The relationship between anemia and the outcome of patients with cirrhosis is not completely clear. Therefore, we performed this large-scale epidemiological study to investigate the prevalence and severity of anemia in patients with cirrhosis and acute decompensation or liver injury and how anemia impacts short-term and long-term outcomes.
Patients and MethodsPatients with cirrhosis and acute decompensation (AD) or acute liver injury (ALI) were enrolled in the Chinese AcuTe on CHronic LIver FailurE (CATCH-LIFE) studies, which consisted of two large, multicenter, prospective, observational cohorts between January 2015 and December 2016 and July 2018 and January 2019. We conducted data analysis on the prevalence of anemia and determined the relationship between anemia and prognosis.
ResultsAmong 1979 patients, 1389 (70.2%) had anemia, among whom 599 (41.3%) had mild anemia, 595 (15.8%) had moderate anemia and 195 (2.4%) had severe anemia. A linear association between hemoglobin level and 90-day or 1-year LT-free mortality was shown, and a 10 g/L decrease in hemoglobin level was associated with a 6.8% extra risk of 90-day death and a 5.7% extra risk of 1-year death. Severe anemia was an independent risk factor for 90-day [HR=1.649 (1.100, 2.473), p=0.016] and 1-year LT-free mortality [HR=1.610 (1.159, 2.238), p=0.005]. Multinomial logistic regression analysis further identified that severe anemia was significantly associated with post-28-day mortality but not within-28-day mortality.
ConclusionsAnemia is common in patients with cirrhosis admitted for acute events. Severe anemia was associated with poor 90-day and 1-year prognoses in these patients.
Anemia, defined by the World Health Organization (WHO) as a reduction in the concentration of hemoglobin (<120 g/L for women and <130 g/L for men) [1], is common in patients with cirrhosis. The underlying mechanism is multifaceted. For example, overt or occult gastrointestinal bleeding from either esophageal or gastric varices and portal hypertensive gastropathy (PHG) play important roles [2], and the cirrhosis-associated chronic inflammatory state resulting from intestinal microbial translocation leads to chronic inflammatory anemia in cirrhosis [3]. In addition, low hepcidin concentration, folate deficiency, malnutrition, hypersplenism, derangement of the hematopoietic niche, loss of hematopoietic stem cells (HSCs), alcohol and bilirubin toxicity and renal insufficiency could also be significant causes of anemia in advanced cirrhosis [4–7]. Several studies have shown the clinical significance of anemia in patients with cirrhosis. A retrospective cohort study reported that anemia is a strong predictor of hospitalization due to hepatic decompensation in outpatients with liver cirrhosis [8]. It has been reported that the prevalence of acute-on-chronic liver failure (ACLF) is significantly higher in patients with anemia than in those without anemia [9], as low hemoglobin concentrations are independently associated with cerebral hypoxia in patients with decompensated cirrhosis and can trigger ACLF. Furthermore, distinct mechanisms of anemia have also been associated with poor prognosis in cirrhosis. For example, macrocytic anemia was found to be associated with the severity of liver damage and might be a predictor of short-term mortality in patients with HBV-related decompensated cirrhosis [10], and increased mortality was observed among patients with spur cell anemia after splenectomy or alcoholism because of progressive lipoprotein impairment [11]. Iron deficiency anemia (IDA), which is common in cirrhosis, was also found to be significantly associated with an increased risk of mortality in cirrhosis [12].
Despite these limitations, most previous studies were retrospective and single-center studies that included only a relatively limited number of cases, and the effect of variations in hemoglobin levels on mortality and time of death has not yet been fully elucidated. Thus, a large prospective cohort study is required to further determine the association between hemoglobin levels and adverse outcomes in patients with cirrhosis. In the present study, we performed a prospective, multicenter cohort study to determine the prevalence and severity of anemia among cirrhotic patients with acute decompensation (AD) or acute liver injury (ALI) and further explored the association between anemia and mortality at their 28-day, 90-day and 1-year follow-ups.
2Material and Methods2.1Study design and populationWe retrospectively used data from the Chinese Acute on Chronic Liver Failure (CATCH-LIFE) study (NCT02457637, NCT03641872), a prospective multicenter cohort study of patients with chronic liver disease and acute exacerbation conducted by the Chinese Chronic Liver Failure (CLIF) Consortium, which is composed of 15 tertiary hospitals in HBV high endemic areas. Patients were included from prospective multicenter cohorts in the CLIF Consortium between January 2015 to December 2016 [13] and July 2018 to January 2019.
We collected patients with cirrhosis who were admitted to the hospital due to acute AD or ALI. The exclusion criteria were acute gastrointestinal bleeding two weeks before admission, hepatocellular carcinoma or other liver malignancies before or during admission, extrahepatic malignancies, hematological diseases or severe chronic extrahepatic disease, age younger than 18 or older than 80 years, pregnancy, and receiving immunosuppressive agents for nonhepatic diseases.
2.2Participant follow-upFollow-up data were collected from all patients during hospitalization within 28 days and obtained regularly from outpatient follow-up or telephone contact after discharge. Patients were considered to be off-study if they died, were lost to follow-up, developed malignancies, received liver transplantation (LT), or withdrew informed consent. The primary endpoint was mortality at 28 days, 90 days, and 1 year. On admission, the patients were recorded for demographic information and medical history. During hospitalization, laboratory parameters, radiological findings, complications, and therapies were recorded at 1, 4, 7, 14, 21, and 28 days (or the last day if the patient was hospitalized for less than 28 days) as well as 24 hours before death or LT (if the patient died or had LT). Models for end-stage liver disease scores, sepsis, and organ failure were evaluated based on available data. After hospital discharge, all patients underwent follow-up regularly via outpatient review or telephone monthly, and clinical outcomes such as death, LT, and development into malignancies were recorded. If patients died, then the time of death and the main cause of death were noted. Up to 28-days, 12 patients were unable to be contacted (loss to follow) by phone for reasons including not answering the phone, wrong number, refusal to answer questions, a number not in service, and other reasons, and up to 90-day, 32 patients were lost to follow, finally, there were 74 patients lost to follow up to 1-year. All patients received routine treatment according to relevant guidelines during hospitalization, and after discharge, healthy life habits, long-term antiviral therapy, alcohol intake restrictions, and other symptomatic therapy were monitored during follow-up.
