Allergic asthma is a complex chronic disease of the respiratory system presenting with cough, dyspnea, wheezing and airway obstruction. More than 300 million people of all age spectrums suffer from asthma worldwide. Immunological and inflammatory processes are main contributors to asthma. Cytokines produced by T helper 2 lymphocytes play main roles in asthma development and progression. Silymarin, a therapeutic agent with anti-oxidative properties, is a main component of Silybium marinum. We herein aimed to compare the anti-inflammatory and anti-allergic effects of two silymarin isomers, isosilybin A and silydianin, in the treatment of allergic asthma.
Materials and MethodsAfter isolating and purifying isosilybin A and silydianin, Balb/c mouse model of allergic asthma was produced using ovalbumin injection. Seventy mice were categorized into five (1 normal and 4 asthmatic) groups (n = 14 per group). Mice in three of four asthmatic groups were treated with either isosilybin A, silydianin or budesonide. The 4th asthmatic group was used as positive control, with the non-asthmatic group serving as negative control. Airway hyperresponsiveness (AHR) and levels of IL-4, IL-5 and IL-13 in the BAL fluid were determined. Gene expressions of IL-4, IL-5, IL-13 and MUC5ac, as well as IgE serum level were also measured. Cellular composition of BAL fluid and lungs histopathology were finally investigated.
ResultsIsosilybin A and silydianin reduced eosinophilic infiltration of lungs, IL-4 and IL-5 levels in BAL fluid, IL-4 and IL-5 gene expressions, as well as AHR in Balb/c mouse model of asthma. However, no significant changes were observed in IL-13 level and mucus hyper-secretion.
ConclusionAccording to our study, isosilybin A and silydianin can control main symptoms of asthma by modulating immune responses.
Allergic asthma is a multifactorial and complex chronic disease of the respiratory system. Asthma development is influenced by genetic, epigenetic and environmental factors. The main symptoms of asthma, which is characterized by airways hyper-responsiveness (AHR) and obstruction, include cough, dyspnea and wheezing. More than 300 million people of all ages suffer from asthma worldwide.1–3
Immunological and inflammatory processes are the main contributors to asthma attacks. In this regard, immunological and inflammatory mediators can be important targets for controlling asthma symptoms. Cytokines produced by T helper 2 lymphocytes such as IL-4, IL-5 and IL-13 play main roles in the development and progression of asthma. IL-4 induces the overproduction of immunoglobulin E (IgE), IL-5 activates eosinophils and directs them toward airways, and IL-13 increases mucus production in bronchia. AHR and airway smooth muscle spasm are finally developed in response to allergens.2–4
B lymphocytes from patients with allergic asthma have a tendency to overproduce IgE (total and allergen-specific). Eosinophilic infiltration and enhanced expression of type 2 cytokines in lungs also contribute to allergic asthma pathophysiology.5–8 Allergic reactions are triggered by binding and cross-linking of allergen bound IgE antibodies to their receptors on mast cells (sensitization) inducing the release of chemical mediators from these cells. The level of sensitization and severity of inflammation in airways (especially eosinophilic infiltration) correlate with the severity of asthma.7–10
Silymarin is a main component of Silybium marinum (milk thistle). The main constituents of silymarin include flavonolignans, silybin A, silybin B, isosilybin A, isosilybin B, silydianin, silychristin, isosilychristin, 2,3-dehydrosilybin and taxifolin.11–13 Silymarin, as an antioxidant therapeutic agent with membrane-stabilizing effects, is used for treating liver inflammation and hepatic disorders. It also has protective effects against inflammatory stress in which leukotrienes are involved. The anti-inflammatory effects of silymarin have been attributed to its inhibiting effects on leukotrienes and prostaglandin production.11,13,14
Airway inflammation is an important event in the development of asthma. In allergic asthma, anti-asthmatic drugs are designed to stabilize mast cell membranes. The roles of leukotrienes and prostaglandins (bronchoconstriction and bronchial hyper-reactivity) have been demonstrated in both intrinsic and extrinsic allergic asthma.15–17 Regarding the anti-inflammatory effects of silymarin and its components, these can be used to alleviate airway inflammation in allergic asthma. In the present study, we compared the anti-inflammatory and anti-allergic effects of two pure isomers of silymarin (isosilybin A and silydianin) in a Balb/c mouse model of allergic asthma.
