus vaccination) appears to enhance mucosal and systemic IgG responses. However, no differences were observed between the 2- and 3-dose vaccination schedule.
Evaluar la presencia de anticuerpos IgA e IgG específicos del SARS-CoV-2 en lágrima de sujetos no vacunados y vacunados contra la COVID-19 con antecedentes de infección SARS-CoV-2. Correlacionar los resultados en lágrima con los de saliva y sangre, datos clínicos y regímenes de vacunación.
MétodosEstudio transversal que incluyó sujetos con antecedentes de infección SARS-CoV-2, tanto no vacunados como vacunados contra la COVID-19. Se recogieron tres muestras: lágrima, saliva y sangre. Se analizaron IgA e IgG frente a S-1 SARS-CoV-2 con ELISA semicuantitativo.
Resultados30 sujetos, edad media 36,4 ± 10, varones 13/30 (43,3%) con historia de infección SARS-COV-2 leve. 13/30 (43,3%) habían recibido un régimen de 2 dosis y 13/30 (43,3%) un régimen de 3 dosis de vacunación anti-COVID-19, 4/30 (13,3%) no vacunados. Todos los sujetos con vacunación completa presentaron IgA detectable en los tres biofluidos. Entre los no vacunados, se detectó IgA en 3/4 sujetos en lágrima y saliva, mientras que no se detectó IgG. No se observaron diferencias entre la pauta de vacunación de 2 y 3 dosis según los títulos IgA-IgG.
ConclusionesAnticuerpos IgA e IgG del SARS-CoV-2 están presentes en lágrima de pacientes con antecedentes de COVID-19 leve, lo que destaca el papel de la superficie ocular como primera línea de defensa frente a la infección. La mayoría de los sujetos no vacunados presentaron IgA a largo plazo en lágrima y saliva. La inmunización híbrida (infección natural más vacunación) parece potenciar las respuestas IgG mucosas y sistémicas. No se observaron diferencias entre la pauta de 2 y 3 dosis.
Since the outbreak of the Coronavirus disease 2019 (COVID-19) pandemic, more than 599 million people have been infected, including over 6 million registered deaths worldwide according to the World Health Organization.1 In addition, more than 12 billions of anti-COVID-19 vaccine doses have been administered globally.1 The mucosa of the ocular surface is directly and indirectly exposed to many pathogens, including the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Consequently, the eye is an organ susceptible to infection, in which ocular mucosa may represent a first line of defense against the virus. SARS-CoV-2 has been detected in tears and conjunctival secretions of patients with COVID-19, pointing to the role of the ocular surface as a possible route of transmission and, consequently, its potential involvement in neutralizing the infection.2,3
Most research to date has focused on the detection of SARS-COV-2 in ocular secretions, rather than on the local immune response on the ocular surface against the virus itself. Given the current phase of the pandemic, the significant spread of SARS-COV-2 infection among the population worldwide and the progress of anti-COVID-19 vaccination have focused our attention on protective immunity against SARS-COV-2 reinfection following either infection or vaccination.
In this study, we have evaluated the presence of two classes of specific anti-SARS-COV-2 immunoglobulins, IgA and IgG, in tears of unvaccinated and anti-COVID-19 vaccinated subjects with previous history of SARS-COV-2 infection. In addition, specific IgA and IgG antibodies to SARS-COV-2 were assessed in two other biofluids, saliva and serum, and the results were correlated with clinical data and vaccination regimens.
Subjects and methodsSubjectsThis Cross-sectional study was approved by the hospital´s Clinical Research Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects.
The inclusion criteria were as follows: subjects over 18 years of age with a previous history of SARS-CoV-2 infection confirmed by real-time reverse transcription-reverse polymerase chain reaction (RT-PCR) from nasopharyngeal swabs. Those subjects with concomitant eye diseases, including ocular surface disease, glaucoma or any other ocular disease that required topical ocular treatments were excluded. Subjects undergoing ocular surgery in the previous 6 months or lacking consent to participate in the study were also excluded. Subjects’ demographic data, medical history, history of SARS-CoV-2 infection and vaccination regimen against COVID-19 were collected.