2.3DefinitionAnemia was defined as a reduction in the concentration of hemoglobin (< 120 g/L for women and < 130 g/L for men) by the WHO as previously described [1] and was divided into different degrees, with mild anemia ranging from 110 g/L to 120 g/L in nonpregnant women (15 years of age and above) and 110 g/L to 130 g/L in men (15 years of age and above), moderate anemia ranging from 80 g/L to 109 g/L, and severe anemia under 80 g/L. Cirrhosis was diagnosed based on CT/MRI scan, laboratory tests, clinical symptoms, and a history of liver disease. Acute decompensation was defined as the acute development of gastrointestinal hemorrhage, hepatic encephalopathy, overt ascites, bacterial infection, jaundice (total bilirubin > 5 mg/dl) or any combination of these within one month before enrollment [14,15], and acute liver injury was defined as alanine aminotransferase or aspartate aminotransferase levels >3 times the upper limit of normal or a total bilirubin level >2 times the upper limit of normal within 1 week.
2.4Statistical analysisAll statistical analyses were conducted using R (version 3.6.0, R Foundation for Statistical Computing, Vienna, Austria). Continuous variables between two groups were compared with Student's t tests or the Mann–Whitney U test and presented as the medians with interquartile ranges. Categorical variables were compared with the chi-squared or Fisher's exact test and are represented as the counts and percentages. For multiple groups, Fisher's exact test or a χ2 test was used for group comparisons for categorical variables. For continuous variables, a comparison was performed by one-way ANOVA for data with normal distribution, and Kruskal‒Wallis one-way ANOVA on ranks was used for data with nonnormal distribution. Post hoc Tukey pairwise comparisons were used for post hoc pairwise comparisons. Multiple imputation methods were used to address missing values. Univariate Cox analysis and multivariate analysis were performed using the Cox proportional hazards model in stratified analyses and were also used to determine the relationship between the severity of anemia and mortality. A generalized additive model (GAM) was used to evaluate the curvilinear association between anemia and LT-free mortality. The 90-day and 1-year LT-free survival curves were plotted, and comparisons between groups were performed by the Kaplan–Meier method. P values less than 0.05 (P < 0.05) were considered statistically significant.
2.5Ethical statementWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of Shanghai Jiaotong University School of Medicine [Approval No. (2014)148k and (2016) 142k].
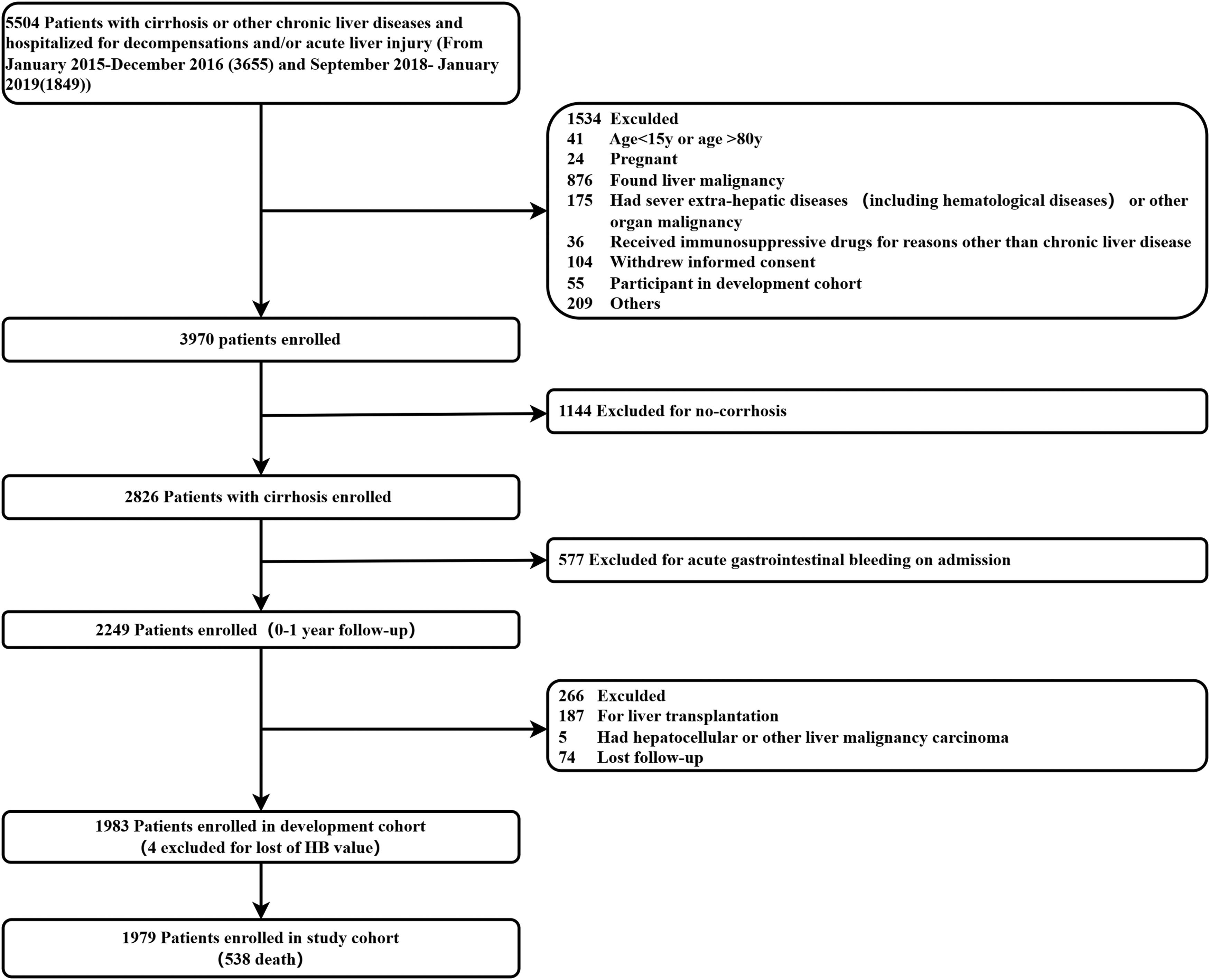
3Results3.1Prevalence and baseline characteristics of patients with cirrhosis and anemiaScreening steps of the study population are shown in Fig. 1. Notably, we first excluded 1144 patients without cirrhosis and 577 cirrhotic patients with acute gastrointestinal bleeding at admission. Finally, 1979 patients with cirrhosis who were admitted to the hospital due to AD or ALI were included in our study.