Material and methodsPreparation of isomersSilymarin and its constituents were extracted and purified from S. marianum using column chromatography (standard silica gel, 230–400 mesh, Merck, Germany) and HPLC preparative high-speed counter-current chromatography (HSCCC) as previously described.18–21 The mass spectra were analyzed by the Data Analysis software version 3.4 (Bruker Daltonics, Billerica, USA). HPLC analyses were carried out using a Shimadzu Prominence LC analytical system (Shimadzu, Kyoto, Japan).20,21 NMR (Bruker 400 MHz) and MS analyses were performed as described previously.22,23
Animal model of allergic asthmaFemale Balb/c mice (6–8 weeks old) were purchased from the Pasteur Institute of Iran (Karaj, Iran). The animals were acclimatized under standard laboratory conditions for one week (12 h light-dark cycle, 24 ± 2 °C temperature, 55 ± 5% humidity, and pathogen-allergen free condition). The experiments were carried out observing all ethical considerations in working with laboratory animals.
Treatment scheduleSeventy mice were divided into five (4 asthmatic and 1 healthy) groups (n = 14 per group, seven for histopathological analysis and seven for bronchoalveolar lavage (BAL) sampling). In the four asthmatic groups, airway inflammation was induced using ovalbumin (OVA, Sigma-Aldrich, Netherlands) as previously mentioned (Athari et al., 2016).10 Briefly, the mice were sensitized by intraperitoneal injection of combination of chicken OVA (20 μg) and aluminum hydroxide (50 μl) (Sigma-Aldrich, Netherlands) on days 1 and 14.
The mice were challenged by inhalation of 1% OVA solution aerosolized by an ultrasonic nebulizer (NE-U07, Omrom, Japan) for 30 min per day on days 24, 26, 28, and 30. The sensitization and challenge experiments were performed in three and one of the four asthmatic groups. The mice in the recent four groups were also treated with pure 1% isosilybin A, 1% silydianin and budesonide solutions (aerosolized by ultrasonic nebulizer for 30 min) on days 25, 27, and 29. Budesonide, as an anti-asthma drug, was used as positive control. The mice that were both sensitized and challenged served as positive control. The mice sensitized and challenged with PBS served as negative controls. At 48 h after the last challenge on day 30, blood, BAL and lung tissue samples were collected for further analyses.
Measurement of AHRTwo days after the last challenge, Methacholine challenge test was performed to assess AHR and determine enhanced pause (the Penh value). All the mice were initially exposed to PBS aerosol to obtain the baseline Penh value. Then the mice were exposed to doubling concentrations of aerosolized methacholine (Mch), (0.5, 1, 2, 4, and 8 mg/mL). Finally, the relative Penh values were determined as percentages (Athari et al., 2016).10 To determine AHR, a catheter was inserted into the trachea and then connected to a mechanical ventilator after mice were anesthetized and tracheotomized (Inspira ASV; Harvard Apparatus).
BAL fluid collectionThe BAL fluid was collected after washing lungs via the trachea using 1 mL PBS. The cells of the BAL fluid were collected on cytospin slides and stained with Wright’s stain solution. Differential cell counting was then performed. The BAL supernatant was stored at −70 °C for cytokine analysis.
Cytokine levels in BAL fluidIL-4, IL-5 and IL-13 levels were measured in the BAL fluid using specific ELISA kits (Abcam, USA).
Quantitative real time PCRTotal RNA of cells in the BAL fluid was isolated using TRIzol (Invitrogen life technologies, NY, USA) according to the manufacturer’s instructions. RNA samples were reverse transcribed to cDNA using a cDNA synthesis kit (Maxima First Strand cDNA Synthesis Kit, Thermo Scientific, Rockford, IL, USA). The recent kit included dsDNase enzyme, which specifically removed contaminating genomic DNA from the samples. Quantitative PCR was performed using a Rotor-Gene SYBR Green PCR Kit and Rotor-Gene Q thermal cycler (Qiagen, Hilden, Germany). The Rotor-Gene SYBR Green PCR Kit was already enhanced to work with Qiagen cyclers (for minimum optimization procedure). Primers for the target (IL-4, IL-5, IL-13, and MUC5ac) and internal control (GAPDH) genes were adapted from Athari et al., 2016.10
Serum IgE levelTwo days after the last challenge, blood samples were obtained, and sera were separated and stored. Total IgE (BD Biosciences, USA) and OVA-specific IgE (Cusabio Biotech, USA) levels were measured using appropriate ELISA kits.
