Acute-on chronic liver failure (ACLF) has been an intensively debated topic mainly due to the lack of a unified definition and diagnostic criteria. The growing number of publications describing the mechanisms of ACLF development, the progression of the disease, outcomes and treatment has contributed to a better understanding of the disease, however, it has also sparked the debate about this condition. As an attempt to provide medical professionals with a more uniform definition that could be applied to our population, the first Mexican consensus was performed by a panel of experts in the area of hepatology in Mexico. We used the most relevant and impactful publications along with the clinical and research experience of the consensus participants. The consensus was led by 4 coordinators who provided the most relevant bibliography by doing an exhaustive search on the topic. The entire bibliography was made available to the members of the consensus for consultation at any time during the process and six working groups were formed to develop the following sections: 1.- Generalities, definitions, and criteria, 2.- Pathophysiology of cirrhosis, 3.- Genetics in ACLF, 4.- Clinical manifestations, 5.- Liver transplantation in ACLF, 6.- Other treatments.
Liver diseases have had an important increase worldwide due to the emerging pandemic of fatty liver disease-associated metabolic dysfunction, with an average prevalence ranging between 25% and 35%; prevalence in the coming years will probably be above 40% in Latin America due to the alarming number of cases of obesity and diabetes. During the natural history of liver disease, with an etiology that can be diverse, the average time of progression from fibrosis to cirrhosis can be 20 to 30 years. Once cirrhosis is established, the compensated phase, when the patient is asymptomatic, lasts five to ten years, after which the disease evolution presents as decompensation (ascites, variceal bleeding, hepatic encephalopathy (HE), renal dysfunction). In this period, the presence of an acute insult can lead to acute-on-chronic liver failure (ACLF) characterized by acute decompensation, multiorgan failure, and increased 28 and 90-day mortality. [1,2].
The growing interest in ACLF has led to more than 500 publications regarding this subject in the last ten years. Likewise, the behavior is different per continent, insults are not the same, coupled with a different compromise in terms of organic failures. Therefore, the wide knowledge of the inflammatory process conditioned by the disease itself, microbiota, the acute trigger, sarcopenia, the progression of the disease, and genetics leads us to deepen the knowledge of this syndrome [3].
Several definitions are available to establish and diagnose ACLF, where the most used classification is that of the CLIF Consortium, established by the European Foundation for the Study of Chronic Liver Failure in patients with Chronic Liver Disease (EASL-CLIF). It is established that ACLF can develop at any stage of cirrhosis, from compensated to decompensated stages, and it can involve a precipitating event that may be hepatic or extra-hepatic, where a non-identified precipitating event represents a high percentage and the most common events worldwide are infections. In this observational study, the behavior of 1,343 hospitalized patients who presented acute cirrhotic decompensation was analyzed (CANONIC study).
The current definitions of ACLF vary worldwide, but despite these differences, patients with ACLF have a uniformly poor prognosis. The role of ACLF prediction, precipitating factors, individual organ failures, management strategies, and impact on liver transplantation, 28 day mortality or end-of-life care is evolving. The current guideline represents the synthesis of the current and emerging data on ACLF as a major entity in patients with chronic liver disease [3].
The main objective of this consensus was to develop a document with updated evidence about the current definition, epidemiology, pathophysiology, diagnosis, and treatment for ACLF, integrating new scientific evidence published worldwide with the aim of providing a basic guide for clinical practice in Mexico. The evidence and the expert panel recommendations were graded according to the Grading Assessment Development and Evaluation (GRADE) system [4].
2MethodFour coordinators were appointed for the elaboration of this consensus. Coordinators carried out a systematization for critical literature assessment. A bibliography review was carried out using the following words as search criteria: «acute-on-chronic liver failure», «cirrhosis», «decompensated cirrhosis», and «chronic liver disease» combined with terms «epidemiology», «incidence», «prevalence», «pathophysiology», «inflammation», «microbiota», «diagnosis», «precipitants», «treatment», «therapy», «management», «liver transplantation», «review», «guidelines», and «meta-analysis», as well as their equivalent terms in Spanish. The search was performed in PubMed from January 1st, 2011 to September 30th, 2022. Publications in English and Spanish were included. Preference was given to consensus, guidelines, systematic reviews, and meta-analyses, but it was not limited to these types of articles. Complementary electronic and manual searches were also carried out on all the publications considered relevant by the coordinators up to February 2022. All the bibliography was made available to the consensus members for consultation at any time throughout the process.
Six working groups were formed to address the main issues of acute-on-chronic liver failure (ACLF):
Group A. Generalities, definitions, and criteria.
Group B. Pathophysiology of cirrhosis: role of inflammation and role of microbiota.
Group C. Genetics in ACLF: ACLF grades and mortality, ACLF in children, ACLF and COVID-19 outcomes.
Group D. Clinical manifestations: ACLF grades and management.
Group E. Liver transplantation in ACLF: mortality-associated factors in transplantation, criteria for transplantation, and results.
Group F. Other treatments: extracorporeal liver support, granulocyte colony-stimulating factor, and stem cells.
After carrying out the review, statements were prepared and submitted to a first anonymous electronic vote that took place from March 7th, 2022 to March 10th, 2022. The consensus participants cast their vote considering the following answers: a) totally agree; b) partially agree; c) uncertain; d) partially disagree; and e) totally disagree. In the event of an agreement equal to or greater than 75%, it was determined that the statement would remain unchanged for the next round of voting. Statements with 75% or more disagreement were removed. Statements with less than 75% agreement or less than 75% disagreement were restated by the coordinator of each working group, taking the participants’ comments into account. The second round of remote electronic voting included statements (from March 15th to 17th, 2022) following the same system. The final vote was carried out through the Zoom platform, on March 18th and 19th, 2022, in which 64 statements were voted on by the consensus group; leaving a total of 60 reviewed, eliminated, and merged statements that were finally decided.
The strength of recommendations of the statements for the vote reflects the quality of underlying evidence. The quality of the evidence was classified into one of four levels: high, moderate, low, and very low considering the confidence in the effect estimate based on current literature. The GRADE system offers two grades of recommendations: strong or weak [4].
GROUP A GENERALITIES, DEFINITIONS, AND CRITERIA
Coordinator: Dr. Mauricio Castillo Barradas
Participants: Dr. Ricardo Sandoval Salas, Dr. María Saraí González Huezo, Dr. José Luis Pérez Hernández, M. Sc. Osvely Méndez-Guerrero.
A 1. Definition of acute decompensation
Progression of the advanced chronic liver disease, characterized by the appearance of one or more of the clinical signs of complications such as ascites, gastrointestinal bleeding, hepatic encephatlopathy (HE) , jaundice, and/or acute kidney injury (AKI). (Key concept / Expert's opinion).
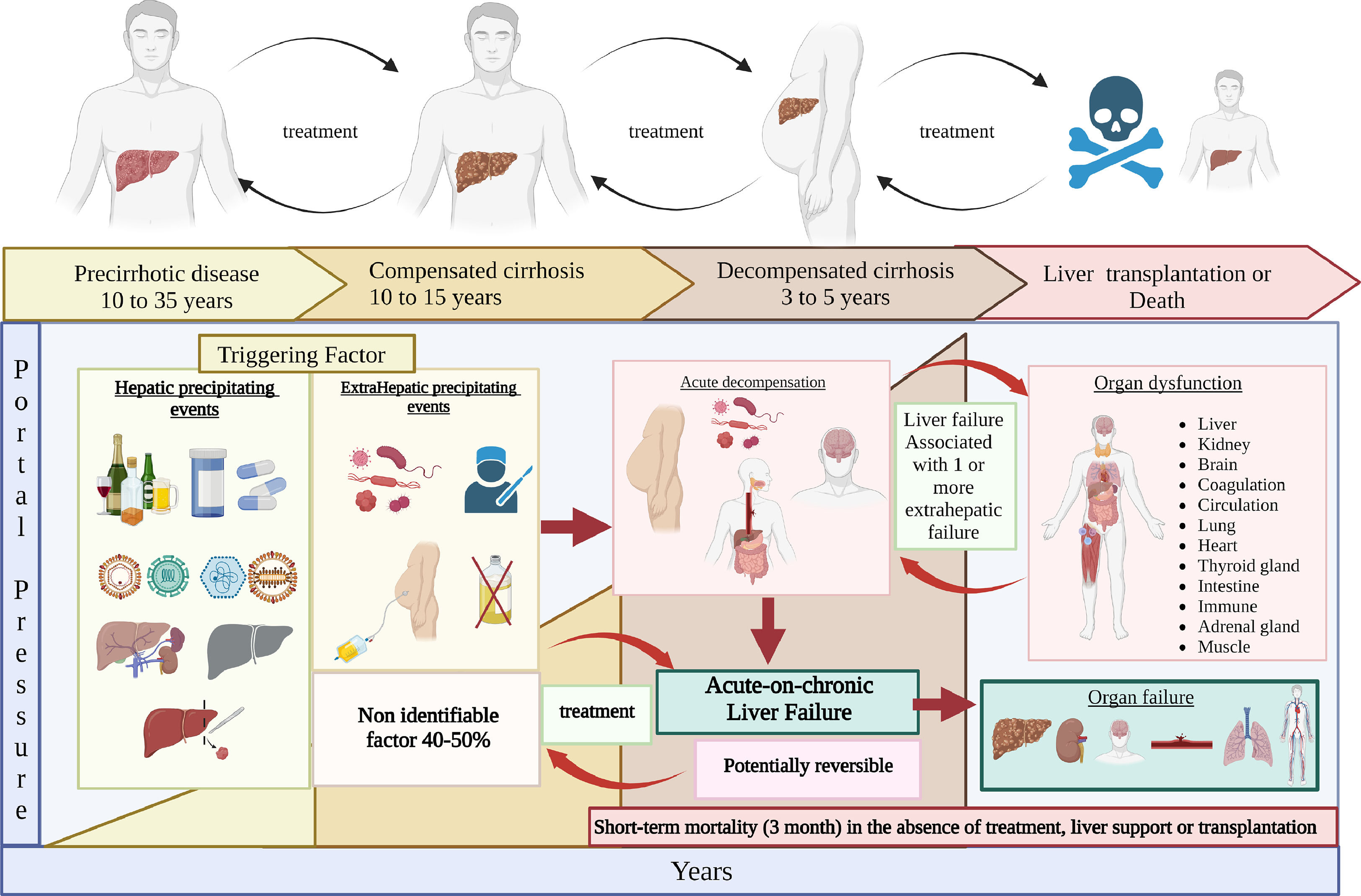
The natural history of cirrhosis is characterized by a silent, asymptomatic course until the increased portal pressure and the worsened liver function produce a clinical phenotype with the onset of cirrhotic complications. In the asymptomatic phase of the disease, generally called compensated cirrhosis, patients can have a good quality of life and the disease can progress undetected for several years [1].
Acute decompensation is characterized by the development of overt clinical signs, the most common of which are ascites, hemorrhage, HE, and jaundice. After the first appearance of any of them, the disease usually progresses more rapidly towards death or the need for liver transplantation (LT). This phase of the disease has been called decompensated cirrhosis [2].
The acute hepatic insult is defined by jaundice (total bilirubin levels of 5 mg/dl or more) and coagulopathy (INR of 1.5 or more, or prothrombin activity of less than 40%) complicated within 4 weeks by clinical ascites, HE, or both [5].
A 2. Definition of acute-on-chronic liver failure (ACLF)
A 2.1. Potentially reversible syndrome that occurs in patients with chronic liver disease, with or without previously diagnosed cirrhosis, characterized by acute hepatic decompensation that may be triggered by an intrahepatic, extrahepatic, or unknown precipitating factor, resulting in liver failure (jaundice and coagulopathy) and associated with one or more extrahepatic organ failure. It has high short-term (3-month) mortality in the absence of treatment of the underlying liver disease, liver support, or transplantation.Fig. 1. (Key concept / Expert's opinion).
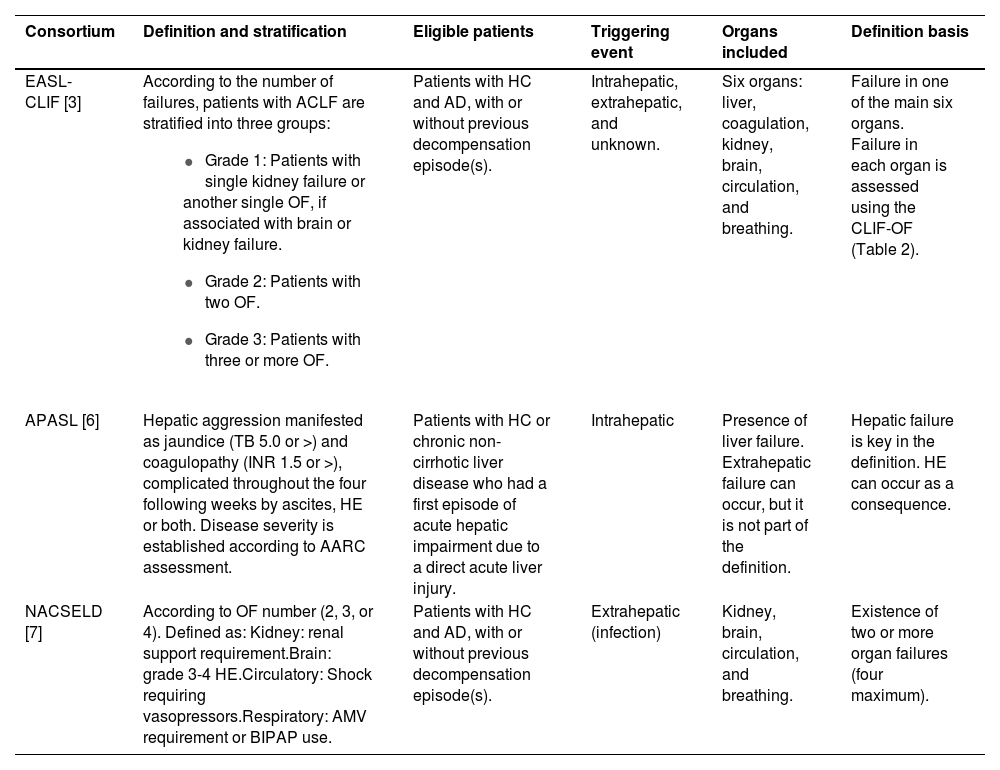
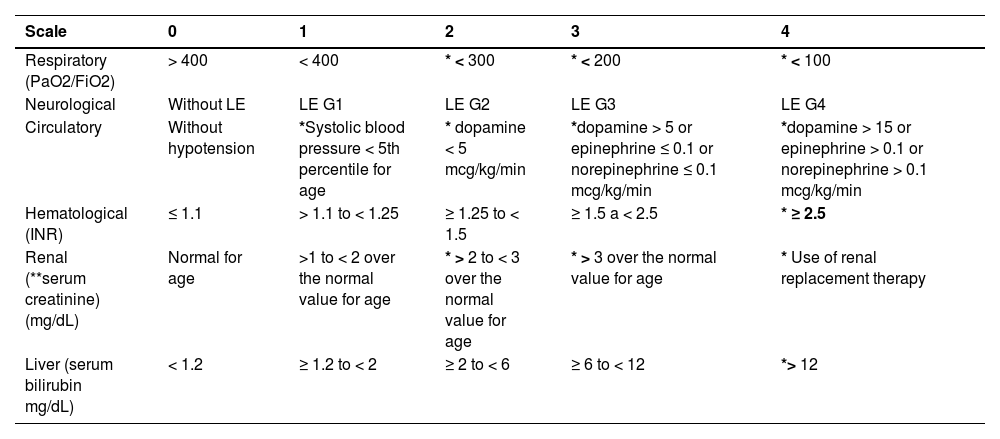
The most cited definitions in literature were developed by three consortia: EASL-CLIF, NACSELD (North American Consortium for the Study of End-Stage Liver Disease), and APASL (Asian Pacific Association for the Study of the Liver); however, the most widely used and validated is that proposed by the European consortium. These definitions differ according to the triggering event, the liver disease etiology, and the definition of organ failure. (Table 1)
Definitions of ACLF by three consortia: EASL-CLIF / NACSELD / APASL.