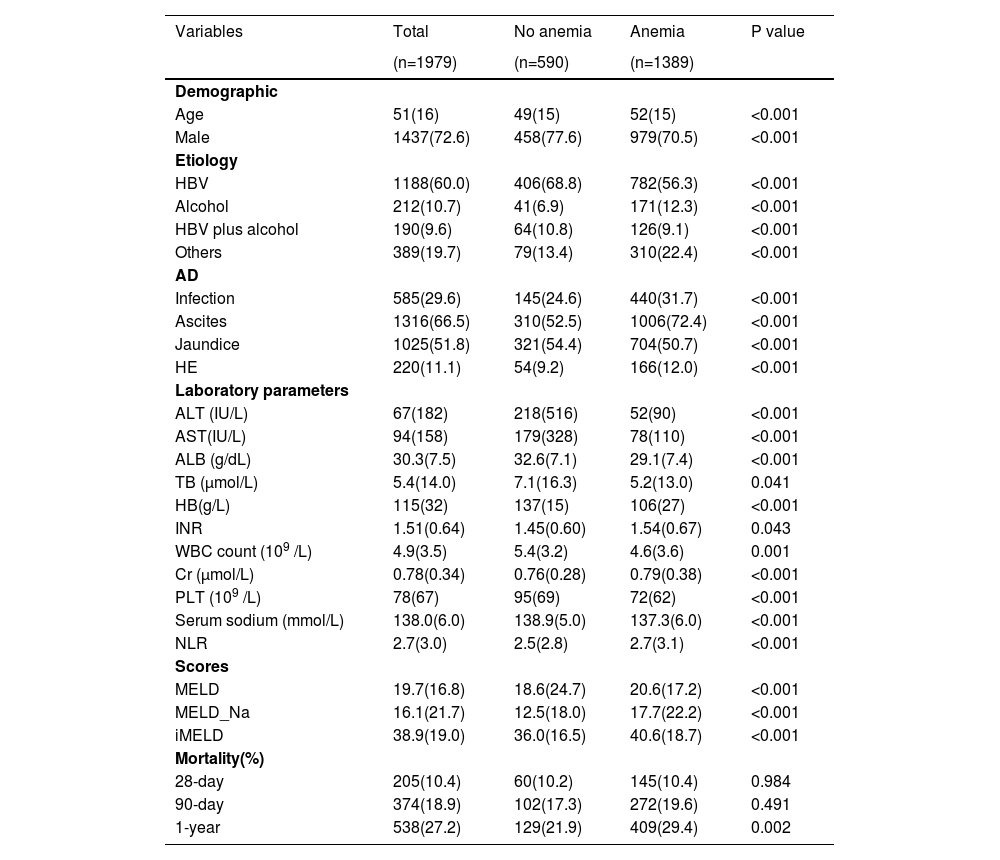
The clinical characteristics of the cohort and subgroups of patients with/without anemia are shown in Table 1. Among 1979 patients, the median age was 51 (IQR 35–67) years, with 1437 (72.6%) patients being male. The median hemoglobin level was 115 g/L (IQR 73–147), and the overall mortality at 28 days, 90 days, and 1 year was 10.4%, 18.9%, and 27.4%, respectively. Compared with patients without anemia, those with anemia had higher 1-year mortality and more severe hepatic dysfunction, as indicated by disease severity scores, as well as laboratory parameters, such as serum bilirubin, international normalized ratio, creatinine, albumin, platelet count and serum sodium. Notably, these patients had more profound systemic inflammation, as indicated by the NLR.
Baseline characteristics of study population.
HBV, hepatitis B virus; HB, hemoglobin; HE, hepatic encephalopathy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; WBC, white blood cell count; NLR, neutrophil-to-lymphocyte ratio; ALB, albumin; MELD, Model for End Stage Liver Disease;INR, International normalized ratio; PLT, platelet count. Data are expressed as mean±SD, median (interquartile range), or number (percent)
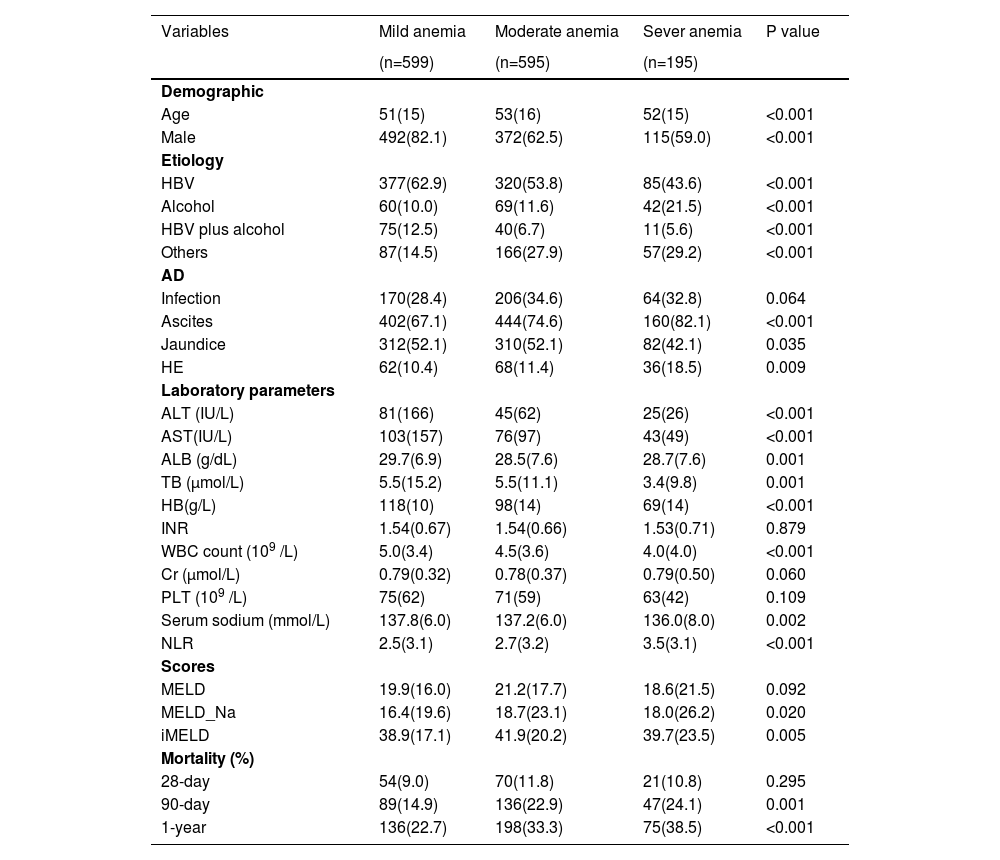
We further compared the baseline characteristics of patients with varying degrees of anemia (Table 2). Among 1389 patients with anemia, 599 (41.3%) had mild anemia, 595 (15.8%) had moderate anemia, and 195 (2.4%) had severe anemia. As expected, there were stepwise increases in 90-day and 1-year mortality, exacerbation of liver diseases, and systemic inflammation with the severity of anemia.