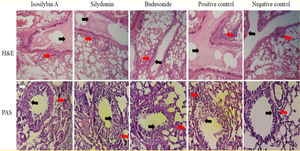
Histological studyLung tissue samples were isolated and fixed in buffered formalin and then trimmed and embedded in paraffin. Tissue sections were stained with H&E solution and periodic acid Schiff (PAS). The ratio of mucus production was scored as the intensity of staining in 10 randomly selected microscopy fields at 400× magnification. The goblet cells were enumerated in several randomly selected microscopy fields, and the result was expressed as the goblet cell index (GCI). Eosinophils were counted in histological lung sections at 1000x magnification. The number of eosinophils was reported as the mean of five repetitions as described by Athari et al. (2016).10
Statistical analysisThe results were expressed as means ± S.E.M. One-way ANOVA followed by Newman-Keuls and two-tailed independent student’s t-test were used for comparing variables between groups. The statistical significance threshold was considered as P < 0.05. The analyses were performed in GraphPad Prism version 5.0.
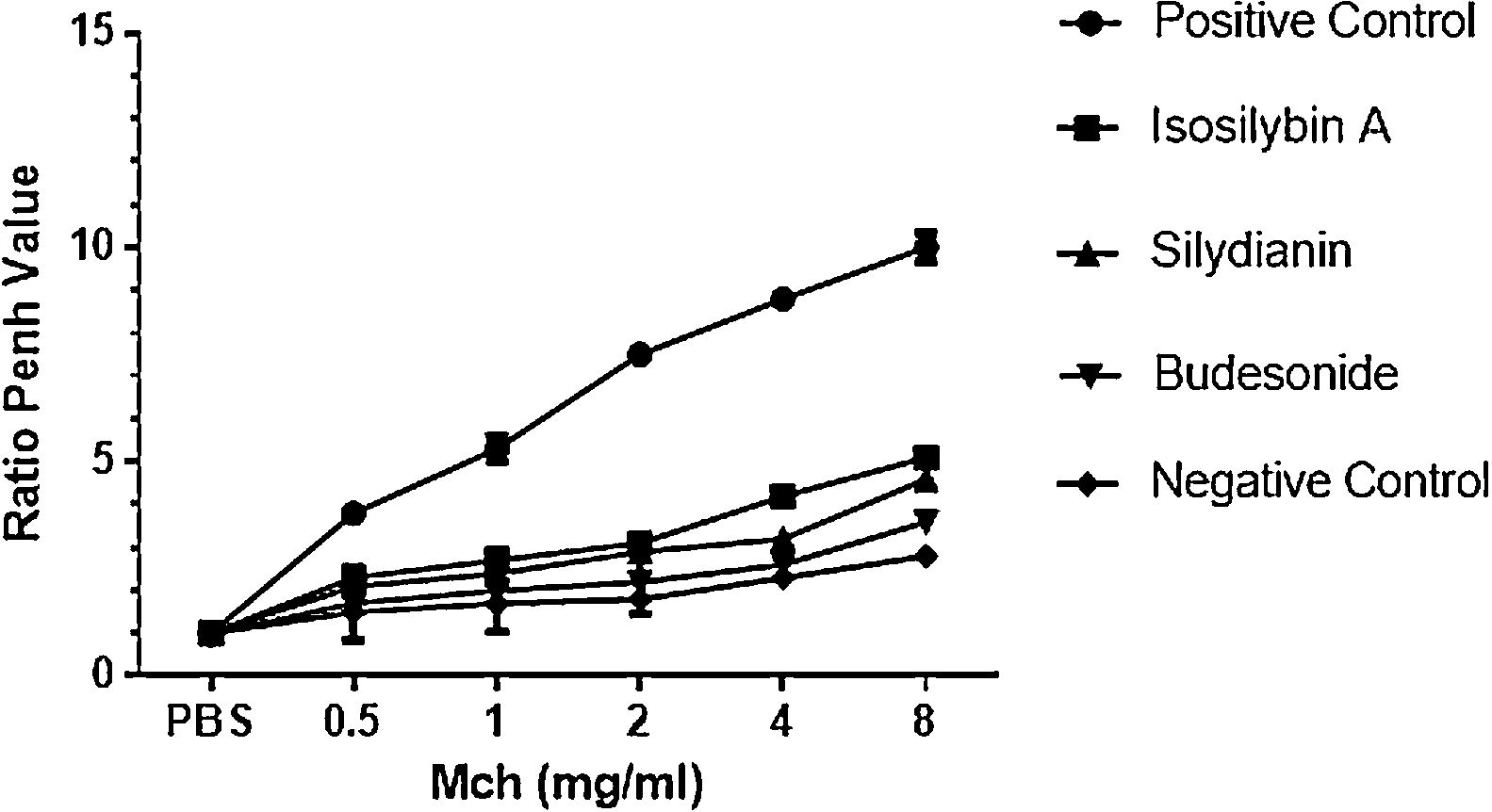
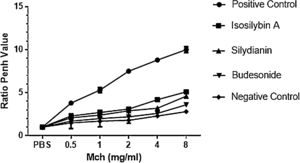
ResultsEffects of silymarin isomers on AHRAHR was significantly higher in the positive (OVA sensitized and challenged) (8.8 ± 0.15) compared with negative (PBS challenged) (2.3 ± 0.08) group (P < 0.05). The two silymarin isomers also reduced AHR (isosilybin A: 4.2 ± 0.15, silydianin: 3.2 ± 0.09) with respect to controls. Budesonide, the commercial anti-asthmatic drug, completely prevented the development of AHR (2.6 ± 0.10) (Fig. 1).
The effects of silymarin isomers on airway hyperresponsiveness measured as the Penh value in response to increasing doses of Mch. The Penh values were higher in the positive compared with the negative control group in all concentrations of Mch. The effects of the silymarin isomers and budesonide on airway hyperresponsiveness have been shown in sensitized and challenged mice. The results have been presented as means ± S.E.M. Statistical significance level was considered as P < 0.05.