| Consortium | Definition and stratification | Eligible patients | Triggering event | Organs included | Definition basis |
|---|---|---|---|---|---|
| EASL-CLIF [3] | According to the number of failures, patients with ACLF are stratified into three groups:
| Patients with HC and AD, with or without previous decompensation episode(s). | Intrahepatic, extrahepatic, and unknown. | Six organs: liver, coagulation, kidney, brain, circulation, and breathing. | Failure in one of the main six organs. Failure in each organ is assessed using the CLIF-OF (Table 2). |
| APASL [6] | Hepatic aggression manifested as jaundice (TB 5.0 or >) and coagulopathy (INR 1.5 or >), complicated throughout the four following weeks by ascites, HE or both. Disease severity is established according to AARC assessment. | Patients with HC or chronic non-cirrhotic liver disease who had a first episode of acute hepatic impairment due to a direct acute liver injury. | Intrahepatic | Presence of liver failure. Extrahepatic failure can occur, but it is not part of the definition. | Hepatic failure is key in the definition. HE can occur as a consequence. |
| NACSELD [7] | According to OF number (2, 3, or 4). Defined as: Kidney: renal support requirement.Brain: grade 3-4 HE.Circulatory: Shock requiring vasopressors.Respiratory: AMV requirement or BIPAP use. | Patients with HC and AD, with or without previous decompensation episode(s). | Extrahepatic (infection) | Kidney, brain, circulation, and breathing. | Existence of two or more organ failures (four maximum). |
EASL-CLIF, European Association for the Study of the Liver–Chronic Liver Failure; APASL, Asian Pacific Association for the Study of the Liver; NACSELD, North American Consortium for the Study of End-Stage Liver Disease; HC, hepatic cirrhosis; AD, acute decompensation; OF, organ failure. CLIF-OF´s: HE: hepatic encephalopathy, AMV: assisted mechanical ventilation, BIPAP: Bilevel Positive Airway Pressure; INR, International normalized ratio; TB, Total bilirrubin.
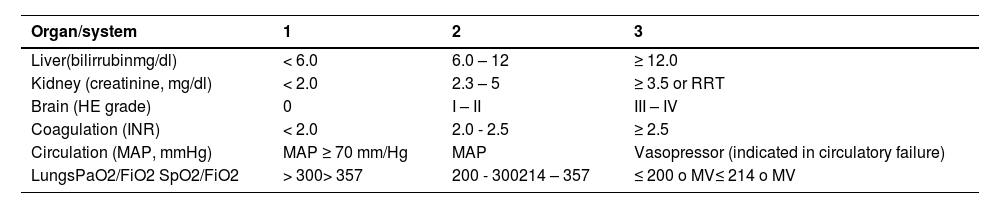
Score for organic failure assessment, proposed by EASL-CLIF.
mg/dl, milligram/deciliter; RRT, renal replacement therapy; HE, hepatic encephalopathy; INR, international normalized ratio; MAP, mean arterial pressure; mmHg: millimeters of mercury; PaO2, partial pressure of oxygen in arterial blood; FiO2, fraction of inspired oxygen; SpO2, oxygen saturation; MV, Mechanical ventilation.
*Organ failure indicated in shaded areas.
APASL
Acute hepatic insult manifested by jaundice (total bilirrubin ≥ 5 mg/dl) and coagulopathy (INR ≥ 1.5 or prothrombin activity less than 40%) complicated within four weeks by ascites, HE or both in a patient with chronic hepatic disease or cirrhosis, whether previously diagnosed or not, associated with high 28-day mortality [6].
NACSELD:
Cirrhosis with two or more severe extrahepatic organic failures from the four described: brain (grade III/IV HEy), renal (renal replacement therapy), circulatory (shock), and respiratory (mechanical ventilation) [7].
EF-CLIF:
Syndrome developed in cirrhotic patients, characterized by acute decompensation (AD), organ failure (hepatic, renal, brain, coagulation, circulation, or respiratory) and high short-term mortality [3].
GROUP B PATHOPHYSIOLOGY OF CIRRHOSIS: ROLE OF INFLAMMATION AND ROLE OF MICROBIOTA
Coordinator: Dr. Nalu Navarro-Alvarez
Participants: Dr. Jesús Alejandro Ruíz Manríquez, Dr. Rafael Trejo Estrada, Dr. Norberto Chávez Tapia, Dr. Luis Carlos Solís Gasca, Dr. José Antonio Caldera.
B 1. Pathophysiology of cirrhosis and portal hypertension Cirrhosis is the final stage of multiple chronic liver diseases that produce a diffuse hepatic fibrosis process where the normal architecture of the liver is replaced by regenerative nodules [8]. In general terms, the disease can remain asymptomatic for a long period of time (called compensated cirrhosis) or manifest through symptoms that are secondary to the progression of the disease (called decompensated cirrhosis). Advanced stages and complications of cirrhosis are characterized by systemic functional alterations, which are consequence of alterations in the liver architecture and liver dysfunction as such.
B 1.1. Structural changes of hepatic cirrhosis are coupled with an alteration of the intrahepatic balance of vasodilator and vasoconstrictor agents, and an increase in splanchnic vasodilator agents that cause a decrease in the effective arterial volume, a decrease in blood pressure, and an increase in portal pressure. (Key concept / Expert's opinion)
B 1.2. The decrease in effective arterial volume causes the activation of compensatory mechanisms and intense vasoconstriction. The increase in portal volume due to sodium and water retention favors ascites formation, and intense vasoconstriction fosters renal dysfunction; these mechanisms cause a hyperdynamic circulation state that, together with splanchnic vasodilation, cause a reversal in the portal flow and dilation of portosystemic collaterals. (Key concept / Expert's opinion)
Portal hypertension is a complex and dynamic process. Initially, structural changes of cirrhosis cause an alteration in the architecture of the vessels, increasing resistance to the portal blood flow, this being the initial factor that causes portal hypertension [9,10].
There is also a dynamic component in hepatic resistance that produces important changes in portal pressure, given by a balance between vasoconstrictor and vasodilator agents. The most studied vasodilator agent is nitric oxide, with evidence that shows less production of nitric oxide in cirrhotic livers; this reduction increases hepatic resistance [8,11].
Consequently, the increase in portal pressure produces circulatory abnormalities; mainly splanchnic arterial vasodilation, which also increases portal pressure and blood flow towards portosystemic shunts [12]. This splanchnic vasodilation reduces the effective arterial volume and produces activation of counterregulatory systems (sympathetic nervous system, renin-angiotensin-aldosterone system, and vasopressin release), producing sodium and water retention, hence an increase in plasmatic volume (culminating in ascites). All this produces a high-expense heart failure and extra-splanchnic compensatory vasoconstriction (splanchnic steal phenomenon) [12] that may end up in hepatorenal syndrome.
B2. Role of inflammation in cirrhosis pathophysiology
Liver inflammation is considered a common trigger for cirrhosis and the main cause of liver tissue damage. Initially occurring in the liver, this inflammatory process spreads to the circulation and contributes to the progression and development of more advanced stages of cirrhosis. Inflammation is, therefore, an important contributing factor to cirrhosis pathophysiology.
B 2.1. Patients with acute decompensation present a significant systemic inflammation grade, which is exacerbated and contributes to the development of ACLF. (Key concept / Expert's opinion)
Cirrhotic patients present a significant immunological dysfunction that leads to the development of systemic inflammation and immune deficiency, which is known as cirrhosis-associated immune dysfunction [13]. Both systemic inflammation and immune deficiency grades are closely related to the cirrhosis stage. In compensated cirrhosis patients, the systemic inflammation grade is low, while it increases progressively in those with acute decompensation and it is severely exacerbated in ACLF patients [13]. The intensity of this cirrhosis-associated immune dysfunction directly contributes to cirrhosis progression and is correlated with liver insufficiency severity, bacterial translocation, and organ failure [14].
Both the CANONIC study and those studies derived from it, such as the PREDICT study, have demonstrated that inflammatory components such as IL-6, IL-8, TNF-α [3,15-17], anti-inflammatories such as IL-10 and TGFβ [16,17], cytokines involved in monocyte migration, macrophages, and chemotaxis pathways such as VCAM-1, ICAM-1, and GM-CSF [3,15,16] exist among the cytokines and mediators that are altered in these patients. Initially, these alterations are moderate in compensated patients, demonstrating a slight elevation of proinflammatory cytokines and a decrease in anti-inflammatory components. However, as the disease progresses to a state of acute decompensation, systemic inflammation increases, reflected in a considerable elevation of inflammatory cytokines, but also of anti-inflammatory cytokines as a compensatory mechanism for the important inflammatory process [16]. Nevertheless, these mechanisms are completely deregulated in ACLF patients, where both are highly elevated, and there is a loss of these regulatory mechanisms, leading to immunological paralysis [16,17]. Some of these markers have been correlated with poor prognosis and mortality [16].
B 2.2. The systemic inflammation observed in decompensated cirrhosis and ACLF patients is a product of the release of pathogen-associated molecular patterns and damage (PAMPS and DAMPS) into the circulation from exogenous precipitants, bacterial translocation, and cell damage. This leads to the activation of immunological and non-immunological cell populations and, as a consequence, inflammatory mediators production and mitochondrial dysfunction that aggravate the disease. (Key concept / Expert's opinion)
The scientific evidence that shows that systemic inflammation in decompensated cirrhosis and ACLF patients are a product of PAMPS release comes from studies carried out in the 90s, where it was shown that endotoxins were detected both in plasma as in ascites fluid in liver disease patients, and that all this was associated with a poor prognosis [18]. It is currently known that there is a close relationship between bacterial translocation and systemic inflammation in cirrhotic patients [19]. Similarly, the important role of DAMPS from cell damage as an important contributor to this systemic inflammation has been demonstrated.
The most recent findings propose systemic inflammation as the common denominator that acts together with mechanisms originally known to be responsible for acute decompensation to contribute to multiple organ failure development, which is present in ACLF patients [20].
This happens through: 1) alteration of the pre-existing circulatory dysfunction that leads to a decrease in effective arterial volume, caused by a deregulation of the endogenous vasodilation and vasoconstriction mechanisms. PAMPS and DAMPS release induces nitric oxide overproduction, causing a decrease in effective arterial volume and, as a consequence and as a compensatory mechanism, vasoconstriction mechanisms are activated, as in the case of acute kidney injury, where there is a significant decrease in renal perfusion and decreased glomerular filtration, with acute kidney injury as a result [21]. 2) immune cells activation mediated by PAMPS and DAMPS from exogenous precipitants, such as bacterial infections and alcoholism, respectively, that cause damage to the organ directly or through their secretion products, leading to dysfunction [3,20,22]. There is activation of the inflammasome in immune cells, which leads to the release of inflammatory cytokines such as TNF-α and IL-1β. TNF-α directly activates apoptotic and necrotic pathways, causing direct tissue damage [23]. IL-1β generated through this activation amplifies inflammation and the production of chemotactic mediators that recruit inflammatory cells, which can also contribute to direct tissue damage. An important example is neutrophils and monocytes, which have been shown to contribute to the progression of liver disease when recruited to different tissues, such as the liver [24]. 3) Mitochondrial dysfunction, caused by excessive consumption of nutrients by the immune cells, which need to continue perpetuating the inflammatory process; this results in less availability of nutrients in the peripheral organs, and therefore a decrease in vital energy production to maintain organ functionality. Using a blood metabolomics study carried out on patients in the CANONIC study, Moreau et al show that ACLF patients have significant mitochondrial dysfunction, represented by a marked decrease in beta oxidation in peripheral tissues and a decreased energy production as consequence [25].
B 2.3. There is no specific marker of systemic inflammation in acute decompensation and ACLF patients. However, both the increase in leukocyte count and C-reactive protein (CRP) could be used as indicators, which are associated with a greater severity of the disease and worsening in the clinical course. (Key concept / Expert's opinion)
Compensated cirrhosis patients present a normal or even decreased leukocyte count, reflecting leukopenia [26]. However, this leukocyte count has been seen significantly increased in decompensated cirrhosis and ACLF patients, and this is accompanied by PCR elevation indicating systemic inflammation.
The first evidence of the above was reflected in the CANONIC study, where both parameters were higher as the ACLF grade increased [3]. Likewise, this systemic inflammation has been associated with a worse prognosis in the clinical course and cirrhosis spectrum evolution from stable decompensated cirrhosis to pre-ACLF grade. Evidence comes from the PREDICT study, where 1071 decompensated cirrhosis patients were analyzed and divided into stable decompensated cirrhosis, unstable decompensated cirrhosis, and pre-ACLF patients. Elevated CRP levels and an elevated leukocyte count were found in all these patients when compared with compensated cirrhosis. Interestingly, patients in the pre-ACLF group who progressed to ACLF had a significant increase in both inflammatory parameters [15]. However, it is important to consider the limitation that both the leukocyte count and PCR have also been used as markers that reflect systemic inflammation, as well as predictors of many other different diseases [27]; therefore they are not specific for cirrhosis and these markers’ values must be cautiously interpreted.
B 2.4. Acute decompensation and ACLF patients present immunological paralysis, which makes them more susceptible to the development of infections. (Key concept / Expert's opinion)
It is well known that, despite having an exacerbated systemic inflammation, decompensated cirrhosis and ACLF patients paradoxically present a significant alteration in their ability to respond to pathogens; consequently, they are more susceptible to the development of infections [3,17].
Said alteration in the response capacity is the well-known immune paralysis, which is the result of immune cells exhaustion and dysregulation [17]. Immune paralysis affects cells of both the innate and the adaptive immune systems. Monocytes in ACLF patients have been demonstrated to show decreased HLA-DR expression within the innate immune system. When stimulated with LPS, these monocytes have a decreased ability to present antigen and to secrete TNF-α [28]. In addition, it has also been observed that ACLF patients have an increase in immunoregulatory monocytes and macrophages that express the MERTK receptor, a receptor that suppresses the innate immune response [29].