SamplesThree samples were collected from each patient: tears, saliva and blood (serum). For the tears sample, standard Schirmer test strips (DINA-HITEX, Czech Republic) were used. Schirmer strips were placed in the lower conjunctival sac between the middle and temporal thirds of the lower eyelid of both eyes without topical anesthesia. The strips were transferred to 600 mL of a 0.9% NaCl solution, the solution was centrifuged at 1000 rpm in centrifuge 5430R (Eppendorf AG, Germany) at 4 °C for 5 min and then the supernatant was collected. Saliva samples were collected in a sterile tube (Greiner Bio One, Spain), centrifuged at 300 g at 4 °C in centrifuge 5430R (Eppendorf AG, Germany) for 10 min and diluted in 0.9% NaCl (1:5, saliva:NaCl). Thirdly, serum samples were collected by venopunture employing dry tubes with gelose of 9 mL (Greiner Bio One, Spain) and centrifuged at 3500 rpm at 4 °C in centrifuge Kubota 5910 (Kubota Corporation, Tokyo) for serum collection. All three samples were collected on the same day and were stored at -80 °C until analysis.
Specific IgA and IgG anti-S1 of SARS-CoV-2 detectionA commercial semi-quantitative ELISA kit specific for the IgA and IgG antibodies against protein S1 of SARS-CoV-2 was used according to manufacturer’s instructions (SARS-CoV-2 IgA and IgG immunoassay, Euroimmun, Lübeck, Germany). Serum samples were analyzed at a 1:101 dilution in sample tampon present in the kit, while tear and saliva samples were analyzed adding directly 100 μL of the proceeded solution to each well. Samples were analyzed and interpreted according to the manufacturer’s instructions (negative: ratio <0.8; borderline: ratio ≥0.8–<1.1; positive: ratio ≥1.1). Borderline results were considered positive for analysis. The limit of detection for IgG was 8.5 RU/mL and for IgA was 6.9 RU/mL. For concentrations above the detection limit of the ELISA, the previous values were assumed for calculation purposes.
StatisticsStatistical analysis was performed using SPSS software for Windows (SPSS, Inc. Chicago, USA, version 25,0). Values are expressed as mean ± standard deviation (SD) and absolute frequency (n) and relative frequency (%) where appropriate. Normality was checked using the Kolmogorov-Smirnov test, followed by Levene's test to assess variance homogeneity. All data complied with a normal distribution. A simple classification analysis of variance (ANOVA) was applied to the data with homogeneity of variance. For the data without homogeneity of variance, a Kruskal-Wallis’s test was performed. Statistical significance was set at p < 0.05.
ResultsThe study included a total of 30 subjects with previous SARS-CoV-2 infection. Demographic and clinical characteristics of SARS-CoV-2 infection are summarized in Table 1. All participants were healthy subjects with history of mild COVID-19 according to WHO classification.4
Demographic and clinical characteristics of SARS-CoV-2 infection in the study subjects.
| N = 30 | ||
|---|---|---|
| Sex | ||
| Male (n, %) | 13 | 43.3 |
| Female (n, %) | 17 | 56.7 |
| Age (mean, SD) | 36.4 | 10.0 |
| SARS-CoV-2 infection: Clinical severity | ||
| Mild (n, %) | 30 | 100 |
| Moderate (n, %) | – | – |
| Severe (n, %) | – | – |
| Days from SARS-CoV-2 infection to sample collection (mean, SD) | ||
| COVID-19 vaccinated group | 93.8 | 35.9 |
| Unvaccinated group | 192.5 | 182.4 |
N: number of subjects; %: percentage; SD: Standard deviation.