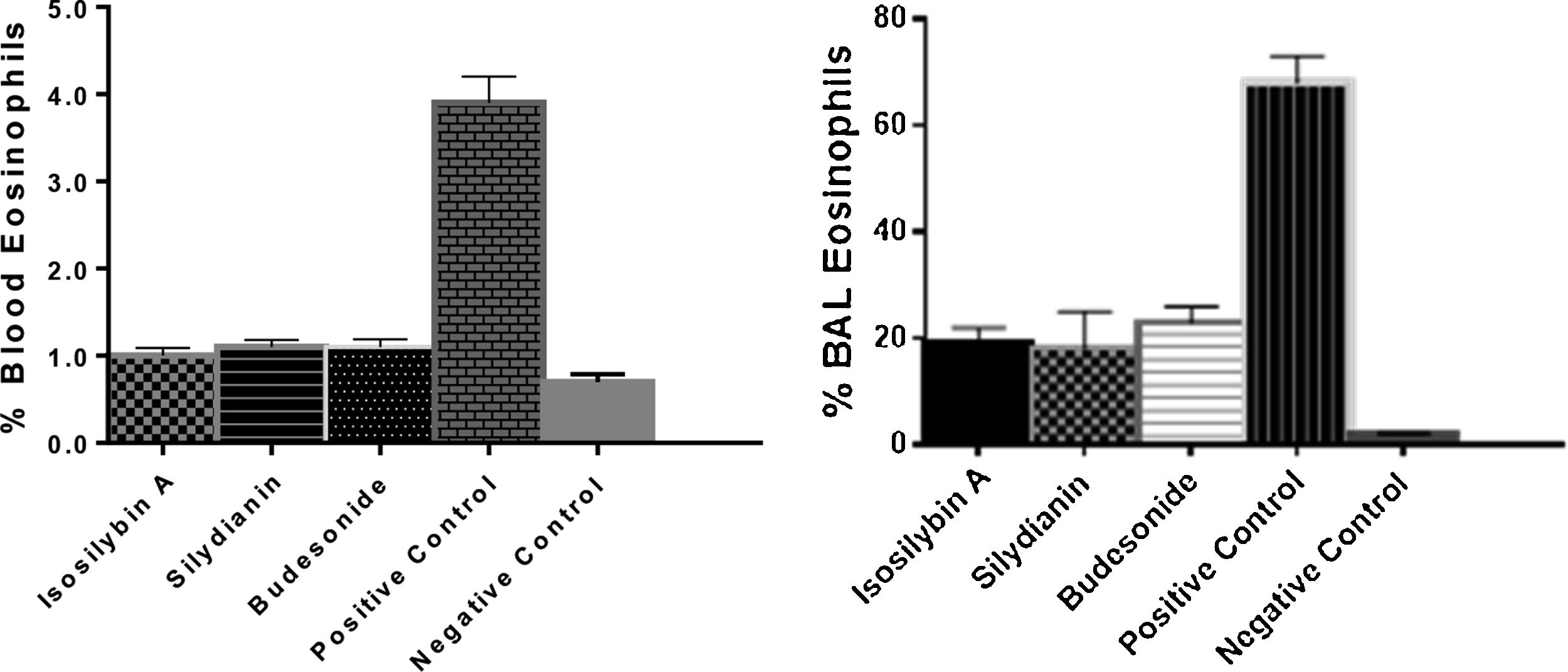
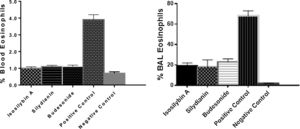
The percentages of eosinophils were higher in blood (4.75 ± 0.11%) and BAL fluid (68.0 ± 4.0%) in positive controls compared with negative control group (0.70 ± 0.09% and 6.0 ± 7.0%, respectively, P < 0.05). Treatment with isosilybin A (1.0 ± 0.08%, P < 0.05), silydianin (1.1 ± 0.11%, P < 0.05) and budesonide (1.2 ± 0.2%, P < 0.05) significantly reduced blood eosinophils. Furthermore, the BAL fluid eosinophil count reduced after treatment with isosilybin A (19.0 ± 5.0%, P < 0.05), silydianin (18.0 ± 0.1%, P < 0.05) and budesonide (23.0 ± 4.0%, P < 0.05) (Fig. 2).
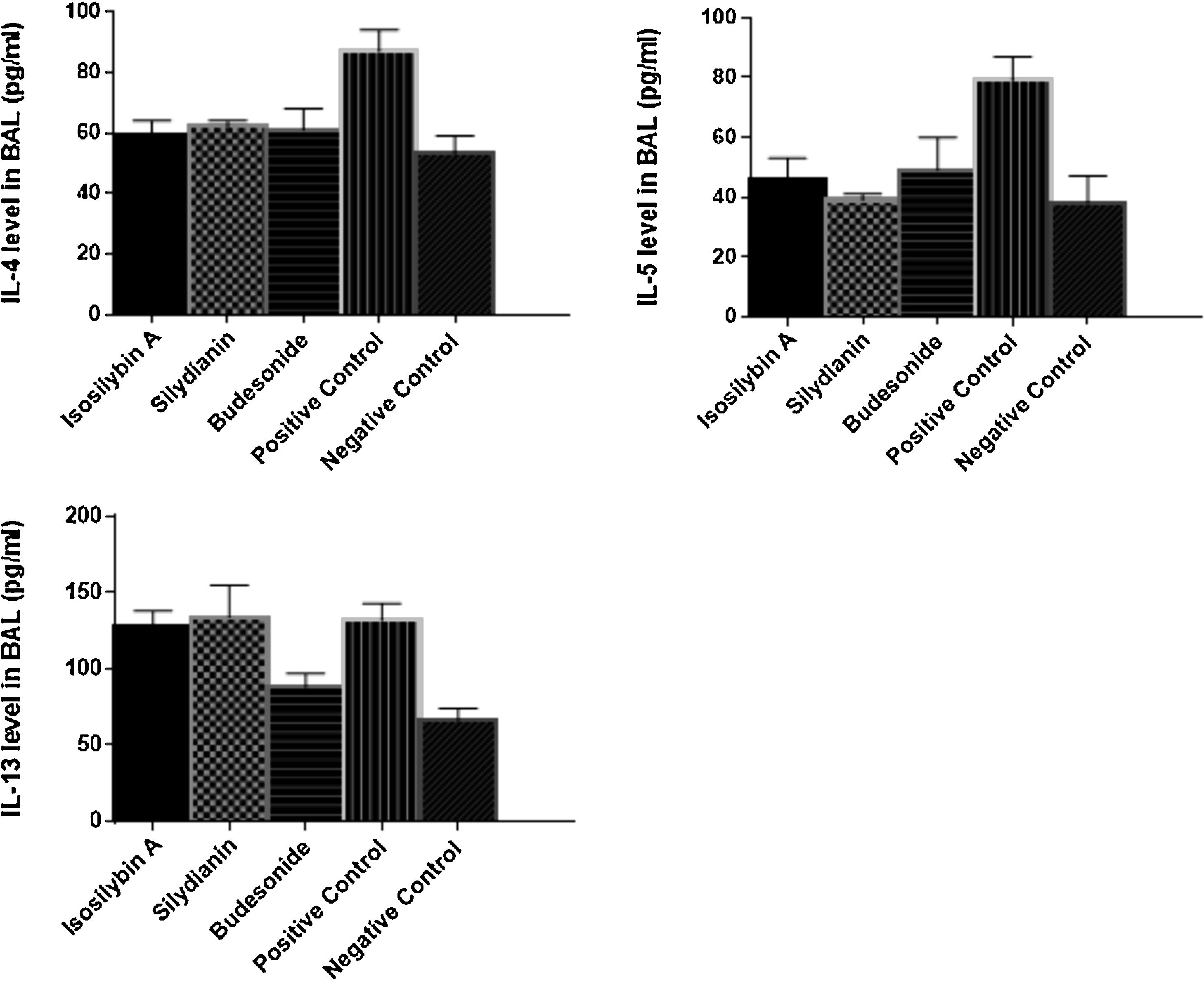
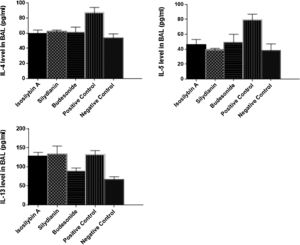
Effects of silymarin isomers on cytokine levels in BAL fluidThe levels of IL-4 (87 ± 7 vs. 53 ± 6 pg/mL, P < 0.05), IL-5 (79 ± 8 versus 38 ± 9 