Baseline characteristics of patients with varying degrees of anemia.
HBV, hepatitis B virus; HB, hemoglobin; HE, hepatic encephalopathy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; WBC, white blood cell count; NLR,neutrophil-to-lymphocyte ratio; ALB, albumin; MELD, Model for End Stage Liver Disease;INR, International normalized ratio; PLT, platelet count. Data are expressed as mean±SD, median (interquartile range), or number (percent)
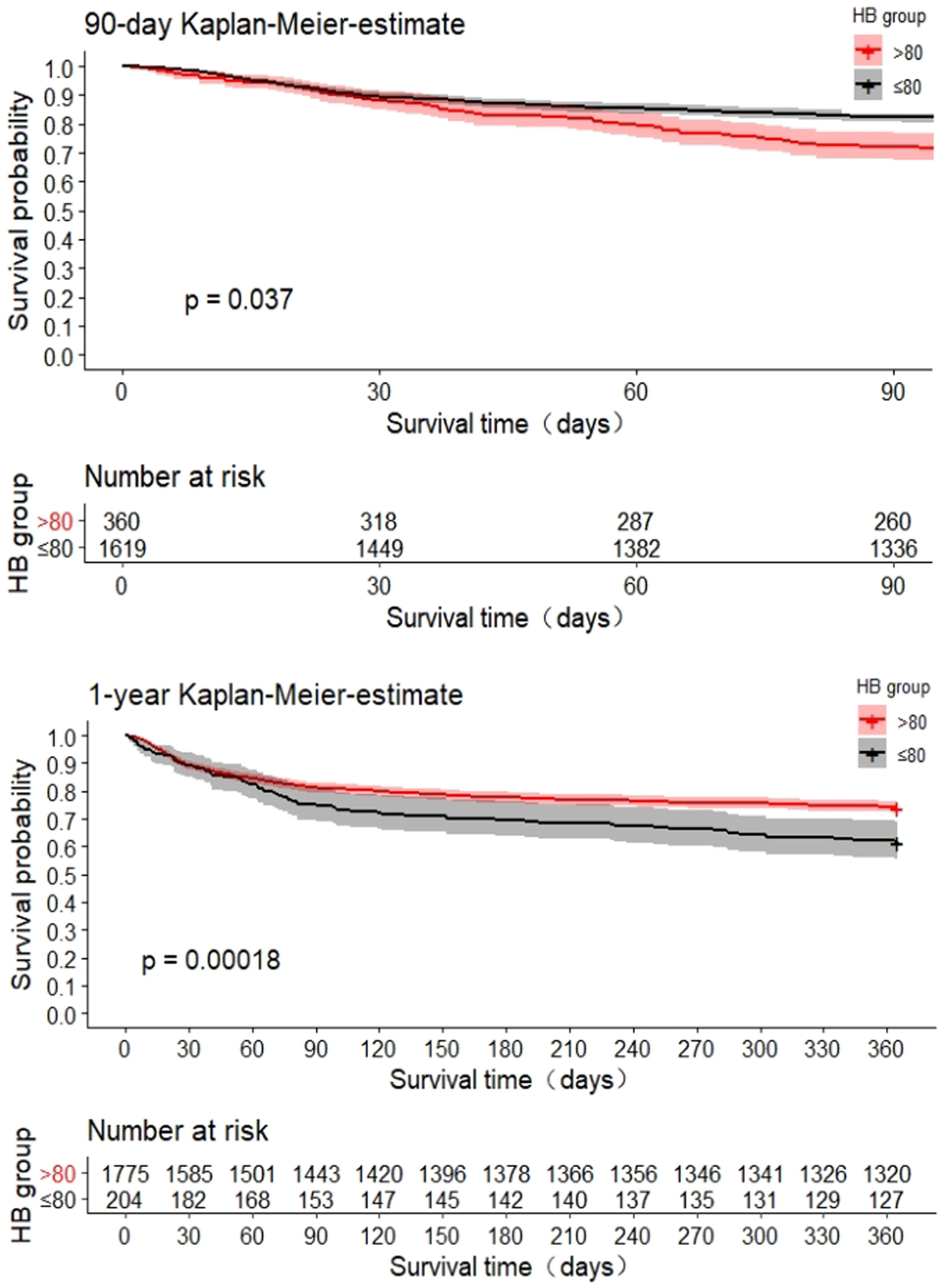
The 90-day and 1-year survival curves showed a significantly lower cumulative survival in patients with severe anemia (p<0.05) (Fig. 2). As shown in Fig. 3, the generalized additive model was used to evaluate the curvilinear association between HB level and 90-day mortality. In the all-adjusted model, the effect of HB level on death was nearly perfectly linear, with an adjusted odds ratio of 0.932 (95% CI, 0.886–980, p=0.006) associated with a 10 g/L decrease in HB at any level in our study population (e.g., a patient with an HB of 90 g/L has a 6.8% lower adjusted hazard of death than one with an HB of 80 g/L, and so on throughout the entire range). Similarly, the effect of HB level on 1-year death was nearly perfectly linear, with an adjusted odds ratio of 0.943 (95% CI, 0.905–0.983, p=0.006) associated with a 10 g/L decrease in HB at any level in our study population (e.g., a patient with an HB of 90 g/L has a 5.7% lower adjusted odds of death than one with an HB of 80 g/L, and so on throughout the entire range).
Association between HB level and 90-day or 1-year mortality by a generalized additive model. Solid lines are predictions from a generalized additive model, and dashed lines represent the corresponding 95% confidence intervals. Data were adjusted for age, sex, etiology, ascites, HE, infection, TB, INR, CR, ALB, ALT, AST, WBC, PLT, and Na.