The study included vaccinated and unvaccinated subjects against SARS-CoV-2 infection: 4/30 subjects (13.3%) unvaccinated against SARS-CoV-2; 13/30 (43.3%) had received 2 doses of anti-COVID-19 vaccine; and 13/30 (43.3%) had received 3 doses. Regarding the type of vaccine administered, the participants enrolled in the study received a vaccination regimen that included: (1) BNT162b2 COVID-19 mRNA Vaccine, Pfizer-BioNTech; (2) Spikevax (mRNA-1273), Moderna; (3) Vaxzevria (ChAdOx1-S), previously COVID-19 Vaccine AstraZeneca; (4) a combination with any of the three previous vaccines. Study participants had received different immunization protocols, which appear summarized in Table 2.
COVID-19 vaccination schedules of the study subjects.
| Number of COVID-19 vaccine doses | N | % |
|---|---|---|
| 0 (n, %) | 4 | 13.3 |
| 1 (n, %) | 0 | 0.0 |
| 2 (n, %) | 13 | 43.3 |
| 3 (n, %) | 13 | 43.3 |
| Mean COVID-19 vaccine doses, SD | 2.2 | 1.0 |
| Type of first dose vaccine | N | % |
| Spikevax® (Moderna) | 2 | 6.7 |
| Comirnaty® (Pfizer-BioNTech) | 22 | 73.3 |
| Vaxzevria® (Astrazeneca) | 2 | 6.7 |
| COVID-19 vaccination schedule | N | % |
| Moderna + Moderna | 1 | 3.3 |
| Pfizer + Pfizer | 9 | 30.0 |
| Astrazeneca + Astrazeneca | 2 | 6.7 |
| Moderna + Moderna + Pfizer | 1 | 3.3 |
| Pfizer + Pfizer + Moderna | 6 | 20.0 |
| Pfizer + Pfizer + Pfizer | 6 | 20.0 |
| Pfizer + Moderna | 1 | 3.3 |