There is also evidence of different immunological populations, such as T cells, within the adaptive immunity branch. Specifically, it has been shown that CD8 cells of decompensated cirrhosis patients have a suppressive phenotype with HLA-DR expression and an increase in inhibitory receptors, such as CTLA-4, PD-1, and TIMP-3 [30]. In general terms, the increase of all these previously described immunological populations has been associated with poor prognostic outcomes [29,30].
B 3. Role of microbiota in ACLF pathophysiology
A better understanding of physiopathogenesis in the evolution of complications, from compensated cirrhosis through decompensated cirrhosis, and finally to its most severe form of damage, ACLF, resides in understanding the bidirectional alterations of the intestine-liver axis.
B 3.1. Dysbiosis begins before detectable liver damage, remains, and exacerbates as the liver disease progresses. Factors promoting said change in bacterial diversity include alterations in the function and permeability of the intestinal barrier, intestinal motility, bacterial overgrowth, immune system, enterohepatic circulation, portal hypertension, and lymphatic drainage. (Key concept / High quality evidence)
In liver cirrhosis patients, intestinal microbiome is affected by multiple intestinal and systemic alterations. Dysbiosis can occur before liver damage, remains, and exacerbates as the liver disease progresses. Factors promoting said change in bacterial diversity include: alterations in the function and permeability of the intestinal barrier, intestinal motility, bacterial overgrowth, immune system, enterohepatic circulation, portal hypertension, and lymphatic drainage [31].
One of the main mechanisms promoting dysbiosis is cholestasis and reduced bile flow, which affects enterohepatic circulation and decreases circulation of intestinal bile acids [32]. In cirrhosis, primary bile acids secretion is decreased and intestinal secondary bile acids are increased, worsening as liver damage severity progresses [32]. Due to cholestasis, bile acids do not reach the intestinal lumen, thus preventing the expression of their antimicrobial properties in the microbiota and favoring bacterial overgrowth [33]. There is also alteration in farsenoid receptor function. Farsenoid X receptor synthesizes antimicrobial peptides and modulates innate immunity, making it a crucial component in epithelial and vascular barrier homeostasis [34].
Through peristalsis, the distal luminal propulsion of the content is a critical factor for intestinal bacterial replication and colonization inhibition [35]. Alterations in intestinal motility have been identified with an increase in migratory motor complex duration [36,37] and an increase in sympathetic tone, as an attempt to counteract splanchnic vasodilatation [38].
Intestinal barrier damage, derived from aspects previously referred to as the resulting bacterial overgrowth, is both physical and immunological and parallels cirrhosis progression [31,34]. Tight junctions are affected and there is peroxidation of the brush border of the membrane and increased macromolecules permeability [34]; as well as alteration in the intestinal vascular barrier, lymphatic translocation, decrease in synthesis and release of antibacterial peptides, IgA, defensins, involvement in innate immunity, alteration in peptides synthesis by Paneth cells, alteration in cell phagocytosis [34], and hypochlorhydria. Hypochlorhydria occurs in cirrhosis even in the absence of the use of proton pump inhibitors and is another factor that promotes dysbiosis [31,39]. Lastly, other factors of bacterial overgrowth include recurrent hospitalizations, use of antibiotics and proton pump inhibitors, and instrumental procedures [40].
B 3.2. In cirrhosis, particularly in advanced stages, there is a significant intestinal dysbiosis. This dysbiosis is characterized by an overgrowth of some potentially pathogenic bacteria, together with a decrease in some beneficial native bacteria. (Key concept / Expert's opinion)
Changes in the microbiota occur early in chronic liver disease development, even before detectable liver damage, especially in alcohol-related chronic liver disease and nonalcoholic fatty liver disease;[41,42] however, the microbiota abnormalities pattern in cirrhosis is independent of etiology [32,43]. Different studies have shown changes in the intestinal microbiome composition in different chronic liver diseases; nonetheless, a common characteristic of these changes, which is easy to assess, is the massive reduction of microbial diversity throughout cirrhosis development and the even higher reduction in decompensation [43,44]. A decrease in the Ruminococcacea family, an increase in Escherichia and Clostridium in non-alcoholic steatohepatitis patients and a decrease in Bacteroidacae, Lactobacillus, Pediococcus, Enterobacteriae, and Lactococcus in patients with alcohol-related damage, and an increase in Prevotella in both etiologies have been observed [41]. Different studies have shown that dysbiosis is accompanied by an overgrowth of some potentially pathogenic bacteria, together with reduced amounts of some beneficial native bacteria, which could contribute to bacterial translocation and increased risk of infections. There is a reduction in autochthonous taxa, including Lachnospiraceae, Ruminococus, and Clostridiales XIV, and an increase in pathogenic taxa, such as Enterococcaceae, Staphylococcaceae, and especially Enterobacteriaceae, an alteration that seems to worsen as the disease progresses [44]. Furthermore, these abnormalities have been shown to be correlated with the development of some complications of the disease, particularly bacterial infection and hepatic encephalopathy [44,45]. As previously mentioned, cirrhosis is characterized by the existence of marked alterations in the intestinal microbiome composition and by an enrichment of pathogenic microbial species in the intestine, which are not the usual ones, particularly enterecoccus species, some of them from the oral flora. The enrichment of patients' stools in taxonomically oral-origin species and Lactobacillaceae seems to be related to the change in the salivary microbiota, proton pump inhibitors, and relatively low levels of gastric acid [46].
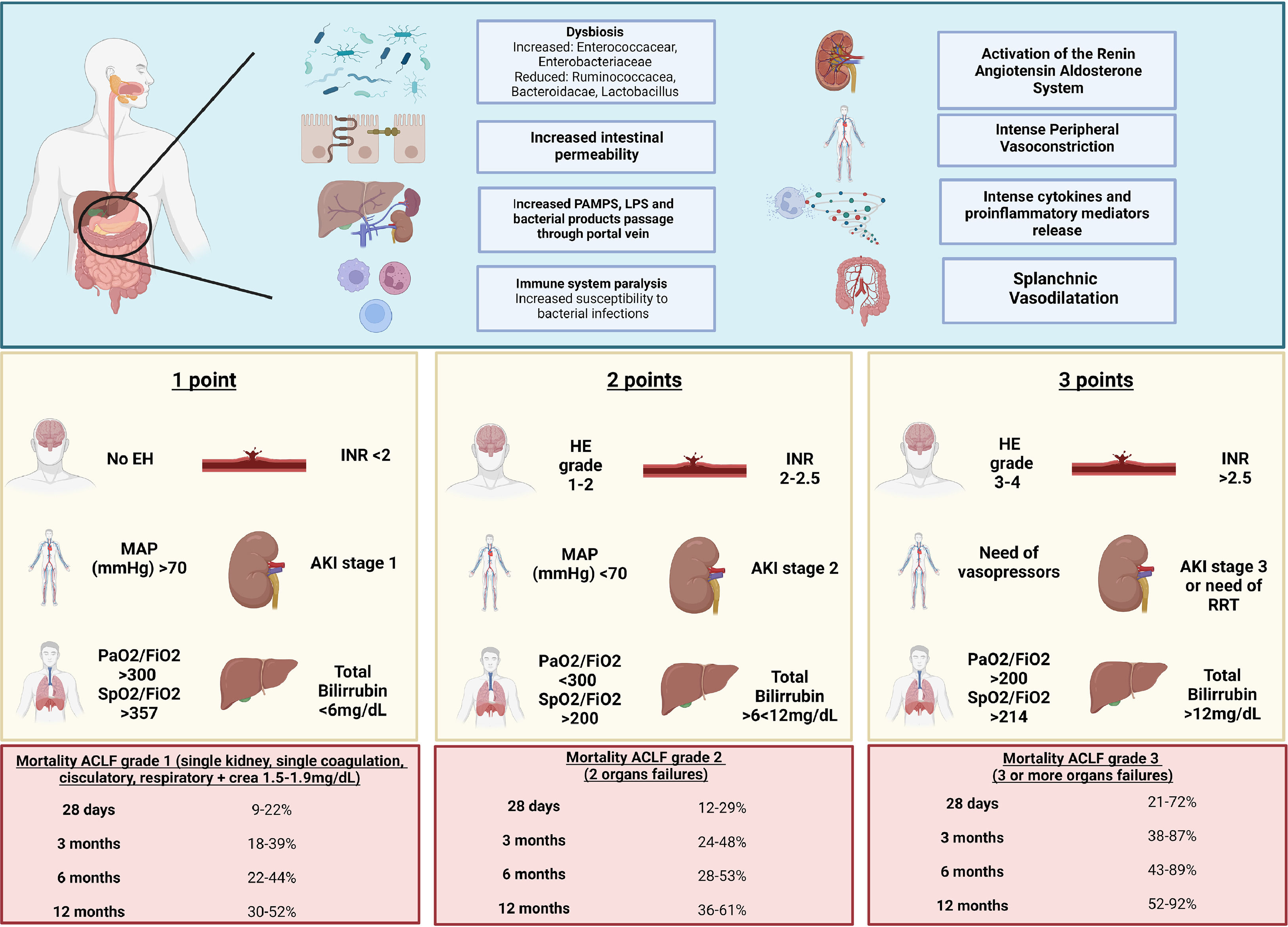
B 3.3. Intestinal barrier function alteration secondary to dysbiosis promotes greater bacterial translocation, development of infections, greater vasodilatation, and systemic inflammation. These factors contribute to acute decompensation and multi-organ failure. Dysbiosis in cirrhotic patients is an important contributor to disease progression. (Key concept / Expert's opinion)
Dysbiosis affects intestinal barrier function and thus promotes greater bacterial translocation, which ultimately leads to the development of infections, more systemic inflammation, and vasodilation. In turn, this contributes to acute decompensation and multiorgan insufficiency [47].
Alteration in the intestinal microbial diversity and the resulting bacterial products cause inflammation and compromise the intestinal barrier, in addition to changing the behavior of hepatic steatosis towards an inflammatory phenotype, even before the detection of event liver damage [41]. Dysbiosis grade increases with liver damage progression and correlates with increase in endotoxemia and clinical manifestations [48].
Dysbiosis worsens during decompensation. Fecal microbial genetic richness, microbial richness, and species diversity decrease in decompensated cirrhosis patients, compared with compensated cirrhosis, and these changes increase as the disease progresses, being maximum in its most severe form: ACLF [49,50]. One possible mechanism is that, as liver disease progresses, the composition and richness of the gut microbiome may be modified by altered bile acid composition and also by the influence of agents responsible for the development of cirrhosis, such as alcohol. In parallel, altered gut microbiome and low gene counts can lead to altered microbiome functionality, which may be a key factor in the induction and maintenance of gut inflammation, intestinal barrier disruption, and translocation of microbial material to the lamina propria and adjacent organs, which aggravates inflammation and systemic and hepatic dysbiosis that exists in cirrhosis and that together can contribute to disease progression [50].
Recent studies have demonstrated that intestinal bacterial translocation, inflammation, and immune disorders play important roles in ACLF pathogenesis [51]. A compromised intestinal mucosal barrier and altered bacteria-mediated immune responses promote liver inflammation in ACLF [52]. Acute inflammatory storms in the liver caused by TB from the intestine, as well as inappropriate responses of the innate immune system and the subsequent development of intra- and extrahepatic circulatory dysfunction ultimately lead to multi-organ failure [51]. It can be concluded that decompensated cirrhosis progression to ACLF is associated with extensive systemic inflammation that activates many inflammatory systems and cytokine pathways [53]. Systemic inflammation and single or multiple organ failure in ACLF patients are significantly associated with intestinal dysbiosis, bacterial translocation, and altered metabolic pathways development, as well as by many of the altered metabolites of microbial dysbiosis [54].
In conclusion, intestinal dysbiosis is associated with a worse ACLF pathogenesis than cirrhosis-associated pathogenesis, with changes in microbiota composition being what correlates with the liver disease severity [55]. Many studies have reported that systemic inflammation from bacterial infection and alcohol are directly correlated with ACLF severity;[52,56,57] yet 40% - 50% of ACLF patients have systemic inflammation without any identifiable precipitating trigger [3]. Systemic inflammation mechanism suggests that metabolites produced by the intestinal microbiome can affect the systemic compartment, via bacterial translocation, and trigger systemic inflammation [58]. Systemic inflammation can induce single or multiple organ failure in cirrhotic patients, where ACLF is its most severe expression. Therefore, the role of gut dysbiosis could be considered an important factor in ACLF precipitating factor, diagnosis, treatment, and prevention management [25].
GROUP C GENETICS IN ACLF: ACLF GRADES AND MORTALITY, ACLF IN CHILDREN, ACLF AND COVID-19 OUTCOMES
Coordinator: Dr. Aldo Torre
Participants: Dr. Carlos Moctezuma, Dr. Jonathan Aguirre, Dr. Judith Flores Calderón
C 1 Genetics in ACLF ACLFis a complex syndrome that develops in cirrhotic patients, and is characterized by acute decompensation, organ failure, and short-term mortality. Imbalance in the immune function is key in pathogenesis and results from an excessive systemic inflammatory response that derives in organ failure and mortality. This hyper-inflammatory state causes an inadequate response to guest at immune level; thus patients are more vulnerable to infections, organ dysfunction, and mortality.
C 1.1.Some genetic variants related to the innate immune system (i.e. NOD-2G908R, MBL_Yx, and MASP2_371) have been associated with an increased mortality risk in ACLF patients with bacterial infections. (Key concept / Expert's opinion).
Systemic inflammation intensity and immune system response to depend on genetic factors. Single nucleotide variants modulate the molecular inflammatory response by inducing changes in pattern recognition receptors (PRRs) or Toll Like receptors (TLRs). Genetic variants encoding these receptors, such as nucleotide-linked oligomerization domain 2 (NOD2), or lectin band-linked ligands (MBL), and MBL associated with serine proteases 2 (MASP) have shown increased short-term mortality in ACLF patients and acute insult associated with infections [59].
Schaapman et al[59] studied 21 single nucleotide polymorphisms (SNPs) in 826 patients with ACLF, included in the CANONIC study. Baseline characteristics, 547 occurrence of infections, and 90-day survival in relation to genetic 548 immunity variants were analyzed.
The NOD2-G908R genetic variant was associated with increased mortality (RR 2.25, p = 0.004), regardless of age and MELD score. This association was also found in a subgroup of bacterial infections (RR 2.78, p < 0.001), along with genetic variants MBL_Yx (RR 1.72, p= 0.008), and MASP2_371 (RR 1.67, p = 0.012).
C 1.2. There are two gene polymorphisms related to inflammation, in particular the IL-1 genetic cluster, which have been associated with a lower inflammatory response and protection against ACLF development. These polymorphisms are rs1143623 for IL 1β, and rs42511961 for IL 1a [60].(Key concept / Expert's opinion)
C 2. ACLF grades and mortality
The difference in ACLF prevalence at a global level, as well as the difference in mortality by regions of the world [61], can be explained by the different definitions of ACLF, its triggers, and chronic liver disease etiology, without being able to conclude on ethnic-genetic differences.