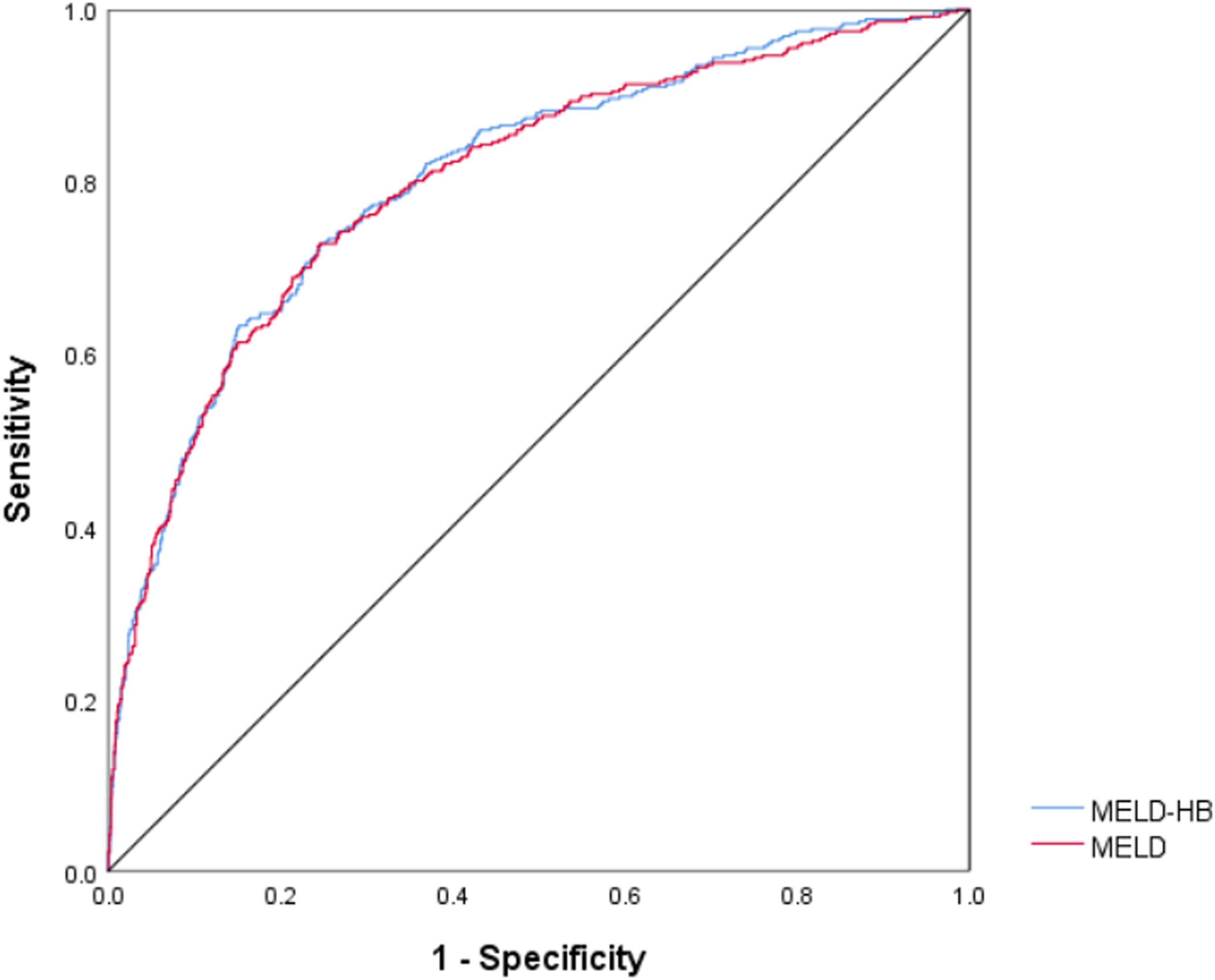
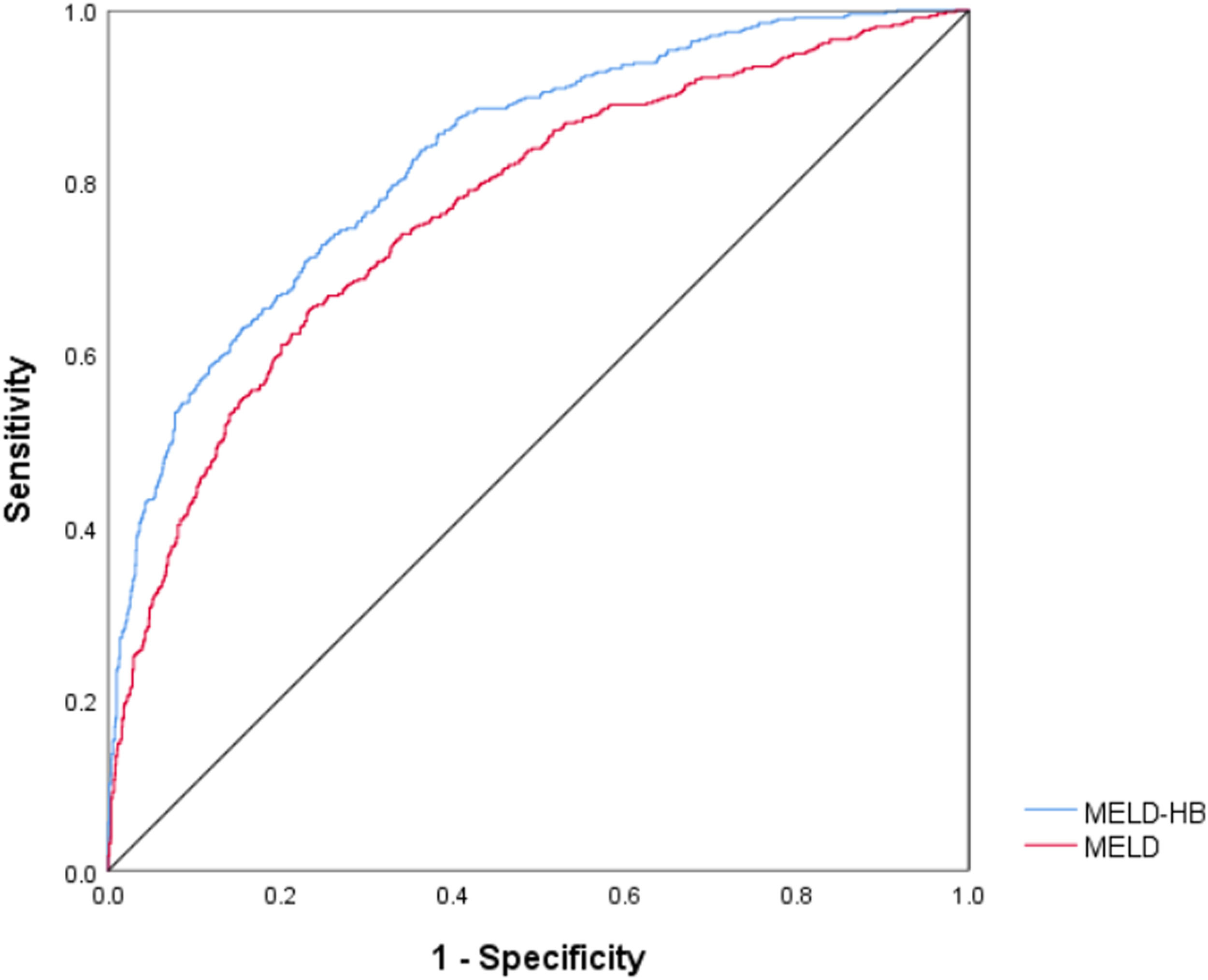
The receiver operating curve curves were generated to evaluate the predictive ability of 90-day and 1-year mortality, and the area under curve (AUC) of 90-day mortality for MELD-HB was 0.804 (95% CI, 0.778–0.830; p<0.001), which was superior to MELD(AUC, 0.801; 95% CI, 0.774–0.828; p<0.001).(Fig. 4) , Simultaneously, the area under curve (AUC) of 1-year mortality for MELD-HB was 0.828 (95% CI, 0.807–0.850; p<0.001), which was also superior to MELD(AUC, 0.768; 95% CI, 0.742–0.793; p<0.001).(Fig. 5)
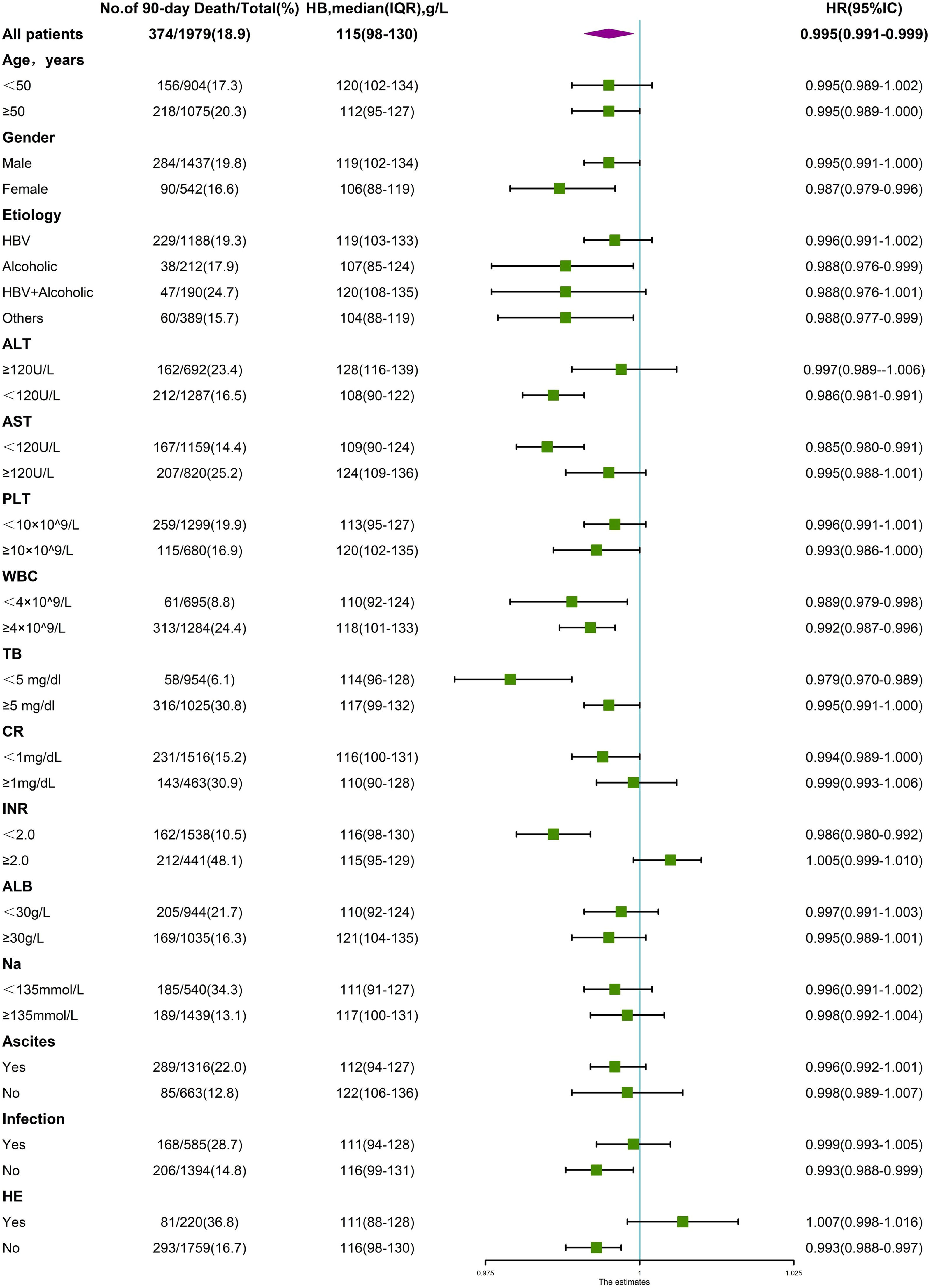
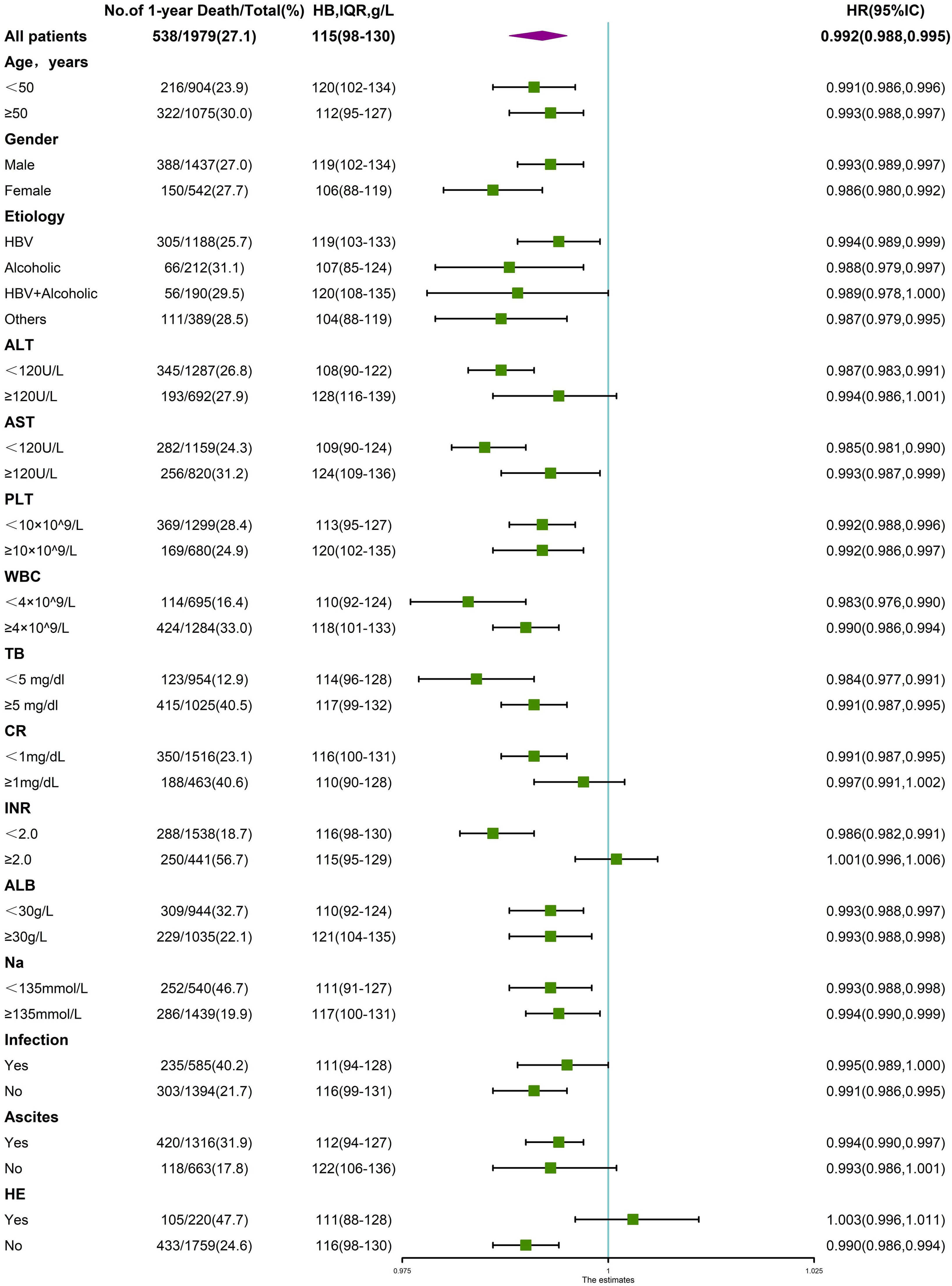
3.4Interaction between anemia and other variablesAs a variety of demographic and clinical parameters significantly differed among patients with varying degrees of anemia and those without, a stratified analysis was performed to analyze the variables that impact the association between hemoglobin levels and LT-free 90-day and 1-year mortality. As shown in Fig. 6, the significant association between hemoglobin level and 90-day mortality was altered in at least one of two subgroups stratified by age, cirrhosis etiology, TB, Cr, INR, ALT, AST, ALB, PLT, presence of infection, ascites or HE, suggesting a potential interactive effect between hemoglobin level and these variables. Similarly, in Fig. 7, the significant association between hemoglobin level and 1-year mortality was altered in one of two subgroups stratified by gender, cirrhosis etiology, ALT, AST, WBC, and presence of ascites or HE, suggesting a potential interactive effect between hemoglobin level and these variables.