| Days from first vaccine to sample collection (mean, SD) | 360.8 | 80.4 |
| Days from the last vaccine to sample collection mean, SD) | 185.2 | 122.7 |
N: number of subjects; %: percentage; SD: Standard deviation.
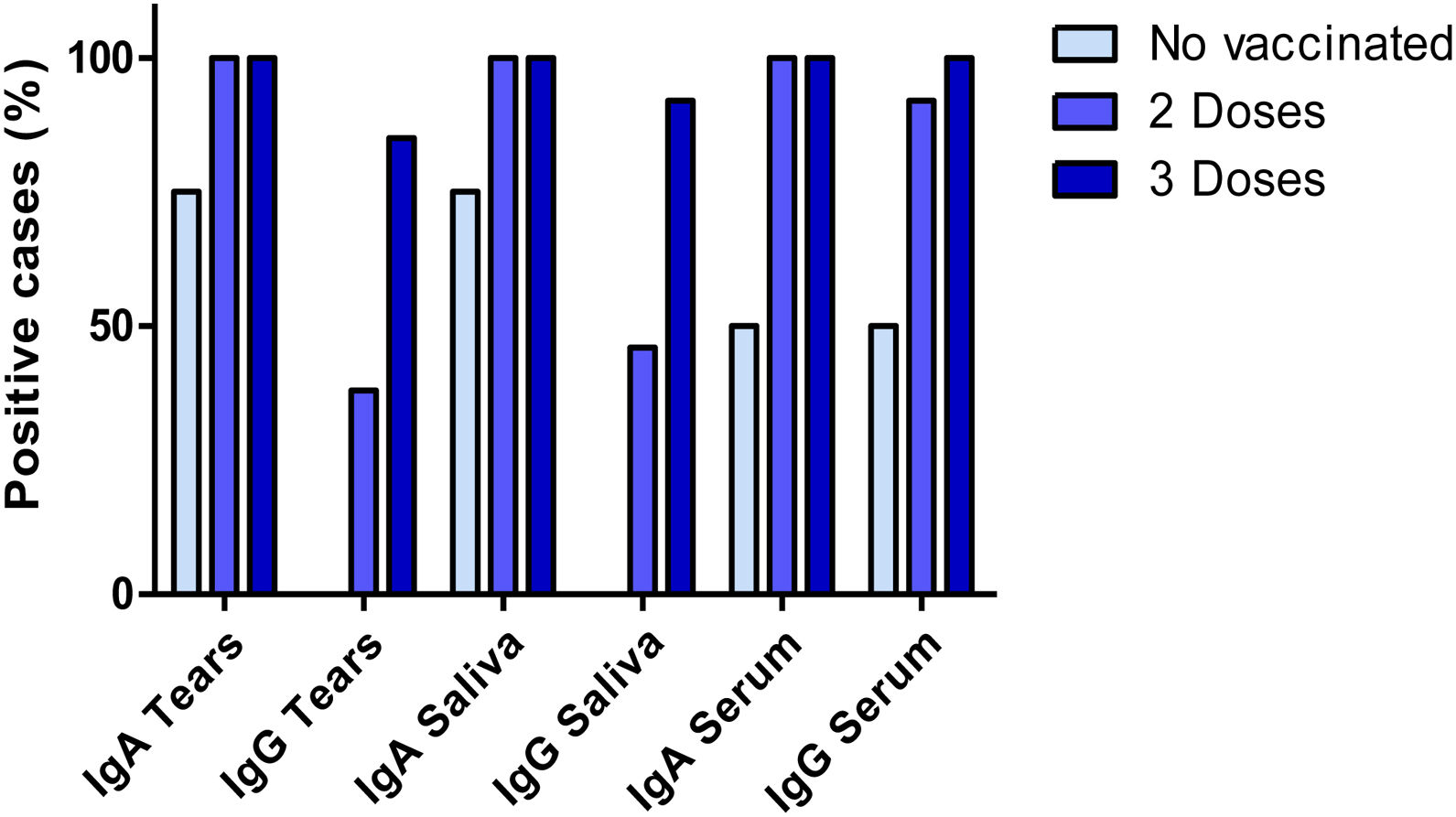
All the participants with full anti-COVID-19 vaccination (individuals with 2- and 3-doses) presented detectable antibody levels for anti-S1 specific IgA in all three biofluids, tears, saliva and serum. In the group of subjects who had received a 2 dose-regimen, we found positive IgG results in 38%, 46% and 92% of the subjects in tears, saliva and serum samples, respectively. In the group of subjects who received a 3-dose regimen, IgG results were positive in 85%, 92% and 100% of the subjects in tears, saliva and serum samples, respectively. On the other hand, all four unvaccinated individuals presented negative results for IgG in tears and saliva samples. Interestingly, IgA anti-S1 antibodies were detected in tears and saliva of three unvaccinated individuals and two of them presented positive anti-S1 IgA and IgG results in serum. These results are shown in Fig. 1. Considering specific IgA and IgG anti-S1 antibodies altogether, significant differences were found between unvaccinated subjects and those who had received a 3-dose schedule (p = 0.024). However, no differences were found between the group of subjects vaccinated with 2- and 3-doses against SARS-CoV-2 (p = 0.151 and p = 0.412 respectively).