ACLF global prevalence is 35% (95% CI, 33% to 38%) amongst liver cirrhosis patients admitted to hospital for decompensation, being the highest in South Asia (65%); on the other hand, 90-day mortality was 58% (95%, CI 51% to 64%), the highest in South America (73%).
ACLF-associated mortality is directly proportional to the number of organic failures established by the different evaluation systems.
As an expert group and Mexican consensus, given the large number of published studies and external validations [62], mortality percentages of the CANONIC group and the NACSELD group are mentioned.
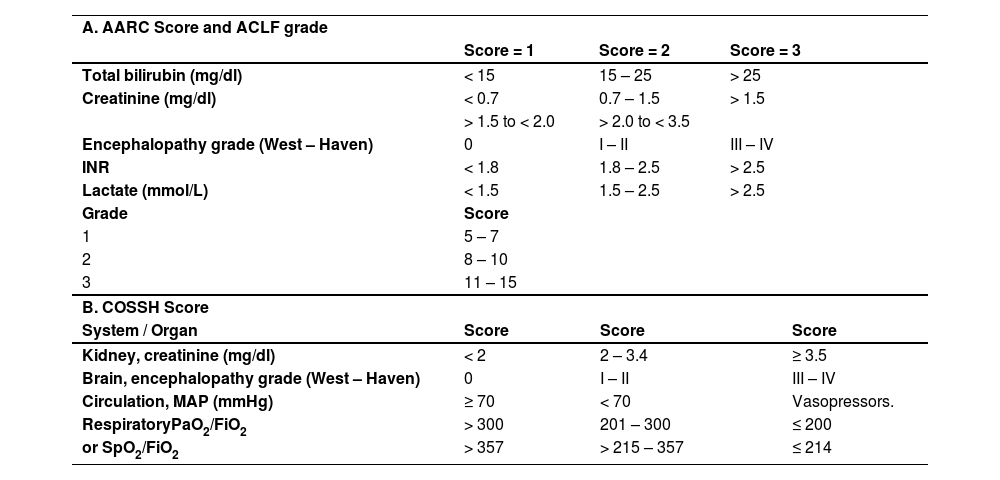
C 2.1. EASL-CLIF establishes 28-day liver transplant-free mortality of 23% in ACLF grade 1, 31% in ACLF grade 2, and 74% in ACLF grade 3; and the showed 30-day mortality of 49% with two organ failures, 64% with three organ failures, and 77% with four organ failures [3,63]. (Key concept / Expert's opinion)
C 3. Sarcopenia and progression to ACLF
Sarcopenia is defined as the pathological muscle loss in chronically ill patients. Different methods have been proposed to diagnose sarcopenia through cross-sectional images of the abdomen.
C 3.1. Sarcopenia has been associated with an increase in the risk of ACLF, postTransjugular intrahepatic portosystemic shunt (TIPS)ACLF, and long-term mortality in observational studies of chronical liver disease patients. ( K ey concept / Expert's opinion)
Univariate and multivariate analyses associated with 1-year survival in ACLF patients suggest an independent association between 1-year mortality and sarcopenia radiological parameters [64].
The presence of sarcopenia defined by the thickness of the transverse psoas muscle at the level of the umbilicus showed significantly higher rates of mortality, ascites, overt liver disease, encephalopathy, and ACLF development after TIPS placement, compared with the group without sarcopenia [65].
Skeletal muscle index determination (the total area of skeletal muscle at L3 level) to define sarcopenia has been associated with higher post-transplant mortality in cirrhotic men who required urgent liver transplantation [66].
Sarcopenia assessment by psoas measurements seems to be less sensitive in men than in women, and for transplanted ACLF patients [64].
C 3.2. Cystatin C > 1.5mg/L, sarcopenia, and MELD are independent ACLF predictors. (Key concept / Expert's opinion)
One relevant study retrospectively evaluated sarcopenia impact, determined by skeletal muscle index, on the impact for ACLF development. In the adjusted competitive risk regression analysis, Cystatin C (CysC) levels ≥ 1.5mg/L, the presence of sarcopenia, and the MELD-Na score were independent predictors of ACLF development in patients on the liver transplant list, while CysC levels ≥ 1.5mg/L, the presence of sarcopenia and albumin were independent mortality predictors. 12-month mortality cumulative incidence was 4% (95% CI, 0% - 16%) in patients with sarcopenia and CysC < 1.5 mg/L; 12% (95% CI, 4% - 25%) in patients without sarcopenia and CysC ≥ 1.5 mg/L; and 34% (95% CI, 18% - 51%) in patients with sarcopenia and CysC ≥ 1.5 mg/L (p <0.001). No patient without sarcopenia and with CysC < 1.5 mg/L died within a 12-month follow-up. Cumulative incidence of ACLF and 12-month mortality was 2% (95 CI, 0% - 10%) in patients without sarcopenia and with CysC < 1.5 mg/L, and 50% in patients with sarcopenia and the presence of CysC ≥ 1.5mg/L [67].
C 4. ACLF and outcomes in COVID 19
COVID 19 is associated with a risk of greater severity in the disease presentation and mortality in cirrhotic patients. Mortality risk is even greater in decompensated cirrhosis patients. Data are limited in liver transplant patients, suggesting that post-transplant mortality depends on age and/or comorbidities [68].
C 4.1. Chronic liver disease patients hospitalized for COVID 19 seem to develop higher ACLF and ACLF-associated mortality when compared with non-electively hospitalized patients for other acute events. (Key concept / Expert's opinion)
COVID 19 can cause decompensation or worsen basal cirrhosis. Lavarone et al showed that severe COVID 19 in cirrhotic patients increases bilirubin, prothrombin time, and creatinine, and decreases albumin levels. Patients with MELD ≥ 15 increase from 13% to 26% (p = 0.037), with ACLF in 28% [69]. On the other hand, Moon et al report that 25% had a new decompensation event after the COVID 19 diagnosis, with mortality higher than 50% [70].
COVID 19-related ACLF is common and is associated with significant mortality. In the study by Chalimar et al, nine patients had ACLF, with 100% mortality. COVID 19-related mortality in ACLF is significantly higher than in historical controls with ACLF [71].
In an Indian study of 57 cirrhotic COVID 19 patients, 20 (35%) presented ACLF. Patients in the ACLF group had a longer hospital stay, more severe COVID 19 forms, longer stay in the intensive care unit, and higher mortality: 30% vs. 5%. Patients who died in the ACLF group had higher CLIF C scores [72].
The study by Marjot et al found that 50% of cirrhosis and acute decompensation patients developed ACLF. Mortality was higher in ACLF patients than in those without ACLF (65% vs. 22%) among cirrhotic patients [73].
C 5. ACLF in Steatotic Liver Disease (SLD)
SLD is currently known to be the second most common underlying liver disease, after alcohol-associated liver disease, in waiting-list patients in the United States, and is also known to be the etiology that has increased the most in relation to transplant patients due to hepatocarcinoma [74,75].
C 5.1.SLDis the fastest growing etiology of underlying liver disease in ACLF patients on the transplant waiting list in recent years, and is expected to be the most common cause in the future. (Key concept / Expert's opinion)
Studies in ACLF patients have determined that around 10% - 20% of them have SLD as the underlying disease [76]. There is little information regarding the temporal trend of the underlying disease in patients who develop ACLF, and available statistics come from the United States.
In a United Network for Organ Sharing (UNOS) database analysis of 20,587 patients enrolled by ACLF in the United States between 2005 and 2017, of whom 20.4% had SLD, it was found that the largest percentage increase in baseline disease had been in SLD, from 134 patients in 2005 to 574 in 2017, representing a 332% increase. When compared with other underlying liver diseases, SLD patients have a higher prevalence of older adults [77], higher percentage of renal organ failure (i.e. 72%), and ACLF grade 1 (59.9%). Regarding the total SLD patients enrolled, this study also found that the proportion of those who are enrolled by ACLF has increased over the years.
In a United States study based on the Healthcare Cost and Utilization Project NIS database, regarding hospitalized patients, the time trend from 2006 to 2014 of hospital admissions due to ACLF was evaluated, both overall and by underlying liver disease.
There was a total of 1,928,764 hospitalizations due to cirrhosis during that period, of which 9.3% were associated with SLD, which was the etiology that increased the most in that period of time, since it represented 6% in 2006 - 2008 and increased to 12% in 2012 - 2014; that is, a 100% increase.
SLD patients were older when compared with other etiologies. Of the total admissions for cirrhosis, 112,174 (5.9%) met ACLF criteria, and of the total MAFLD admissions, 5% corresponded to ACLF, which was the etiology that presented the greatest increase in the period studied, being 3.5% in 2006 - 2008 and 5.7% in 2012-2014.
When compared with other underlying liver diseases, MAFLD and ACLF patients had a greater tendency to hemodynamic failure and to present an associated infection, as well as to develop sepsis and septic shock [78].
One of the factors that may be contributing to the increase in MAFLD-associated ACLF is obesity. A study of the UNOS database in 100,382 decompensated cirrhosis patients found that morbid obesity, by promoting a persistent state of low-grade inflammation, was a risk factor for ACLF development [79]. In this study, the presence of ACLF upon admission to the waiting list was more common in patients with morbid obesity when compared with grade I and II obesity patients and non-obese patients (23% vs. 16.5% vs. 15.9%, respectively, p<0.001). Relevantly, the most common etiology of underlying liver diseases in morbidly obese patients was MAFLD.
On the other hand, of the patients who did not have ACLF at the time of enrolling in the list, 7,630 had ACLF at the time of OLT; in multivariate analysis, both obesity grade I and II (HR 1.12, 95% CI, 1.05 - 1.19) and morbid obesity were associated with ACLF development (HR 1.24, 95% CI, 1.09 - 1.45). These results were replicated in an analysis of 287,502 hospitalized patients with decompensated cirrhosis from the NIS database.
C 5.2. MAFLD ACLF patients have lower 90-day and 30-day and in-hospital mortality when compared with ACLF in other etiologies. However, those MAFLD ACLF patients older than 60years have higher 1-year mortality when compared with ACLF in other etiologies. (Key concept / Expert's opinion)
In the previously mentioned study by Axley et al, which evaluated patients hospitalized for ACLF in the United States, in-hospital mortality was lower in MAFLD ACLF patients when compared with ACLF in other underlying liver diseases; in the multivariate analysis, MAFLD was associated with lower mortality when compared with viral hepatitis (OR 0.48, 95% CI, 0.45. - 0.51). However, MAFLD patients had a longer hospital stay, which translated into higher hospital costs per patient [78].
In the UNOS-based study by Sundaram et al, as discussed in the previous section, MAFLD ACLF patients on the liver transplant waiting list had lower mortality in multivariate analysis at 28 days (SHR 0.85, 95% CI, 0.76 - 0.96) and 90 days (0.84, 95% CI, 0.77 - 0.92) when compared with patients with HCV as the underlying disease, when the total number of patients listed by ACLF between 2005 and 2017 was analyzed.
However, when the analysis was limited to older adults enrolled for ACLF as of 2014, higher 1-year mortality was found in MAFLD ACLF patients when compared with patients with ACLF associated with alcohol-related liver disease (SHR 1.19, 95% CI, 10.04 - 1.34) or HCV infection (SHR 1.20, 95% CI, 1.02 - 1.39).
When performing a sub-analysis by ACLF severity, it was precisely in older adults that higher mortality was found on the waiting list in ACLF grade 1 patients, when compared with ACLF in alcohol-associated liver disease (SHR 1.24, 95% CI, 1.05 - 1.44) or HCV (SHR 1.35, 95% CI, 1.08 - 1.71), not so in ACLF grades 2 and 3, where mortality was similar regardless of the underlying disease.
Importantly, this study also analyzed 1- year post-transplant survival, which was lower in MAFLD ACLF patients when compared with that of alcohol-associated ACLF patients (88% vs. 92%, p = 0.002) [79].
C 6. ACLF in children
ACLF is an acute liver event associated with failure of other organs in patients with chronic liver disease (CLD), with or without cirrhosis, with high mortality rates. Criteria to define ACLF used in pediatric studies have been based on definitions used in adult patients [80,81] without having a consensus as such in the pediatric population [20].
C 6.1. ACLF in the pediatric population is not well characterized. In children, it has been defined by the presence of an acute hepatic event in patients without or with a previouschronic liver diseasediagnosis manifested by jaundice (total serum bilirubin ≥ 5 mg/dL), coagulopathy (INR ≥ 2.0), clinical and/or radiological ascites, and/orHEwith in the first four weeks. (Key concept / Expert's opinion)
In the European pediatric population, the term has been used according to the recommendations of the EASL-CLIF. Under this definition, a multicenter study was carried out including 130 cases between 1 month and 16 years of age, diagnosed with cirrhosis, who presented impaired liver function due to a precipitating factor that caused at least two organ failures;[82] in North America, following the NACSELD definition, a study was reported in 20/66 cirrhotic children and a mean age of four years. The criteria used was the presence of acute deterioration in liver function and at least one extra-hepatic organ failure (instead of two organ failures, as denoted by the original definition), within the first 24 hours of hospitalization [83].
The APASL recommended that the adult definition can be used in children, with modification for the recognition of both clinical and/or radiological ascites due to the difficulty for its identification in children, and the use of evaluation scales for children under 3 years of age for HE [6]. Under this criterion, three studies have been carried out in Asia where around 30 cases of children over 2 years of age in each report were included. The inclusion criteria was presenting acute liver injury with chronic liver disease or cirrhosis, with or without a previous diagnosis, with jaundice (total serum bilirubin (≥ 5 mg/dL), coagulopathy (INR ≥ 2.0), clinical and/or radiological ascites, and/or HE in a 4-week period [82,84,85].
Studies in ACLF children reported to date are scarce and vary according to the geographical region, the patients’ age, liver disease progression grade (chronic liver disease, with or without cirrhosis), and different diagnostic criteria, so there is no universal definition that can be recommended.
Certain parameters, different from those for adults, must be taken into account in the future in order to establish an adequate definition for pediatric patients, such as chronic liver disease etiology and age. The main cause of cirrhosis in children under two years of age is bile ducts atresia or metabolic diseases; the latter can occur without jaundice. Other factors to be considered are creatinine levels and blood pressure values, which must be modified according to the child's age, as well as the HE grade assessment with an appropriate scale, according to the patient's age [81–83].
A greater number of multicenter studies is required to validate and define ACLF criteria based on specific modifications for pediatric patients.
C 6.2. ACLF occurs between 10% to 13% in children with chronic liver disease. (Key concept / Expert's opinion)
ACLF prevalence in the pediatric population is not exactly known; however, taking three studies evaluated with the APASL and NACSELD definition into account, in which a greater number of cases was included, results showed that between 10% and 13% of chronic liver disease patients can develop ACLF [60,63,64], with around 60% survival with native liver [86,87].