pg/mL, P < 0.05) and IL-13 (132 ± 11 vs. 66 ± 8 pg/mL, P < 0.05) were significantly higher in the positive than the negative control group (Fig. 3). Treatment with isosilybin A and silydianin did not affect OVA-induced IL-13 production (128 ± 10 and 134 ± 21 pg/mL, respectively). On the other hand, isosilybin A, silydianin and budesonide significantly attenuated IL-4 (59 ± 5, 62 ± 2, and 61 ± 7 pg/mL respectively) and IL-5 (46 ± 7, 39 ± 2, and 49 ± 11 pg/mL respectively) levels (P < 0.05).
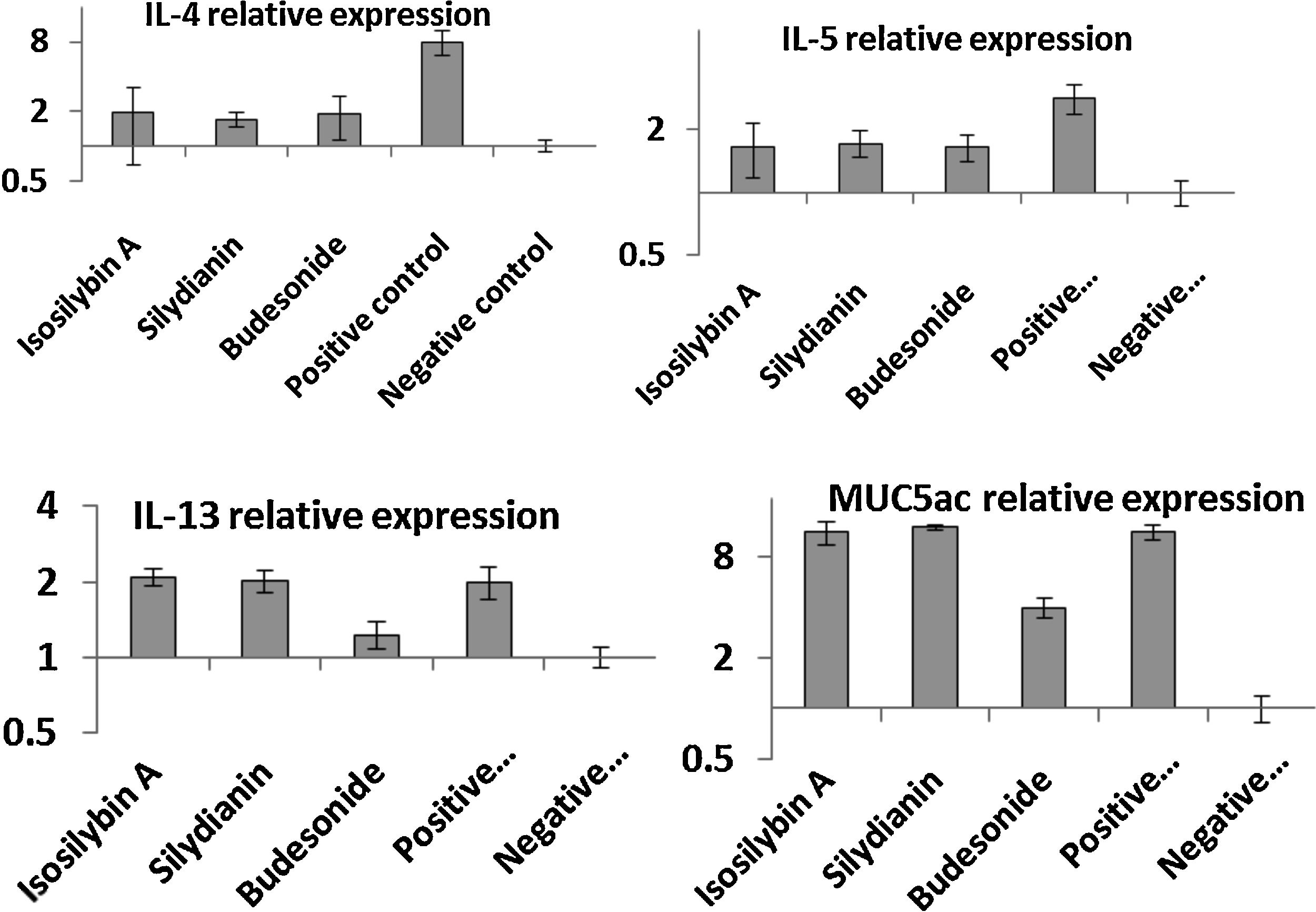
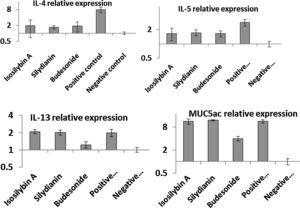
Effects of silymarin isomers on gene expressions of cytokines and mucinThe mRNA levels of IL-4 (8.11 ± 1.37 vs. 1.00 ± 0.12), IL-5 (3.12 ± 0.22 vs. 1.00 ± 0.10), IL-13 (2.11 ± 0.13 vs. 1.00 ± 0.12) and MUC5AC (12.11 ± 1.09 vs. 1.00 ± 0.19) were significantly higher in the positive compared with the negative control group (P < 0.05, Fig. 4). In mice treated with isosilybin A, silydianin and budesonide, there were significant reductions in mRNA expressions of IL-4 (1.91 ± 0.24, 1.74 ± 0.11, and 1.90 ± 0.32, respectively) and IL-5 (1.61 ± 0.24, 1.72 ± 0.14, and 1.60 ± 0.18, respectively) compared with the positive control group (Fig. 4) (P < 0.05 for all comparisons). However, in mice treated with either isosilybin A or silydianin, there were no significant differences in mRNA expressions of IL-13 (2.01 ± 0.21 vs. 2.08 ± 0.03, respectively) and MUC5AC (11.33 ± 1.11 vs. 12.12 ± 0.10, respectively) compared with the positive control group (Fig. 4) (P < 0.05 for all comparisons).
The effects of silymarin isomers on the mRNA expressions of IL-4 (A), IL-5 (B), IL-13 (C) and MUC5AC (D) in BAL cells using quantitative RT-PCR. The results were expressed as means ± S.E.M (n = 7). Sensitized and challenged mice treated with the isomers and budesonide had decreased levels of IL-4 and IL-5 mRNAs. The expressions of IL-13 and mucin reduced only in the budesonide group.