Stratified analyses of the risk of 90-day death according to hemoglobin levels. The unadjusted hazard ratio of death per unit increment in standard deviation of hemoglobin is plotted for the entire cohort and according to strata of baseline covariates. Subgroups were stratified by age, sex, etiology, ALT, AST, PLT, WBC, TB, CR, INR, ALB, Na, presence of ascites, infection and HE.
Stratified analyses of the risk of 1-year death according to hemoglobin levels. The unadjusted hazard ratio of death per unit increment in the standard deviation of hemoglobin is plotted for the entire cohort and according to strata of baseline covariates. Subgroups were stratified by age, sex, etiology, ALT, AST, PLT, WBC, TB, CR, INR, ALB, Na, presence of ascites, infection and HE.
We next evaluated whether the impact of anemia was independently associated with 90-day and 1-year mortality after adjusting for the interacting variables. As shown in Table 3, when divided into various degrees of anemia, severe anemia was shown to be independently associated with 90-day and 1-year mortality adjusted by different models, with hazard ratios of 1.649 (95% CI, 1.100–2.473, p=0.016) and 1.610 (95% CI, 1.159–2.238, p=0.005) in the fully adjusted model (adjusted for age, sex, etiology, ascites, HE, infection, TB, INR, CR, ALB, ALT, AST, WBC, PLT, Na).
Univariate nd multivariate Cox analysis of the realtionship between anemia and 28-day,90-day and 1-year mortality.
Model I Un-adjusted;
Model II Adjusted for age gender etiology;
Model Ⅲ Adjusted for age gender etiology HE ascites infection, TB INR Cr
Model Ⅳ (full-adjusted) Adjusted for age, gender, etiology, ascites, HE, infection, TB, INR, CR, ALB, ALT, AST, WBC, PLT, Na
Statistical ananlysis was performed using univariate and multivariate Cox analysis.