C 6.3. There is no enough evidence for an ideal prognostic scoring system in ACLF children. The measurement scale based on the presence of organ failure for the pediatric population pCLIF-SOFA ≥ 11 predicts 28-day mortality and a value ≥ 7 for the need for LT. (key concept / Expert's opinion)
ACLF in children has been evaluated with different prognostic scales. A prospective study that included 31 CLDL children who developed ACLF reported 19.4% mortality; the study showed that a 6.50 cut-off value for SOFA (sequential organ failure assessment) scale to predict 28-day mortality had 100% sensitivity and 76.9% specificity [84]. Another scale evaluated in Pediatrics is CLIF-SOFA (Chronic Liver Failure-Sequential Organ Failure Assessment), with creatinine value modification according to the child's age, HE assessment with the West Haven scale for older children, and a modified scale for children under 3 years of age [88,89].
With a CLIF-SOFA cut-off value of 8.5 to predict mortality, Alam et al found 100% sensitivity and 64.7% specificity [87]. These scales have also been used to predict the need for LT in a study where patients with or without LT were compared; the average CLIF-SOFA values at the time of admission were 6.80 and 6.09 (p = .028), respectively [90].
Bolia et al[91] conducted a prospective study in 2018 for the assessment of the pCLIF-SOFA that included 110 chronic liver disease children, reporting a 28-day mortality of 33.6%. Risk factors for mortality were elevated INR (HR 1.17; 95% CI, 1.04 – 1.31; p < 0.001), serum bilirubin (HR 1.04; 95% CI, 1.01–1.08; p < 0.001), low levels of low serum sodium (HR 0.93; 95% CI, 0.89 – 0.98; p = 0.01), and serum albumin (HR 0.46; 95% CI, 0.27 – 0.77; p = 0.03). Those of greatest significance were the absence of treatable etiology (HR 2.00; 95% CI, 1.40 – 2.87; p = 0.001) and the presence of organ failure (HR 3.22; 95% CI, 1.98 – 10.58; p < 0.001).
In the multivariate analysis, organ failure and hyponatremia were independent factors of poor prognosis. From the evaluations carried out to determine the 28-day mortality prognosis, the pCLIF-SOFA (Table 3) showed to be better than Child Pugh and PELD. A ≥ 11 score was observed to predict 28-day mortality with 94.9% sensitivity and 91.5% specificity [89] and a ≥ 7 value for LT need. Similarly, Claude et al found in 130 studied children that a pCLIF-SOFA value > 7 on days 28 and 60 after the onset of the symptoms predicts the need for LT with a 77.3% and 44% sensitivity and 75.9% and 47.6% specificity, respectively [82].
Organ failure assessment: pCLIF-SOFA Score.
LE (liver encephalopathy) *Values indicative of organ failure. Dopamine, epinephrine, and norepinephrine values are expressed in mcg/kg/min. Kidney failure is defined as a 60% elevation of the normal mean creatinine value according to age and renal dysfunction with a 30% elevation.
Serum creatinine values according to age **: Infants < 1 year: (Cr > 0.5 mg/dl); children 1 - 12 years: (Cr > 0.9 mg/dl); adolescents (12 – 18): (Cr > 1.3mg/dl).
Studies are few; however, these results suggest that measurement based on the presence of organ failure, with the corresponding modifications for the pediatric patient, is an adequate tool to assess the prognosis and the need for LT in chronic liver disease children who develop ACLF.
C 6.4. ACLF mortality in children varies between 19% and 34% at 28days, and from 30% to 59% at three months; 49% to 80% survive with native liver, and between 8.9% and 24% undergoLT. (Key concept / Expert opinion)
ACLF mortality reported in children at 28 days is between 19.4% and 34% [82,84,87,92] and between 30.4% and 59% at three months, due to liver and multi-organ failure [86,88,93]. The number of organ failures is a determining factor; an increase in mortality of up to 29% has been reported when two organs are involved, 33% with three organ failures, and up to 66% with four organ failures [83]. In a pediatric transplant center, no deaths were reported in 24 cases. LT was carried out in 24.14%, with 75% native-liver survival. In these cases, a CLIF-SOFA cut-off level of 7.5 on the fifth day from admission early predicted the need for LT. The most significant biochemical parameter studied was INR determination, with a cut-off value of 3.04 (100% sensitivity and 79.6% specificity, 0.89 ROC; 95% CI, 0.76 - 1.00, p < .01) [90].
Survival with native liver is variable: 48% to 60% at 28 days [82,84,87,91] and 22% to 60% at 90 days [87,87,92]. The feasibility of receiving care in a center specialized in LT is an important factor.
C 6.5. ACLF treatment in children is supportive and the severity assessment with the prognostic evaluation systems can be useful to decide LT multiple organ failure development or advancedEH. (Moderate quality / Strong recommendation)
ACLF can be reversed using standard therapy; liver transplantation is the only alternative treatment for those patients with no response or progress. The recognition of the entity and the precipitating events before organ failure development is vital for patient survival [81,82,86].
ACLF precipitating factors in children are acute infections by endemic hepatotropic viruses (viruses A and E), for which there is no specific treatment [82,85], and other bacterial infections and sepsis susceptible to adequate antimicrobial therapy [82,84,92].
Other factors that have been considered to trigger ACLF are gastrointestinal bleeding [82,84,93] and underlying disease exacerbation, especially associated with lack of adherence to treatment in cases of autoimmune hepatitis and Wilson's disease, which is potentially preventable [85–87]. It is important to investigate the use of alternative therapies or hepatotoxic drugs; it is known that cirrhotic patients who develop a DILI-related (drug-induced liver injury) acute event are less susceptible to recovery [94]. This condition has been reported in children associated with herbal therapy and drugs [84,87,95].
Evaluation systems application for the recognition of extrahepatic organ failure is important for the detection of the need for LT and the timely initiation of vasoactive drugs, mechanical ventilation, and/or replacement therapy. A study in ACLF children showed that respiratory failure occurred in 74%, cardiovascular failure in 52%, renal failure in 30%, and HE in 39% during hospitalization; 56% of these cases presented more than two organ failures [83].
Extracorporeal liver support systems and plasmapheresis have been recommended as bridges to LT. These can be alternatives for children; however, they are not routinely recommended due to scarce expertise [81,85]. The lack of an intensive care unit, the possibility of performing LT, and a pCLIF-SOFA assessment greater than 7 are alerts for sending the patient to a specialized center [91,96].
Adapted and modified from [91].
GROUP D CLINICAL MANIFESTATIONS: ACLF GRADES AND MANAGEMENT
Coordinator: Dr. María Fátima Higuera de la Tijera
Participants: Dr. Ignacio García Juárez, Dr. Nancy Canedo, Dr. René Malé V, Dr. Iaarah Montalvo Gordon
D 1. Clinical manifestations and circulatory approach
D 1.1. In ACLF patients, circulatory failure is considered a decrease in mean arterial pressure (MAP) < 70mm Hg or when vasoconstrictors are required to maintain MAP at values > 70mm Hg. (Key concept statement)
Regardless of the precipitating event, in acute-on-chronic liver failure (ACLF) there are pathophysiological changes induced by a pro-inflammatory state that leads to acute deterioration of liver function associated with multiple organ failure development. ACLF is associated with different cellular mediators that produce profound alterations in macro and microcirculation, resulting in multiple organ failure, including circulatory failure [97]. An acute increase in portal pressure has been described in ACLF patients, with alterations in systemic and pulmonary hemodynamics; in fact, portal pressure significant basal elevation has been considered a poor independent prognostic factor in ACLF patients. Control of systemic and portal pressure has been strongly related to clinical and biochemical improvement in ACLF patients at 3-month follow-up [98,99]. A hyperdynamic state is observed in ACLF patients’ peripheral circulation, with decreased mean arterial pressure (MAP) and systemic vascular resistance (SVR) compared with those of compensated cirrhosis patients. The cardiac output (CO) has been detected elevated both in ACLF patients or in decompensated cirrhosis, compared with the measurements detected in compensated cirrhosis patients. Cirrhotic patients present particular and distinctive changes in hemodynamics that differentiate them from subjects without cirrhosis. In ACLF patients with an infectious process, these hemodynamic changes further accentuate septic shock-associated PVR decrease. PVR decrease is mainly mediated by an increase in nitric oxide (NO) induced by NO synthetase induction [100,101].
Vasodilation increase leads to an abnormal plasma volume distribution with hypervolemia in the splanchnic vascular bed and effective hypovolemia in the systemic circulation, with an increase in intrahepatic vascular resistance induced by the activation of endogenous vasoconstrictors (renin-angiotensin-aldosterone system), which promote water and sodium retention. In fact, NO, which is significantly elevated in peripheral circulation, is decreased in intrahepatic circulation. Alteration in vasoconstrictors and vasodilators balance has a fundamental role in the development of increased portal pressure in decompensated cirrhosis patients [100,101].
A decreased cardiac ventricular function under physiological conditions or surgical or pharmacological stress has been observed in liver cirrhosis patients. Cirrhotic patients affected by spontaneous bacterial peritonitis (SBP) present a ventricular function decrease, which favors hepatorenal syndrome (HRS) development [102].
D 1.2. The use of vasopressors, such as norepinephrine, and the administration of balanced crystalloid solutions to recover circulating volume is recommended for ACLF patients with circulatory failure associated with liver cirrhosis and acute decompensation. (Key concept statement)
Circulatory failure is one of the organic alterations that define ACLF syndrome as described by EASL-CLIF and by NACSELD. The most widely used definition is that described by EASL-CLIF, where a reduction in MAP < 70 mm/Hg or when drug use (dopamine, dobutamine, norepinephrine, epinephrine, terlipressin, or vasopressin) is necessary to maintain said pressure of 70 mm/Hg are considered significant [3]. In the presence of circulatory failure, the cautious use of crystalloid solutions is recommended, trying to avoid volume overload. Colloids use, including albumin, can be associated with vascular congestion, so their use is recommended with caution. The use of norepinephrine is preferably recommended to control MAP because it has a safety profile and greater efficacy compared with other drugs [80,103,104].
D 2. Clinical manifestations and renal approach
D 2.1. Acute kidney failure is the most frequent organ failure in ACLF patients; it is considered when kidney failure occurs, according to theAcute Kidney Injury(AKI) classification. (Key concept statement)
Kidney failure pathophysiology in ACLF is associated with the hemodynamic changes previously explained in the circulatory failure description [100,101]. These hemodynamic changes are not only associated with peripheral vasodilatation and intrahepatic vasoconstriction, but also in cases of HRS with renal vasoconstriction and alteration in renal tubular microcirculation [105]. Renal failure in ACLF is the most frequent failure and usually occurs in isolation in up to 20% of the cases described in the CANONIC study [3]. There is a reduction in creatinine production and in muscle mass in cirrhotic patients, so creatinine levels can overestimate the glomerular filtration rate (GFR). Because of this, dynamic changes over time in serum creatinine (SC) have been used to define renal damage in cirrhotic patients. AKI has been defined according to the International Club of Ascites (ICA) criteria, when an acute reduction in renal function is observed, assessed by an increase in serum creatinine (SC) of 0.3 mg/dl or more in less than 48 hours, or when there is an increase equal to or greater than 50% of SC baseline value [106].
Traditionally there are three types of acute kidney injury:
- a)
Prerenal azotemia that occurs due to renal hypoperfusion without tubular or glomerular damage that improves with volume administration.
- b)
Intrinsic renal failure secondary to tubular necrosis (ischemic or toxic), glomerulonephritis, or interstitial nephritis.
- c)
Post-renal failure, which occurs in cases of urinary tract obstruction and hydronephrosis.
Liver cirrhosis patients can develop these traditional types of acute kidney injury, but are also susceptible to developing HRS, a type of acute prerenal injury that is unresponsive to volume expansion and is seen exclusively in advanced liver injury patients, with or without acute decompensation [107].
According to ICA [106], acute kidney injury severity is categorized into the following stages:
- •
AKI Stage 1A, 0.3 mg/dL increase in SC over 48 hours or 1.5 to 2-fold increase in SC from baseline, with an absolute value < 1.5 mg/dL.
- •
AKI Stage 1B, 0.3 mg/dL increase in SC over 48 hours or 1.5 to 2-fold increase in SC from baseline, with an absolute value > 1.5 mg/dL.
- •
AKI stage 2, SC increase > 2-3 times from baseline.
Kidney failure is considered from AKI stage 2; according to EASL-CLIF, kidney failure is considered when SC is greater than or equal to 2 mg/dL. It is important to differentiate whether kidney failure is associated or not with HRS (HRS AKI or Non-HRS-AKI) [106].
Diagnosis criteria to consider the presence of HRS-AKI are the following:
- 1.
Cirrhosis with ascites.
- 2.
AKI 2 or 3.
- 3.
Lack of response (decrease by at least 0.3 mg/dL SC) after 48 hours of suspending diuretics and volume expansion with albumin 1gr/kg/day for two days.
- 4.
Absence of hypovolemic or septic shock that requires vasoactive drugs to maintain blood pressure.
- 5.
Negative history for the current or recent use of nephrotoxic drugs.
- 6.
Proteinuria <500 mg/dl or microhematuria < 50 red blood cells/mL.
D 2.2. Albumin and vasoconstrictors (terlipressin or norepinephrine) use is recommended in patients with ACLF and acute kidney failure AKI-HRS 2 and 3. (Low quality / conditional recommendation)
As a single treatment, albumin has not shown effectiveness in HRS AKI treatment, but its use is recommended as a complement in HRS treatment due to its anti-inflammatory effect and as a volume expander. Vasoconstrictors improve splanchnic and systemic hemodynamics, with subsequent improvement in renal function in type 1 HRS patients. Controlled studies, meta-analyses, and systemic reviews have shown the usefulness of vasoconstrictors such as norepinephrine and terlipressin combined with albumin in HRS AKI treatment, especially when increasing MAP above 10 mm Hg from the baseline value is possible. Vasoconstrictor drug administration for up to 14 days is recommended, as long as there is a response, as well as considering suspension on the fourth day in case of lack of response (< 25% reduction in SC). The most studied drug is terlipressin. If its use is considered, it is important to know its side effects, which include ischemic events, so its use would be contraindicated in patients with coronary or peripheral vascular disease. Likewise, terlipressin can be associated with respiratory failure in patients with lung damage, particularly in ACLF grade 3 patients. Patients who respond to terlipressin have a better survival rate compared with non-responders or patients who did not receive terlipressin [107–116].
The use of an evidence-based protocol for the treatment of HRS translated into higher survival. The authors suggest that the use of evidence-based protocols for the diagnosis and treatment of HRS could reduce cost and mortality in tertiary hospitals [117].