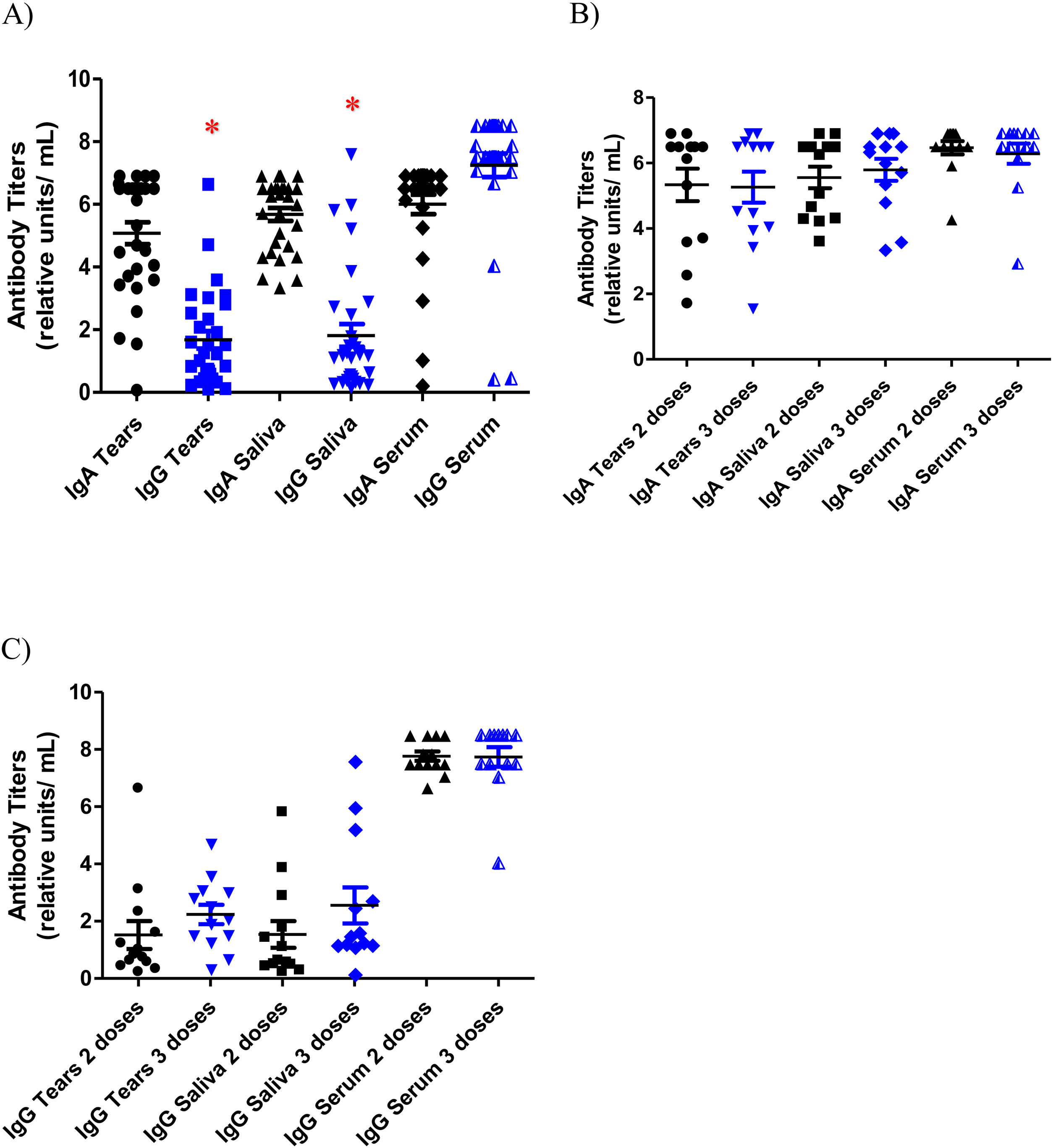
As for antibody titers, the lowest values were registered in the anti-S1 IgG titers of tears and saliva samples in both groups: vaccinated group with mean values of 1.76 ± 1.4 and 2.06 ± 2.0 RU/mL respectively and unvaccinated group with mean values of 0.37 ± 0.3 RU/mL and 0.32 ± 0.04 respectively. The highest specific anti-S1 IgG antibody titers were found in serum in also both groups (mean values of 7.76 ± 0.9 RU/mL in the vaccinated subjects and 4.08 ± 3.9 RU/mL in the unvaccinated subjects). Significant differences were found between IgG titers in serum and mucosal samples (tears p = 0.001 and saliva p = 0.008). These results are depicted in Fig. 2A. Specific anti-S1 IgA antibodies titers in tears, saliva and serum were 5.25 ± 1.7 RU/mL, 5.64 ± 1.2 RU/mL and 6.37 ± 0.9 RU/mL respectively in the vaccinated individuals, and 3.65 ± 2.7 RU/mL, 5.73 ± 0.9 RU/mL and 3.55 ± 3.4 RU/mL respectively in those not vaccinated. Among the group of subjects vaccinated against SARS-CoV-2 infection, IgA and IgG titers were compared between subjects vaccinated with a 2-dose and a 3-dose regimen. No differences were found between the IgA and IgG titers of the two groups in any of the three samples (Fig. 2B and C).