We further evaluated whether the adverse impact of severe anemia on the prognosis of cirrhosis was limited to the postacute period. Therefore, a multinomial logistic regression analysis was performed to identify significant risk factors for early death and delayed death. On multivariate analysis, age, ALB, ALT, TB, INR, Cr, WBC, Na, and presence of HE but not severe anemia were predictors of 28-day mortality. Severe anemia was significantly associated with post-28-day mortality, and other independent variables included age, male sex, ALB, ALT, TB, INR, Na, and presence of HE or ascites (see Table 4). The above findings suggested that severe anemia had no impact on short-term prognosis but determined the long-term outcome.
Risk factors associated with early/delayed death by multivariate multinomial logistic regression analysis.
Statistical ananlysis was performed using multinominal logistic regression model to identify risk factors associated with multiple outcomes (survival, early/delayed death)
We performed this large multicenter prospective cohort study to describe the prevalence of anemia among cirrhotic patients with AD/ALI and then investigated the impact of anemia on patient outcomes. We found that hemoglobin levels were independently associated with increased 90-day and 1-year mortality, as patients had a 6.8% lower adjusted hazard risk of death on 90-day mortality and a 5.7% lower adjusted hazard risk of death on 1-year mortality with a 10 g/L decrease in hemoglobin at any level throughout the entire range. With regard to the severity of anemia, severe anemia was an independent risk factor for increased 90-day and 1-year mortality in cirrhotic patients with AD/ALI; however, it had no impact on 28-day prognosis.