D 2.3. Renal replacement therapy use in ACLF patients is indicated only as bridging therapy for those who are potential candidates for liver transplantation. (Key concept statement)
Patients with kidney failure who do not respond to treatment should be considered candidates for liver transplantation. Renal replacement therapy (RRT) is considered for patients who are candidates for liver transplantation or for patients with a reversible factor of kidney damage. Patients who are not candidates for liver transplantation or patients with non-reversible kidney damage should be considered for palliative treatment. Liver transplantation should not be delayed in renal failure patients, since the main factor that predicts recovery of renal function after liver transplantation is the use of RRT for less than 14 days. Combined liver and kidney transplantation is considered recommendable in patients with a history of prolonged renal failure, in patients on RRT greater than 90 days, in patients older than 60 years of age, in chronic kidney damage patients, creatinine clearance ≤ 30 ml/min, renal biopsy with > 30% of glomerulosclerosis or fibrosis [115,116,118].
D 3. Clinical manifestations and coagulation approach
D 3.1. Coagulation failure in ACLF patients is defined as the presence of INR > 2.5. (Key concept statement)
Conventional coagulation tests such as PT/INR show poor prediction of bleeding risk and do not provide enough information to optimize the management of blood products in bleeding events [118,119].
Although INR may concomitantly increase as hepatic decompensation occurs, its usefulness is more related to the risk of short-term death than to the risk of bleeding [120,121]. PT/INR measurement corresponds to the formation of thrombin as a function of procoagulant factors and does not take into account the circulating anticoagulant factors that maintain homeostasis (rebalance theory) that justifies why the patient does not bleed [121]. Meta-analyses that combine data on different invasive procedures show that INR is not correlated with bleeding risk [121,122].
D 3.2. Conventional coagulation tests such as PT/INR show prediction of bleeding risk and do not provide enough information to optimize blood products management. Viscoelastic testing (TEG/ROTEM) provide a more physiologic assessment of coagulation and should be used to guide blood product requirements. (Moderate quality / Conditional recommendation)
Tests that measure thrombin generation reveal important information; however, their availability is limited, hence viscoelastic tests have become the point of treatment [121,123].
Viscoelastic tests’ importance lies in the fact that they dynamically reflect, in vivo, the cell-based coagulation theory with plasma interaction (coagulation factors), platelets, fibrin production, speed and hardness of the clot, as well as lysis. Result quality depends on sample pretreatment [124]. Coagulation and the need for blood products should be evaluated with dynamic viscoelastic tests (TEG/ROTEM) in all patients with failure and bleeding exacerbation who require an invasive procedure [119,124–127].
Measurement of protein C, protein S, and factor VII is recommended in cases of hypercoagulability with thrombosis in unusual regions, recurrence, or refractory to oral anticoagulation [119].
D 4. Clinical manifestations and pulmonary approach
D 4.1. Respiratory failure in ACLF patients is defined as a pO2/FiO2(PAFI) ratio ≤ 200 or SO2/ FiO2(SAFI) ≤ 214 or the need for invasive mechanical ventilation. (Key concept statement)
Pulmonary clinical manifestations accompany advanced liver disease [128]. Invasive ventilatory management is necessary in cases of West-Heaven III-IV encephalopathy [129,130].
D 4.2. To reduce pneumonia risk associated with invasive mechanical ventilation, maintaining the head position at 30° and subglottic suction are recommended. (Key concept statement)
Mechanical ventilation should be protective [129,131]. Ventilator-associated pneumonia (VAP) occurs in 10% - 20% of patients with invasive mechanical ventilation; [132] the early development of this entity is the main cause of morbidity and mortality in comatose patients [133].
A bundle of interventions is recommended to prevent VAP, including 30%-45% elevation of the head with the aim of limiting micro-aspirations of oropharyngeal or gastric contents, [132,134] subglottic aspiration, [132,133,135] daily interruption of sedation, and evaluation of the possibility of ventilation withdrawal [132,135–137]. The use of prophylactic systemic antimicrobial treatment may be useful to reduce pneumonia incidence in this population [138]; however, in the meta-analysis of systemic antimicrobials use in 10,988 comatose patients, it is associated with a decrease in early VAP (RR 0.32; 95% CI, 0.19 – 0.54) and a decrease in ICU stay (SD −0.32; 85% CI, 0.56 − 0.08), but without a significant difference in mortality (RR 1.03; 95% CI, 0.7 – 1.53) or in mechanical ventilation time (SD −0.16; 95% CI, −0.41 - 0.08) [133].
D 5. Clinical manifestations and brain approach
D 5.1. Neurological failure in ACLF is defined by the presence of West-Heaven grade III-IV encephalopathy. (Key concept statement)
Neurological failure is an independent prognostic factor for death [139,140]. Meta-analyses prove the prognostic value of encephalopathy in acute failure (OR 5.62, 95% CI, 6.30 – 9.82; p = <0.001) [141]. West-Heaven grade III-IV encephalopathy involves inflammatory etiology edema, hyperammonemia, and decreased jugular venous saturation, characterized by euphoria, bradypsychia, confusion, disorientation, disorientation in space, drowsiness, daytime hypersomnia, strange behaviors, clonus, nystagmus, Babinsky's sign, lethargy, and coma without verbal or visual response [142,143]. Extrapyramidal and hepatic myelopathy signs are rare but should be considered especially in men with documented large shunts and a history of multiple episodes of severe encephalopathy [144,145]. Excluding causes of HE of other etiology is necessary, such as neurological ones: cerebral infarcts, delirium, and withdrawal syndrome; metabolic such as hypothyroidism, hyperglycemic crises or hypoglycemia, dysnatremia, especially hyponatremia; infectious: especially urinary tract infection; pulmonary: hypoxemia; drugs: opioids and benzodiazepines [146,147]. Perform a simple head CT scan if the clinical state is unusual, if the onset of symptoms is abrupt and severe if there are focal neurological symptoms, and if response to treatment or anti-ammonium measures is limited [103,144]. The EEG should be carried out in order to stage encephalopathy severity; it is useful to monitor the patient and to allow the inclusion of the patient as a candidate for transplantation [104,144,147]. In the case of severe coma, the combination of somatosensory evoked potentials will inform about the residual cortical and subcortical activity [144,148].
D 5.2. The use of short-acting sedatives such as dexmedetomidine or propofol is preferable, preferably in bolus versus continuous infusion. (Very low quality, conditional recommendation)
The cerebral edema grade will indicate the dosage. The use of light sedation RASS -2 to 1 is feasible, safe, allows daily neurological evaluation, early decannulation, more days free of mechanical ventilation, shorter ICU stay, and decreased related adverse effects, lower hospital and 90-day mortality [104,149,150]. The use of dexmedetomidine preserves cognitive function, specifically attention [151]. A recent meta-analysis showed that the use of dexmedetomidine in inflammatory processes was associated with a marked reduction in the duration of mechanical ventilation (SD –0.53, 95% CI, −0.85 to −0.21, p = 0.001, I2 = 0%) and inflammatory mediators such as TNF-α: SD −5.27, 95% CI, −7.99 to −2.54, p < 0.001, I2 = 0% and IL-1β: SD −1.25, 95% CI, −1.91 to –0.59, p < 0.001, I2 = 0% [152]. Regarding the adverse effects of bradycardia and hypotension, both propofol and dexmedetomidine present the same prevalence [153].
D 5.3. The airway should be protected with orotracheal intubation with a Glasgow score ≤ 8 points or in the presence of West Haven grade III-IV encephalopathy. (Key concept statement)
Cerebral edema is the most frequent cause of death, so neurocritical care measures must be implemented to reduce intracranial pressure: head at 30°, neutral position, pCO2 and pO2 control, hypercapnia and hypoxemia must be avoided, which cause cerebral vasodilation and increase in intracerebral vascular content with concomitant elevation of intracranial pressure, MAP must be maintained between 85 - 90 mmHg to maintain cerebral perfusion pressure, if necessary with vasopressor use, preferably norepinephrine [103].
D 5.4. Enteral nutritional support is preferably recommended; parenteral nutritional support should be considered in selected cases. (Key concept statement)
Enteral nutritional intake is safe and feasible [154]. Prescribing a low-protein content diet should be avoided [154–156]. Prioritizing the intake of branched-chain amino acids such as valine, isoleucine and leucine is desirable, as well as avoiding aromatic amino acids consumption [154,155]. Branched chain amino acids improve symptoms (RR 0.73, 95% CI, 0.61 - 0.88; n = 827 patients; 16 studies). Regarding HE, a beneficial effect of branched amino acids was observed in meta-regression (RR 0.76, 95% CI, 0.63 - 0.92); they are also well tolerated, but nausea and vomiting are the most frequent adverse effects (RR 5.56; 95% CI, 2.93 - 10.5; low quality of evidence), no deleterious effects on the patient's nutrition or HE worsening; however, the evidence lacks robustness regarding mortality decrease [157]. Omega 3 fatty acid lipid emulsion will be effective in reducing endotoxemia and sepsis in ACLF [158].
D 5.5. Managing the triggering factors of hepaticHEand initiating empirical management with anti-ammonium measures is recommended. (Key concept statement)
Most HE episodes are related to precipitating factors ranging from increased ammonium production, increased ammonium diffusion through the blood-brain barrier, reduced toxin metabolism, increased GABA activation, and toxin consumption [159]. The mainstay of encephalopathy treatment is lactulose, which decreases the systemic absorption of ammonia, facilitating its elimination and preventing recurrence of episodes [160–166]. The most recent meta-analysis demonstrated benefit of lactulose on encephalopathy severity with NNT = 4, encephalopathy prevention NNT = 6, and mortality (RR 0.36, 96% CI, 0.14 – 0.94) in six randomized trials, n = 172 [160]. Rifaximin effect is the secondary encephalopathy prophylaxis; it also reduces bile acids production and inflammation [167,168]. Its use, together with lactulose, improves survival and decreases sepsis-related mortality and hospital stay length [169].
D 6. Prognostic stratification (ACLF grades and organs involved in the failure for prognosis)
D 6.1. The 28-day ACLF forecast is determined by different scores, CLIF being the most validated. (Key concept statement)
ACLF prognosis is determined by the presence of precipitating factors, in addition to a potentially reversible severe liver dysfunction of multiple organs, characterized by high 28-day mortality [4,170].
Several scales are proposed to determine the prognosis of this group of patients that can help the clinician in decision making. The European group determined the CLIF-C ACLF scale, (Chronic Liver Failure-Consortium), Table 4. Initially, this scale only included the six organ failures; however, it currently includes age and leukocytes creating the CLIF-C ACLF based on the formula = 10 * [0.33 * CLIF-C OFs + 0.04 * age + 0.63 * Ln (white blood cell count) - 2], same as found in its electronic version or as an app at: https://www.efclif.com/scientific-activity/score-calculators/clif-c-aclf [4,170].
Scores for ACLF prediction.
These variables come from the CANONIC study data, where it was shown that death prediction was better with CLIF-C ACLF than with other scales (MELD, MELD-Na, Child-Pugh, CLIF-C OF), even higher than the critical care scales of patients, such as SOFA or APACHE II [4,170].
It is understood that the higher the score in this system, the worse the prognosis. Multicenter studies have shown that a CLIF-C ACLF > 70 is associated with 90-day mortality in the absence of transplantation of > 90%. [166] However, it must be remembered that ACLF can be dynamic and that even up to 40% of patients can improve at least one grade in a 72-hour period, as mentioned in the study by Karvellas et al, where interventions can improve the outcome of the patient; therein lies the importance of detecting them [171,172]. Fig. 2.
In turn, the Asia Pacific Association for the Study of Liver Disease, ACLF Research Consortium (AARC) proposed and validated the AARC ACLF score (Table 4); in this scenario, variables such as total bilirubin (mg/dL), HE grade, INR, serum lactate (mmol/L), and creatinine (mg/dL) were considered as 28-day mortality predictors, with a score ranging from 5 to 15 points, creating grades 1, 2, and 3, which represent a 28-day mortality of 13%, 45%, and 86%, respectively. This scale proved to be superior to MELD and CLIF-SOFA in predicting short-term mortality [4]. Available at: http://www.aclf.in/?page=doctor_aarc_grade_cal[6].
Other scores that have been developed are those of the Chinese Group for the Study of Severe Hepatitis B (COSSH), based and modified from the CLIF-C OF, including specific risk factors for hepatitis B virus (HBV) (Table 4). This scale includes the formula: 0.741 × INR + 0.523 × HBV-sequential organ failure assessment score + 0.026 × age + 0.003 × TB (μmol/L), which is known as HBV-SOFA and takes characteristic variables of ACLF-HBV patients into account. This score shows a greater predictive value at 28 and 90 days than those previously commented [173].
These scales allow for critically ill patient identification in whom liver transplantation may be a therapeutic option. Although the scales will not identify those patients who have a contraindication for surgery, they can help change the clinical perception of futility in order to be able to determine timely admission to intensive care, continuous support grade, and even determining the best time for transplantation [167].
D 7. Treatment of precipitating event: bacterial or fungal infections; alcoholic hepatitis; acute variceal bleeding
D 7.1. Bacterial or fungal infections Performing cultures and starting empirical therapy with broad-spectrum antibiotics is recommended in ACLF patients, whenever an infection is suspected. (Low quality, conditional evidence)
ACLF is related to a high incidence of endotoxemia, systemic inflammatory response syndrome, sepsis, and mortality [53,58,172]. Up to 45% of ACLF patients develop bacterial infections at 30-day follow-up [57,173]. The main isolated organisms triggering ACLF are Gram-positive bacteria, followed by Gram-negative bacteria. Spontaneous bacterial peritonitis, pneumonia, urinary tract infections, and skin infections are prevalent infections that trigger and complicate ACLF [174]. Bacterial infection development is also an important predictive factor associated with high mortality in ACLF patients [56,175]. ACLF patients can benefit from establishing antimicrobial strategies early, taking local bacterial resistance into account and always adjusting antimicrobial schemes, according to the symptoms and bacterial cultures results [54,176,177].
D 7.2. Albumin administration is necessary to prevent AKI development in SBP and ACLF patients. (High quality / strong evidence)
Albumin has been shown to decrease mortality and acute kidney injury (AKI) incidence in patients with spontaneous bacterial peritonitis (SBP) [178,179]. Intravenous albumin administration at a dose of 1.5 g/kg on day 1 and 1 g/kg on day 3 is recommended, with respective maximum doses of 150 g and 100 g for body weight > 100 kg and minimum doses of 90 g and 60 g for body weight < 60 kg [180]. However, evidence is still lacking to support albumin administration in bacterial infections other than SBP to prevent HRS-AKI development and reduce mortality [181,182].
D 7.3. Empirical antifungal therapy should be implemented exclusively when there is enough evidence to suspect fungal infection as ACLF trigger or adjuvant. (Low quality / conditional recommendation)
Although most infections in ACLF are bacterial, fungal infections are increasingly recognized and are related to the increasing and rampant use of antibiotics, as well as being more frequent in those patients with multiple readmissions or instrumented procedures, in those who also have diabetes, with prolonged stay, with admission to the intensive care unit, and the presence of acute kidney injury (AKI). Fungal infections are associated with poorer 30-day survival, where the fatality rate has been reported to be 30% with most fungal infections, but > 50% for fungemia and fungal peritonitis [183].