In addition, differences in antibody titers in the group of vaccinated subjects were analyzed according to sex. No significant differences were found between the mean IgA and IgG antibody titers in any of the three samples between females and males (p = 0.774 for IgG and p = 0.793 for IgA).
Regarding the correlation between IgA and IgG antibody titers and the date of infection in all subjects, and the date of vaccination in the vaccinated group, no correlation was found in any of the three samples analyzed.
DiscussionThe unstoppable spread of SARS-COV-2 infection and the rise in vaccination rates have led us to focus on immunity following COVID-19 and vaccination, particularly on local protective immunity at the ocular surface. Our work showed interesting results regarding the detection of anti-SARS-COV-2 antibodies (IgA and IgG) in three different biofluids, tears, saliva and serum, collected from unvaccinated and anti-COVID-19 vaccinated previously infected subjects. In our vaccinated subjects, all of whom had hybrid immunization (natural infection plus vaccination), specific IgA anti-S1 antibodies were detected in all three samples, which might suggest enhanced specific humoral responses over natural immunization. Moreover, as expected, the prevalence of subjects with detectable IgA and IgG in all three samples was higher in subjects vaccinated with a 3-dose regimen than in those with a 2-dose regimen, and this in turn was higher than in non-vaccinated subjects. Specifically, SARS-COV-2 IgG antibodies were not detected in tears and saliva samples from any of the unvaccinated subjects, which might suggest that vaccination boosts the IgG response in the mucosa. Mucosal immunity, both ocular and respiratory, has gained significant interest considering its role as the first defensive barrier against SARS-COV-2 infection. A number of SARS-COV-2 entry receptors, such as angiotensin-COnverting enzyme receptor 2 (ACE2) and cellular transmembrane serine protease serine 2 (TMPRSS2) have been identified on the ocular surface.5 Consequently, it has been suggested that the ocular surface may represent a viral entry route, as the mucosal surface of the eye is directly exposed to infectious aerosols.6,7 Variable and generally low rates of positive results have been detected with real-time reverse transcription-polymerase chain reaction (RT-PCR) of tears and conjunctival swabs.2,8,9 Humoral immunity to SARS-CoV-2 play an essential role in protection against COVID-19.10 Humoral immune responses are most often measured in the blood. However, little is known about the presence of SARS-CoV-2 specific antibodies in the mucosa following infection and COVID-19 vaccination.11–13
IgA antibodies in dimeric form are the most abundant antibody isotypes in the human mucosa and play a critical interplay between the immune system and environmental pathogens to provide mucosal protection, often acting as the first line of defense.14 Sterlin et al. found that IgA antibodies may dominate the early SARS-CoV-2 specific antibody response compared to serum and saliva IgG and IgM concentrations.15 This same study found that serum dimeric IgA was more potent than IgG in neutralizing SARS-CoV-2, highlighting the potential role of IgA during early SARS-CoV-2 infection and providing a critical remark given the emerging information on the types of antibodies associated with optimal protection against reinfection. Our work detected the presence of specific SARS-CoV-2 IgA antibodies in all three biofluids, tears, saliva and serum of COVID-19 vaccinated subjects. Specifically, on tear samples, we observed that all COVID-19 vaccinated subjects presented IgA and IgG anti-S1 antibodies. Most unvaccinated subjects presented anti-S1 IgA antibodies in tears and saliva after 192.5 days after primary infection, highlighting the importance of natural infection in blocking virus entry. Noteworthy, the only subject of the 4 unvaccinated subjects who did not exhibit IgA anti-S1 antibodies was a male who had been infected with SARS-CoV-2 464 days prior to sampling, which might explain the fact that IgA was not detected. Moreover, all 4 unvaccinated individuals presented negative results for IgG in tears. These results are congruent with those of other authors who observed that vaccination, in contrast to natural infection, induced an IgG-dominated response, with reduced levels of other Ab isotypes (IgM, IgA). Because of the different role of these isotypes in mucosal (IgA) versus systemic (IgG), these findings raise the possibility that, despite enhanced IgG responses, the quality of protection against reinfection conferred by vaccines may differ from that of natural infection.15,16 Another noteworthy finding of our work is the fact that no differences were found in IgA and IgG antibody titers between the group of subjects vaccinated with a 2-dose regimen and those with a 3-dose regimen. These preliminary results might question the value of COVID-19 vaccine boosters, especially in young, healthy subjects, as is the case in our sample in terms of humoral responses. These results provide quantitative guidance for the vaccination of unvaccinated but previously infected individuals, as well as for the implementation of booster vaccination programs. Given the strong immune response and lesser severity of disease shown in young adults,17,18 determining whether, for previously infected individuals, when and how many doses of COVID-19 vaccines would be necessary will require ongoing surveillance.
On the other hand, although there is some evidence of sex differences in antibody response, with females being more likely to be IgG positive post-vaccination than males and neutralizing antibodies decaying more rapidly in males than in females after natural infection and after vaccination, in our study no sex differences in IgA and IgG titers were observed in any of the three samples.19–21
Some limitations of the present study must be acknowledged. First, this study included mostly young and healthy subjects with a history of mild SARS-CoV-2 infection, which might result in higher and longer-lasting antibody titers than those found in older subjects or subjects with comorbidities.22,23 Different studies have shown a correlation between the antibody titers and COVID-19 severity.24,25 However, we could unfortunately not relate antibody titers to the severity of COVID-19, as all subjects included in the study had mild disease. On the other hand, the number of subjects included is limited, especially the group of unvaccinated subjects due to the difficulty in recruiting them in the public health hospital setting in Spain, where 92.7% of the population is fully vaccinated.26 Another limitation is that the Euroimmun Anti-SARS-CoV-2 ELISA Assay is intended for the qualitative detection of IgG and IgA class antibodies to SARS-CoV-2 in human serum or plasma, but is not validated for analysis of saliva or tears. Nevertheless, its off-label application has been used in several studies.12,27,28 Finally, tears have proved to be an acceptable sample for the detection of antibodies against SARS-CoV-2 by standard ELISA. Since both tears and saliva, unlike serum, may be collected readily and non-invasively, these biofluids may be a promising alternative strategy for detecting Anti-SARS-CoV-2 antibodies in large population screening programs.
In conclusion, this study provides valuable data on the presence of SARS-CoV-2-specific IgA and IgG antibodies in tears after mild COVID-19 in young adults, highlighting the role of the ocular surface as a first line of defense against infection. Our preliminary results reveal that most naturally infected unvaccinated individuals exhibit long-term specific anti-IgA in tears and saliva, whereas hybrid individuals developed specific IgA and IgG antibodies. Hybrid immunization appears to enhance mucosal and systemic IgG responses. These findings emphasize the importance of COVID-19 vaccination in boosting protective humoral immunity, not only in the serum, but also in the mucosa, as this constitutes an important portal of entry to cope with future reinfections.
DeclarationsEthics approval and consent to participate: The study was approved by the Hospital Clinico San Carlos Clinical Research Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Committee’s reference number 22/309-E.
Written informed consent was obtained from all subjects.
Consent for publication: All authors have approved this manuscript and agree with submission to Archivos de la Sociedad Española de Oftalmología
Declarations of interestNone.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributionsAll authors approved the final version of the manuscript.
We thank Ana María González-Nava and Helga Yolanda Tallón Avila who assisted in the collection of certain data.