Anemia is common in patients with cirrhosis, and previous studies have demonstrated a remarkable decrease in hemoglobin concentration, with a prevalence ranging from 21%-84% among patients with varying severity of cirrhosis [2,10,16,17]. The results are in line with our study, as we reported a prevalence of up to 70.2%, with 9.9% combined with severe anemia. The potential causes of anemia include portal hypertension, chronic inflammatory conditions, bone marrow suppression, malnutrition, and imbalances in iron homeostasis, which are more common in cirrhosis, as previously described [2–7]. Remarkably, patients with alcohol-related cirrhosis had a higher prevalence of anemia than those with HBV-related cirrhosis, with a prevalence of anemia of 80.7% to 65.8% in our study population. Possible explanations are as follows: first, bone marrow toxicity of alcohol could be an important reason for the development of anemia [15]; second, hemolytic anemia was shown in patients with alcohol-related cirrhosis as previously described through altering the structure and metabolic pathways of the red-blood-cell membrane; third, malnutrition is common in patients with alcohol-related cirrhosis, as chronic alcohol consumption may lead to micronutrient deficiencies [18]; and finally, spur cell anemia caused by alcohol-related cirrhosis has also been reported by previous studies [19,20].
A strong linkage between anemia and adverse outcomes was also found in patients with cirrhosis. First, anemia was significantly associated with illness severity, as patients with anemia often have higher prognostic scores; higher levels of laboratory parameters, such as serum bilirubin, international normalized ratio, creatinine, and NLR; and lower levels of laboratory parameters, such as albumin, platelet count and serum sodium, which could indicate the severity of the disease. This result is in line with a previous study that demonstrated the close relationship between anemia and ACLF [9]. Moreover, our study confirmed that HB level is an independent risk factor for increased mortality among patients with cirrhosis. Specifically, severe anemia is associated with increased 90-day and 1-year mortality, rather than 28-day mortality, among patients with cirrhosis is the central viewpoint of this study, which may be related to the following factors.
First, anemia may accelerate the progression of liver cirrhosis by affecting the hemodynamic status. It has been shown that low hemoglobin levels result in hemodilution and a reduction in blood viscosity, aggravate tissue hypoxia, increase cardiac output, worsen hyperdynamic circulation and contribute to the development of portal hypertension [21].
Second, anemia may increase the risk of decompensation events. For example, in addition to exacerbating portal hypertension, a reduced level of hemoglobin leads to a decline in NO scavenging and subsequent guanylyl cyclase activation, which impairs platelet aggregation [22] and increases the risk of bleeding. Additionally, anemia is associated with an increased risk of HE, which is an independent factor for adverse outcomes in cirrhosis. In our study, the prevalence of HE in patients with moderate to severe anemia was 16.5% compared with 9.7% among those without anemia, in line with a prospective cohort study that reported a relationship between anemia and HE in ambulatory cirrhotic patients without recent overt gastrointestinal bleeding at baseline [23]. Anemia is associated with hyperammonemia due to occult gastrointestinal blood loss in cirrhosis [24]. Furthermore, patients with anemia have an increased risk of infection, with a prevalence of up to 31.7% compared with 26.5% in patients without anemia in our study. Previous studies have shown that low hepcidin levels impose increased vulnerability to bacterial infection in patients with cirrhosis and IDA [25].
In addition, anemia may indicate dysregulation of the hematopoietic niche and loss of HSCs, which are correlated with the severity of cirrhosis and cause hematological and immunological dysfunctions in patients with advanced cirrhosis [5]. These mechanisms suggest that anemia is a critical risk factor in the temporal course of disease progression in patients with cirrhosis and could result in increased long-term mortality.
The strength of the study is the high-quality data based on a multicenter, prospective, national cohort. However, there are also several limitations. First, the diagnosis of anemia was based on the hemoglobin level obtained at admission, and we did not include those who developed anemia during hospitalization. Second, the possible causes of anemia were not further explored based on our limited available data. Finally, we did not assess the recurrence of decompensated events after hospital discharge during the 1-year follow-up; thus, the effect of anemia on the risk of recurrent decompensation remains to be determined.
5ConclusionsIn conclusion, anemia was common in patients with cirrhosis with a variety of acute events. Hemoglobin level was linearly correlated with 90-day and 1-year mortality, and severe anemia was an independent risk factor for poor prognosis among cirrhotic patients with AD/ALI. The potential clinical benefit of correcting anemia, such as the use of erythropoietin, warrants assessment.
FundingThis work was supported by the Fundamental Research Funds for the Central Universities (2021FZZX001-41,226-2023-00127), Chinese National Natural Science Foundation (No.81870425), Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No.2022490480), the National Science and Technology Major Project (2018ZX10723203 and 2018ZX10302206), the National Key Research and Development Program of China (2017YFC0908100), and the Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support and Shanghai Hospital Development Center Funding (16CR1024B). This study was supported in part by the National Natural Science Foundation of China (GZ1263, 81770572, 81470842, 81470869, 81670576, 81330038, 81571978, 81401665, 81270533, 81470038, 81271884, 81461130019, 81774234, 81700561, 81870425, and 81473641), the Chongqing Natural Science Foundation (cstc2014jcyjA10118), the Department of Science and Technology of Guangdong Province (2015B020226004), the Foundation for Innovative Research groups of Natural Science Foundation of Hubei Province of China (2018CFA031), and the Fundamental Research Funds for the Central Universities.
Author contributionsHaotang Ren, Shan Yin, Wenting Tan, Yixin Hou, Shue Xiong, Liyuan Long, Beiling Li, Sen Luo, Weituo Zhang collected and analysed the data; Yu Shi, Hai Li, Guohong Deng, Xianbo Wang, Xin Zheng,Yan Huang, Jinjun Chen, Zhongji Meng, Yanhang Gao, Zhiping Qian,Feng Liu, Xiaobo Lu, Jia Shang and Shaoyang Wang designed the research study; Haotang Ren wrote the paper and Yu Shi, Hai Li, Guohong Deng, Xianbo Wang, Xin Zheng,Yan Huang, Jinjun Chen, Zhongji Meng, Yanhang Gao, Zhiping Qian,Feng Liu, Xiaobo Lu critically reviewed the manuscript. All authors approved the final version of the manuscript.
Hai Li, Guohong Deng, Xianbo Wang, Xin Zheng, Yan Huang, Jinjun Chen, Zhongji Meng, Yan-hang Gao, Zhiping Qian, Feng Liu, Xiaobo Lu, and Yu Shi are members of the Chinese Chronic Liver Failure Consortium, China.