D 7.4. PPIs use should be avoided in ACLF patients as they may increase bacterial infection risk, unless there is a clear indication for PPIs prescription. (very low quality / conditional recommendation)
PPIs administration in cirrhotic patients has been associated with an increased risk of bacterial infections development, including SBP [184] and other nosocomial and healthcare-associated infections [185]. Zhang M et al demonstrated that PPI therapy increases the risk of developing SBP in ACLF patients. According to this study, the MELD score, advanced age, male sex, and high leukocyte count could serve as predictors of SBP development in PPIs users. Caution should be taken with PPIs use, especially for patients with MELD scores > 30 [186]. A meta-analysis that included eight studies, with a total of 3,815 patients, observed that the risk of hospitalized cirrhotic patients developing SBP increased when acid-suppressive therapy was used. The risk was greater with treatment with PPIs (n = 3815; OR 3.15, 95% confidence interval: 2.09 - 4.74) compared with those receiving treatment with type 2 histamine receptor antagonists (ARH2 = (n = 562; OR 1.71, 95% CL: 0.97 - 3.01)) [187]. Although this association may be controversial, since another meta-analysis in which a total of ten case-control studies and six cohort studies were analyzed, with a total of 8,145 patients, failed to establish causality between PPIs use and a higher SBP-related incidence or mortality [188]. Finally, a more recent meta-analysis, where a total of 23 studies were analyzed, which included a total of 10,386 patients, showed a significant association between SBP development and PPIs use, although substantial heterogeneity was observed in the studies. This study suggests caution regarding PPIs use in cirrhotic patients with ascites [189].
D 8. Alcoholic hepatitis
D 8.1. In patients with MDF > 32 (MELD > 20 and < 51) without contraindications to receive steroids, treatment with prednisone 40 mg per day decreases short-term mortality. (Moderate quality / Strong recommendation)
Pentoxifylline did not improve survival in patients with severe alcoholic hepatitis (AH) in the STOPAH study. Prednisolone was associated with a reduction in 28-day mortality, but no improvement in 90-day or 1-year outcome [190]. Regarding the definition of AH severity, MELD has currently been shown to perform better than Maddrey's discriminant function to predict short-term mortality risk; therefore, MELD is the preferred model to use [191]. Corticosteroids are effective in severe AH, defined by a MELD > 20; however, there are patients who may be too sick to benefit from this therapy. A recent global cohort study demonstrated that corticosteroid use was associated with increased 30-day survival, but not at 90 or 180 days. The maximum benefit was seen in patients with a MELD score between 25 and 39. Nonetheless, this benefit was lost in patients with the most severe liver disease (MELD score greater than 51) [192].
D 8.2. The need for liver transplantation should be considered for AH patients who do not respond to steroid therapy, considering each center's expertise, resources, and specific criteria. (Low quality / conditional recommendation)
Severe AH patients who do not respond to corticosteroids after seven days, according to the Lille model resulting in a score > 0.45, present poor survival estimated at less than 25% in the following six months [193]. In these patients who do not respond to corticosteroids, liver transplantation offers the greatest survival benefit [194]. Several studies have shown that there were no changes in graft survival at 1-year and 3-year follow-up among patients with six months of sobriety compared to those transplanted before the six-month abstinence, or versus those transplanted for other different indications [195–198]. Selection criteria are a key component for a successful transplant, where multidisciplinary evaluation, including addiction experts, can be very useful in the context of AH patients [194]. A study by Lee BP et al showed four variables strongly associated with relapse risk in alcohol consumption in post-transplant AH patients; the authors integrated the SALT score (Sustained Alcohol use post-Liver Transplant) with these variables, where a score < 5 had a very highly negative predictive value, as well as a substantial specificity to identify people with the lowest relapse risk in alcohol consumption after liver transplantation [199]. The SALT score requires further validation to be widely recommended, but it shows that there are other criteria of greater relevance (consumption of > 10 drinks/day at initial hospitalization, history of multiple prior rehab attempts, history of previous alcohol-related legal problems, previous illicit substance abuse) to be taken into account in order to define AH patients admission to the transplant list, beyond the ± 6-month abstinence criteria. In Mexico, local committees request 6 months of abstinence for the transplant, this indication we know that's not requested worldwide, in this type of patient (ACLF) the transplant is decided by psychosocial evaluation.
D 9. Acute variceal bleeding
D 9.1. Bleeding control is a priority to maintain adequate MAP, since this strategy preserves tissue perfusion and reduces the risk of ACLF development. (Low quality / conditional recommendation)
Hemodynamic stability must be maintained. In the event that a transfusion of packed erythrocytes is required, a restrictive approach is recommended, maintaining hemoglobin levels between 7 and 8 g/dL, considering other factors where a higher goal may be required, such as in the case of active bleeding or patients with concomitant cardiovascular diseases. Vasoactive drug therapy should be started early (terlipressin or octreotide) and continued for 48 to 120 hours. Endoscopic therapy should be carried out within the first 12 hours after the patient has been hemodynamically stabilized. Antibiotic prophylaxis reduces the risk of bacterial translocation and infections in ACLF patients and should be started at the time of patient admission, generally with intravenous ceftriaxone 1g every 24 hours, but taking reported local bacterial resistance into account is recommended. When bleeding cannot be controlled, the Sagenstaken-Blakemore catheter or the placement of self-expanding stents can be used as a bridge to more definitive therapies, such as TIPS placement. Variceal bleeding is a trigger for episodic hepatic encephalopathy; early administration of lactulose can prevent this complication. Finally, in patients with acute variceal hemorrhage, it is important to look for and, where appropriate, treat splanchnic vein thrombosis or hepatocellular carcinoma using a contrast-enhanced imaging study, ideally tomography or magnetic resonance imaging [200].
D 9.2. Pre-emptive TIPS placement can be performed in ACLF patients who meet specific criteria; likewise, the placement of rescue TIPS can improve survival in ACLF patients with acute variceal bleeding. (Low quality / conditional recommendation)
Rebleeding and 42-day and 1-year mortality risk increases in ACLF patients and, as expected, the higher the ACLF degree, the higher the risk; but a study by Trebicka J et al has shown that these associated risks can be reduced with the placement of pre-emptive TIPS (pTIPS) [201].
The specific criteria for pTIPS placement are: patients with variceal bleeding from esophageal or gastroesophageal varices 1 or 2, Child-Pugh C < 14 points, or Child-Pugh B > 7 points, with active bleeding on initial endoscopy or hepatic venous pressure gradient (HVP) > 20mmHg at the time of bleeding [200].
D 10. Precipitating event treatment: B virus reactivation, other viruses,DILI There are no records of ACLF by DILI in Mexico; however, the most frequent potential causes are herbs, antibiotics, and anti-tuberculous drugs. (Key concept statement)
In other countries’ records, up to 10% of ACLF cases are secondary to DILI. There is no such record in Mexico; however, due to its alternative medicine culture, its behavior is probably similar to countries like China, where 79% is secondary to supplements and herbs. In India, the most common cause is anti-tuberculous drugs, which are also frequently used in our population. The most frequent cause of ACLF due to DILI in Western countries is antibiotics [81,202,203]. This is why educating patients in terms of avoiding herbs use and limiting medication use is essential. Patients with chronic diseases should be monitored when new medications with hepatotoxic potential are prescribed [81,202].
D 10.1. Liver failure usually occurs after approximately four weeks from the start of the agent, although it can occur up to 12 weeks later. (Key concept statement)
Hepatic failure has usually been found to occur approximately four weeks after the initiation of the agent's use and up to 12 weeks thereafter. There are few studies that provide information regarding DILI as a trigger for ACLF. The largest carried out in the Asia Pacific region, observed that the average time for the development of symptoms was 84 days [202].
D 10.2. DILI-related ACLF mortality is approximately 50% . (Key concept statement)
The study carried out in the Asian Pacific population observed that the most frequent manifestations were jaundice (100%), ascites (88%), and hepatic encephalopathy (46%), as well as high MELD (approximately 30). 90-day mortality was higher in DILI-related ACLF patients (46.5%) than in ACLF patients due to another cause (38.8%) [202].
D 11. HBV and other viruses
D 11.1. Differential diagnoses must consider hepatotropic virus infections as triggering causes in ACLF patients. (Key concept statement)
Hepatotropic viruses can be a cause of ACLF and should be considered a cause of acute decompensation. Viruses A and E are endemic in Mexico, which are transmitted orofecally, so educating patients about the importance of food hygiene and not consuming raw food is important. If they are no longer immune, vaccinating patients with chronic liver diseases against hepatitis A and B viruses is suggested. In Asian countries, HBV infection or activation is the main cause of ACLF. This cause is rare in Mexico; however, it should be taken into account as a possible etiology and, if documented, antiviral treatment should be started promptly. HBV reactivation can occur spontaneously or by antiviral treatment discontinuation [81,204]. ACLF development in patients with HBV infection seems to be triggered by sterile and non-sterile inflammation [200], and the viral DNA increase in decompensation is not associated with higher mortality or progression to ACLF [205].
Finally, bacterial infections are a common trigger for ACLF in patients with HBV liver disease and seem to be associated with higher mortality than in patients with liver diseases from other etiologies. These patients must be monitored and treated expeditiously if an infectious focus exists [81,206,207].
GROUP E LIVER TRANSPLANTATION IN ACLF: MORTALITY-ASSOCIATED FACTORS IN TRANSPLANTATION, CRITERIA FOR TRANSPLANTATION, AND RESULTS
Coordinator: Dr. Mario Vilatobá
Participants: Dr. Gustavo Varela Fascinetto, Dr. Ernesto Márquez Guillén, Dr. Nayelli Cointa Flores García, Dr. Godolfino Miranda Zazueta
E 1. ACLF patients should preferably be treated in hospital centers with liver transplantation programs; otherwise, the transfer must be sought as soon as possible. (Key concept statement)
Given the high short-term mortality of ACLF patients and the lack of specific treatment for this entity [208], a multidisciplinary assessment of patients by the liver transplant team is recommended in order to determine if they are candidates for transplantation as soon as possible.
E 1.1. The CLIF-C ACLF score is currently the one with the most advantages for its use in patients on the waiting list and is superior to MELD-Na. (Key concept statement)
Due to the various definitions of ACLF, multiple scores have been developed for diagnosis and prognosis. The different comparisons amongst these scores have highlighted the advantages and disadvantages of each, which makes the selection of one over the other impossible. In the absence of studies designed for transplantation, it is impossible to select one as the ideal score. On the one hand, the MELD score has not been shown to be a good mortality predictor in ACLF patients [209] and should not be considered in decision-making for patients with this entity. Choudhury et al showed in a study that the AARC score (APASL ACLF research consortium) has a greater area under the curve in predicting 28-day mortality when compared to CLIF-C ACLF, SOFA, and MELD; [210] it can be easily calculated and seems to be a good option, in accordance with these results. However, it does not have external validation to be used universally. Regarding the CLIF-C ACLF score, it can be said that it is the most used score to identify ACLF patients. When used dynamically (5-7 days), it can predict 30-day and 90-day mortality more accurately and establishes a threshold that defines therapeutic futility [211], so it can currently be considered the score that presents the most advantages for staging severity and prognosis in ACLF patients.
E 1.2. Patients with bleeding, uncontrolled infection, respiratory failure, refractory shock, and CLIF-C ACLF > 64 who do not show improvement are not candidates for liver transplantation. (Key concept statement)
The decision to continue with the management of an ACLF patient is related to the actual post-transplantation survival chances. In some occasions, despite the transplant, the patient is so sick that unfortunately he or she will die [212].
Gustot et al did not initially find a cut-off point for CLIF-C-ACLF that would reflect a mortality close to 100%. Therefore, futility can only be decided once the initial treatment has been established and the evolution of the patient is known. These authors did find that the number of organ failures and the CLIF-C-ACLF at three and seven days after ACLF-3 diagnosis were useful to determine futility. 28-day and 90-day mortality was 90% and 100% in 25 patients with four or more organ failures and 100% in 24 patients with CLIF-C ACLF > 64 [206].
Choudhury et al. define ineligibility for LT in ACLF in the following scenarios: Sepsis with 2 or more organ failures: uncontrolled sepsis, 4 or more organ failures at a time point, serum creatinine > 4 mg/dl, increase in creatinine by 300% from baseline, need for renal replacement therapy, respiratory failure or HE with ventilatory support > 72 hours. All these parameters will be considered, and the patient needs optimization, but if CLIF C ACLF > 64 despite treatment and hemodynamic control, they are not candidates for liver transplantation [210].
It is important to mention that this evidence can be considered a guide or algorithm; however, the final responsibility to continue with the management of patients and offer them a liver transplant falls on the multidisciplinary group of each program.
E 1.3. Prognosis of an ACLF patient correlates better with the clinical course than with the ACLF grade at diagnosis. Survival can be predicted at three and seven days after diagnosis by calculating the ACLF grade. (Key concept statement)
When prognosis is assessed in ACLF patients, it is important to consider that this is a dynamic syndrome that may improve or worsen during hospitalization.
It has been observed that most patients reach their final ACLF grade within the first week; hence assessing at days 3 and 7 after diagnosis is recommended because this allows for a more accurate 28-day and 90-day mortality prediction than ACLF grade at diagnosis [213].
A general agreement considers that transplantation should be avoided in patients with severe circulatory or respiratory failure and ongoing sepsis [58].
Independently associated factors with poor survival after liver transplantation are lactate levels > 4 mmol/L (HR 3.14; 95% CI, 1.37 – 7.19; p = 0.0069), need for renal replacement therapy at the time of transplantation (HR 2.74; 95% CI, 1.37 – 5.51; p = 0.0046), recipient's older age, use of marginal organs, and infections with multidrug-resistant organisms (HR 3.67; 95%, CI 1.63 – 8.28; p = 0.0017) while the patient remains on the waiting list [212].
E 1.4. After initial stabilization and adequate infection control, ACLF patients should undergo rapid assessment for liver transplantation. (Key concept statement)
If there are no contraindications, all patients who are hospitalized with ACLF diagnosis should be evaluated for liver transplantation; however, liver transplantation in this context is difficult due to donor scarcity and also due to the frequency of these patients being found too ill to be transplanted [213].
When infections are present, it is important to note that they must be adequately controlled in order to consider patients for liver transplantation. On the other hand, recent data suggest that the respiratory failure degree (partial pressure of oxygen [PaO2] / fraction of inspired oxygen [FiO2] > 150), especially when not due to pneumonia or acute respiratory distress syndrome (ARDS), is an alteration that could be acceptable for transplantation, with adequate results [214].
Patients transplanted after seven days on the waiting list but who improved from ACLF grade 3 to ACLF grade 0 to 2 at the time of transplantation have been observed to have better survival than candidates transplanted within seven days, but who remained with ACLF grade 3 from the time they enrolled until they were transplanted (88% vs. 83%, P < 0.001) [214].
Therefore, identifying the right time to carry out transplantation is crucial since ACLF patients may have a very narrow window of opportunity, due to the risk of developing multiple organ failure [213].
ACLF is a rapidly progressive disease and risk stratification within the first week of hospitalization is needed. 'Emergent LT' should be defined in the first week in the ACLF patients; the transplant window for improving survival in a live donor setting [210].
E 1.5. Due to the severity of these patients, any type of donor should be considered, including marginal donors. (Very low quality / conditional recommendation)
Due to the high mortality on the waiting list and the urgency to carry out liver transplantation in ACLF patients, especially ACLF 2 and 3, considering any possibility of donors is necessary, whether living donor, deceased donor, or split liver, as long as there is expertise [215].
In Asia, where the number of deceased donors per million inhabitants is low, the living donor has been considered a good option for ACLF patients, with a good 5-year survival ranging from 74% to 90% [216]. With this type of donor, the risk of the donor will always have to be pondered with the possible futility of the transplant.
In the West, where there is a higher ratio of donors per million inhabitants, the deceased donor is considered a better option [58]. However, there are also important factors to consider that have been shown to affect the results of liver transplantation in ACLF. These factors are elderly donors, grade II steatosis, donor without heartbeat, and ABO group incompatibility. This is probably related to the severity of these patients, who hardly tolerate initial graft dysfunction [217].
Other authors have also indicated that a donor risk index (DRI) ≥ 1.7 is a poor prognostic factor in ACLF patients and should be avoided [218].
In practical terms, it is difficult to determine which organ is no longer adequate and which other could be the last option for critically ill patients like these. In the end, the decision falls on each transplant program based on its multidisciplinary group and its expertise, always remembering that mortality on the waiting list can be as high as 70%.
E 1.6. Transplanting ACLF-3 patients is appropriate, even if this implies greater resource expense and a longer hospital stay. (Very low quality / conditional recommendation)
Several retrospective studies have shown post-transplant results in ACLF patients.
A study carried out in Europe, in which 73 ACLF-3 transplanted patients were included, demonstrated a 1-year survival of 83.9% compared to 7.9% of non-transplanted control patients, p <0.0001. One-year survival of transplanted ACLF-3 recipients was the same as for controls with no ACLF (90%), ACLF-1 (82.3%), or ACLF-2 (86.2%). The group of ACLF-3 transplant recipients had a higher rate of complications (100%) and a longer hospital stay [219]. In another European report which analyzed 234 ACLF patients' data, 98 of them with ACLF-3, the 1-year survival probability after transplantation was 81% (95% CI, 74 - 87%) [212].
There are also studies from North America. An analysis of the UNOS database, retrospectively identifying ACLF transplanted patients, found that ACLF-3 transplanted patients had a lower 1-year survival (81%), compared with other categories of ACLF or no ACLF: 88.1% – 91%, p < 0.001 [217]. In another report, information from ten transplant centers in the United States and Canada was included, incorporating the results of 212 ACLF transplanted patients, 77 of them with ACLF-3; 1-year survival of ACLF transplant recipients was 85%, while that of transplant recipients without ACLF was 94.3%, p=0.02. No differences in survival amongst the different grades of ACLF were found [220].
Interpretation of these data should be cautious given the potential biases in patient selection, ACLF heterogeneity, and even the different ways of categorizing ACLF among different studies.
ACLF–3 is a rapidly progressive disease and risk stratification within the first week of hospitalization is needed, to properly select the patient for transplant [209].
GROUP F OTHER TREATMENTS: EXTRACORPOREAL LIVER SUPPORT, GRANULOCYTE COLONY-STIMULATING FACTOR, AND STEM CELLS
Coordinator: Dr. Laura E. Cisneros Garza
Participants: Dr. Belinda Isela Martínez Saldívar, Dr. Víctor Manuel Páez Zayas, Dr. Linda Elsa Muñoz Espinosa, Dr. Francisco Alfonso Solís Galindo
F 1. The use of extracorporeal liver support systems in ACLF patients has shown its usefulness by eliminating albumin-bound toxic products, but without showing improvement in survival. (Key concept statement)
Among the therapeutic alternatives proposed for ACLF there are extracorporeal liver support devices that use dialysis techniques to remove both water-soluble and fat-soluble substances from the plasma (albumin-bound); which objective is to serve as a bridge, definitive therapy and reducing MELD until liver recovery and/or liver transplantation [80]. Among them, the most widely used is the Molecular Adsorbent Recirculation System (MARS), although there are two other similar systems: PROMETHEUS (fractionated plasma separation and adsorption system) and single pass albumin dialysis (SPAD). Several studies have been published to date, reporting controversial results on the usefulness of MARS and Prometheus in ACLF patients, observing improvement in cholestasis, hepatic encephalopathy, liver and kidney function, circulatory and immune dysfunction, but without showing a 28-day survival benefit compared with standard medical therapy [221,222]. However, more recent studies show that making an adequate selection of patients and increasing the number of MARS sessions is associated with an increase in 14-day and 28-day survival [223–225]. This should motivate new studies to analyze MARS role in patients with different degrees of ACLF.
F 1.1. Plasma exchange could be associated with increased survival in ACLF patients when compared with standard medical therapy. (Key concept statement)
In recent years there has been growing interest in the use of plasma exchange in ACLF patients due to its effect on plasma cytokines and systemic inflammatory mediators elimination, improving survival compared with standard medical treatment [225,226]. Plasma exchange in ACLF is an emerging therapy and can be considered in absence of liver transplant in patients with ACLF [227]. However, there are no studies to date that have evaluated plasma exchange usefulness in ACLF patients with etiologies other than HBV, so more studies that include a more homogeneous ACLF patient population will be necessary to establish its usefulness.
F 2. Liver dialysis device (DIALIVE)
A clinical trial was published in 2021 using the DIALIVE device that removes dysfunctional albumin from the patient and infuses fresh albumin, whereby circulating endotoxin is adsorbed, leading to significant attenuation of systemic inflammation and having a direct impact on failure recovery of other organs. DIALIVE is being evaluated in a phase II study in ACLF patients [228].
F 3. Granulocyte colony-stimulating factor (G-CSF) administration has not shown to improve survival of acute-on-chronic liver failure patients. (Very low evidence / conditional recommendation)
The efficacy of G-CSF administration has been hypothesized as therapy in patients with acute-on-chronic liver failure, through an increase in CD34+ cells in peripheral blood.
Garg et al conducted the first randomized, placebo-controlled trial in 47 ACLF patients; 23 were assigned to the G-CSF treatment group (5 μg/kg, for the first five days, then every third day thereafter until completing twelve doses), and 24 patients only to standard medical therapy. Alcohol-related liver disease was the main etiology (57.4%, alcoholic hepatitis), followed by HBV reactivation (21.2%). The 60-day survival was evaluated, as well as the differences regarding the score on the Child-Turcotte-Pugh (CTP) scale, Model for End-Stage Liver Disease (MELD), and Sequential Organ Failure Assessment (SOFA) scale. An improvement in survival was observed in this period (66% vs. 26%, p = 0.001) for the treatment group, and secondarily, a progressive improvement in CTP, MELD, and SOFA scores, in addition to preventing the development of hepatorenal syndrome, hepatic encephalopathy, and sepsis. Adequate tolerance to G-CSF was observed, with mild adverse events [229].
Subsequently, Duan et al published the results of a randomized, blinded, and controlled trial, in which G-CSF (5 μg/kg, six days) therapy efficacy and safety were evaluated in a cohort of 55 chronic HBV infection-associated ACLF patients; 27 in the treatment group and 28 in the control group [230]. Similarly to the study by Garg et al, they observed an improvement in 90-day survival in the treatment group when compared with the group receiving standard treatment (48.1% vs. 21.4%, p = 0.0181), also showing a decrease in Child-Turcotte-Pugh and MELD (Model for End-Stage of Liver Disease) scales scores, with minor adverse effects (p = 0.004) [229].
The results of the GRAFT study were published in 2021, the first multicenter phase 2 study designed to evaluate G-CSF efficacy and safety in 176 ACLF patients (defined by the EASL-CLIF criteria), randomized into two groups of 88 to receive G-CSF (5 μg/kg, for the first five days, then every third day until day 26), plus standard medical therapy compared with standard therapy alone, with the main objective of assessing 90-day transplant-free survival and 360-day secondarily, as well as the development of ACLF-related complications and the impact on MELD and CLIF-C OF scores. However, this study failed to demonstrate a beneficial effect in the treatment of ACLF patients, in their complications, or in improving the scales scores, so it had to be terminated prematurely. Serious G-CSF-related adverse effects (seven patients) were reported. Therefore, according to the results of this study, G-CSF therapy use in ACLF patient management is not suggested [231,232].
We can conclude that, despite the fact that there is evidence derived from small cohorts mainly composed of patients with hepatitis B and alcohol-related chronic disease, which report evidence in favor of G-CSF usefulness in improving ACLF patient survival and other clinical outcomes, more robust evidence has not been able to reproduce these results, so G-CSF use for purposes other than research through clinical trials is not recommended.
F 4. Hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs), mesenchymal cells (MSCs), and embryogenic cells have a greater pluripotential capacity to differentiate into hepatocytes. This is a therapeutic strategy that is currently under development and its use outside of research protocols is not recommended. (Very low quality / conditional recommendation)
Stem cell therapy in ACLF patients has generated interest due to the potential immunomodulatory and repair effect that this therapeutic strategy presents [233]. Different clinical trials have been carried out for 15 years to date; the results of these studies have shown that stem cell therapy is safe and has beneficial effects; however, most of these studies are low-quality since they include small groups of patients, there is no control group in any of them, and the administration protocols (type of stem cell used, dose, frequency, and application route) vary considerably [234,235].
A meta-analysis carried out in 2018 found 78 clinical trials published in full text, of which only ten met evaluation criteria (seven carried out in China, two in Iran, and one in Switzerland). Results demonstrated an increase in albumin levels and a decrease in bilirubin levels, ALT and MELD score that were maintained up to twelve months after stem cell transplantation; mortality was not evaluated. However, these conclusions are limited by the heterogeneity of the studies: only four of the ten clinical trials were randomized, different administration protocols were used, and ACLF etiology varied considerably [236].
Granulocyte-stimulating factor G-CSF is a glycoprotein encoded by the CSF3 gene. Its central physiological role is in neutrophil production regulation in health, and particularly in emergency response situations to infections and in bone marrow aplasia. G-CSF serum concentration is not detectable or only at very low levels in healthy humans; most tissues are capable of secreting it after a stimulus; for example, with IL-1, lipopolysaccharide, TNF-α secreted by macrophages, epithelial cells, fibroblasts, and mesenchymal cells. IL-17 is an important G-CSF regulator, especially in the bone marrow [237].
There are stem cells niches in the Hering canals on the liver (oocytes). The liver is the organ with the greatest capacity for regeneration, probably due to this fact. In chronic liver diseases, liver stem cells can be depleted over many years. There is evidence in cirrhotic patients that CD34+ cells stimulation by mobilizing them with G-CSF can improve the course of the disease, decreasing ascites fluid and improving albumin and bilirubin synthesis [238].
G-CSF is believed to improve the liver synthetic function and decrease fibrosis based on the concept that bone marrow CD34+ cells mobilization into the liver microenvironment transforms them into progenitor cells and are able to restore lost liver volume. Angiopoietin expression reduction decreases neoangiogenesis, thus reducing fibrosis.
The increase in CD133+ fibrolytic activity induced by G-CSF in monocytes and bone marrow activation, possibly through IL-10, a Stat3-mediated regeneration, has been postulated [238]. G-CSF stimulation in healthy people produces a neutrophilic response within the next four hours and mobilizes activated stem cells from the bone marrow after three days, peaking at five days, which may be associated with spleen enlargement (10 μg/kg/day). CD34+ cells are a hematopoietic stem cells marker. In carbon tetrachloride (CCL4)-cirrhosis animal models, treatment with MSCs transplantation and CD34+ cells mobilization has been seen to produce a possible improvement to F2 in the first case and to F3 in the second case [239].
Various clinical trials have been published with CD34+ cells mobilization, as well as with autologous bone marrow cell transplantation, with improvement in liver function, which initially most likely included: hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs), mesenchymal cells (MSC), and embryogenic cells (ESCs), which are the ones with the greatest hepatocyte-like differentiation capacity, known as pluripotential capacity in decompensated liver cirrhosis with improvement [237–239].
In a multicenter study on ACLF, no improvement was found with stem cell mobilization, which is why it is not recommended as standard therapy [226]. A meta-analysis showed the heterogeneity of the studies were patients with decompensated cirrhosis and ACLF were analized [236]. A meta-analysis on treatment with G-CSF for CLD, where two studies were analyzed, concluded that CD34+ mobilization can be a therapeutic alternative, when liver transplantation is not possible [240]. Regarding ACLF treatment with G-CFS, a meta-analysis concludes that it may be useful [241]. Since another meta-analysis concluded that G-CSF was not useful in ACLF, more studies are undoubtedly needed [242].
Many questions remain open to be resolved before CD34+ cells mobilization is an accepted therapeutic option in cirrhosis and ACLF, such as what the ideal number of CD34+ cells is, how often they should be mobilized, and how to determine success and its duration. Albumin levels, INR, and clinical improvement (such as decreased ascites, bilirubin, and ammonium) are some parameters to follow in functional classifications.
Therefore, with the current evidence, our recommending its that CD34 cell mobilization and use of GCSF in patients of ACLF is in the research domain or selected cases when no other feasible therapy available.
3ConclusionsACLF has emerged as a major cause of mortality in patients with cirrhosis and chronic liver disease worldwide. The varying definitions that focused on established organ failure have reduced generalizability and potential for prevention of ACLF in different settings. Prevention of major precipitating factors is critical in improving the prognosis of individual organ failures (circulatory, renal, brain, respiratory, and coagulation), and judicious use of antibiotics and antifungal medications is required. Critical care management strategies and LT potential listing should be balanced with futility considerations in those with a poor prognosis. The approach and treatment of ACLF must be personalized and represents a challenge for physicians.
External reviewersAshok Choudhury
Jonathan Soldera
Paul J. Thuluvath
Author contributionsCoordination and preparation of manuscript: responsible for the work: Torre Aldo; preparation of the manuscript: Navarro Alvarez Nalu; participation in the guidelines: Cisneros Garza LE, Castillo Barradas M, Sandoval Salas R, González Huezo MS, Pérez Hernández JL, Méndez Guerrero O, Ruiz Manríquez JA, Trejo Estrada R, Chávez Tapia N, Solís Gasca LC, Moctezuma Velázquez C, Aguirre Valádez J, Flores Calderón J, Higuera de la Tijera F, García Juárez I, Canedo N, Malé Velázquez R, Montalvo Gordon I, Vilatobá Chapa M, Márquez Guillén E, Córdova-Gallardo J, Flores García NC, Miranda Zazueta G, Martínez Saldívar BI, Páez Zayas VM, Muñoz Espinosa LE, Solís Galindo FA.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.