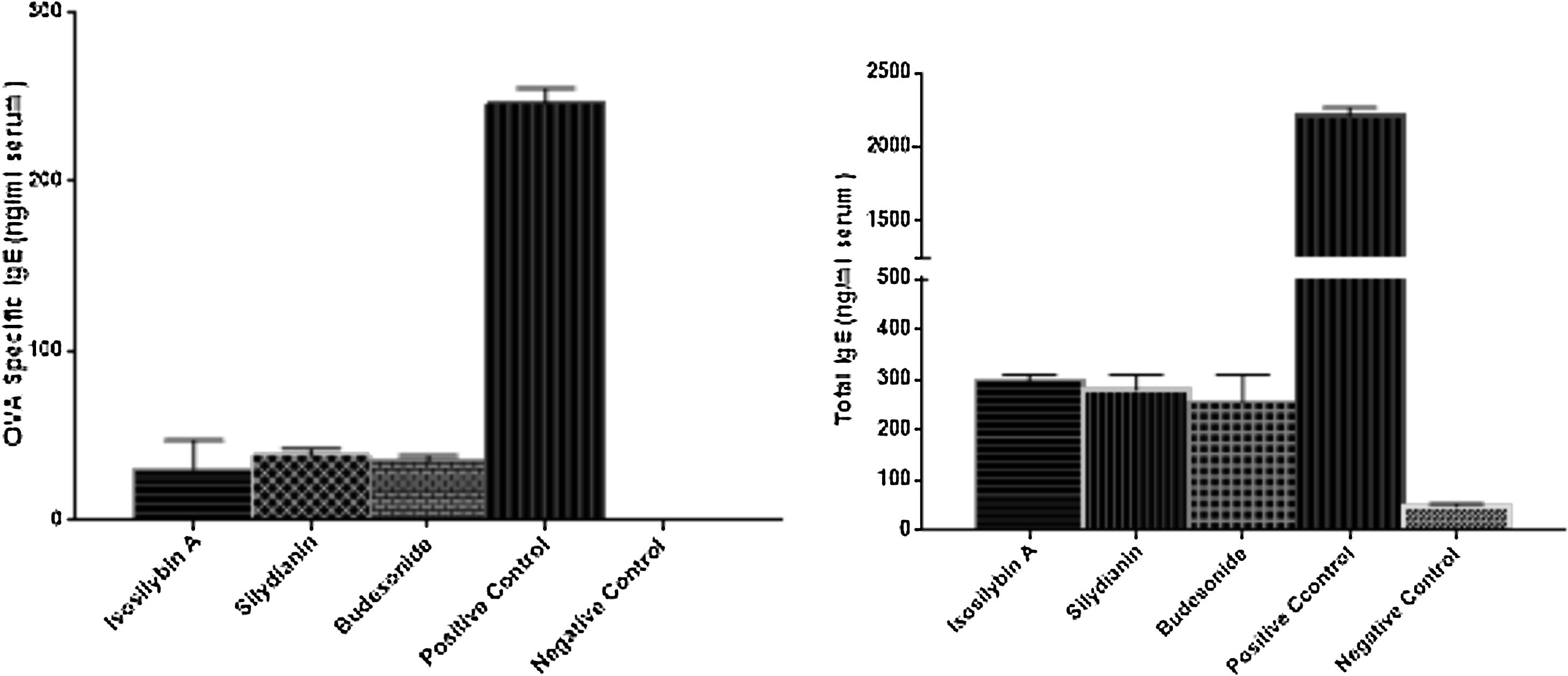
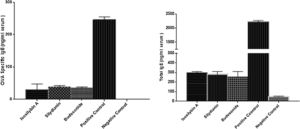
The levels of total and OVA-specific IgE were significantly higher in the positive (2210 ± 57 and 246 ± 9 ng/mL, respectively) compared with the negative (48 ± 5 and 0 ± 0 ng/mL, respectively) control groups (P < 0.05). Treatment with isosilybin A (total IgE: 298 ± 11, OVA-specific IgE: 30 ± 17 ng/mL, P < 0.05), silydianin (total IgE: 280 ± 29, OVA-specific IgE: 38 ± 4 ng/mL, P < 0.05) and budesonide (total IgE: 256 ± 54, OVA-specific IgE: 35 ± 3 ng/mL, P < 0.05) reduced IgE levels in OVA sensitized and challenged groups (Fig. 5).
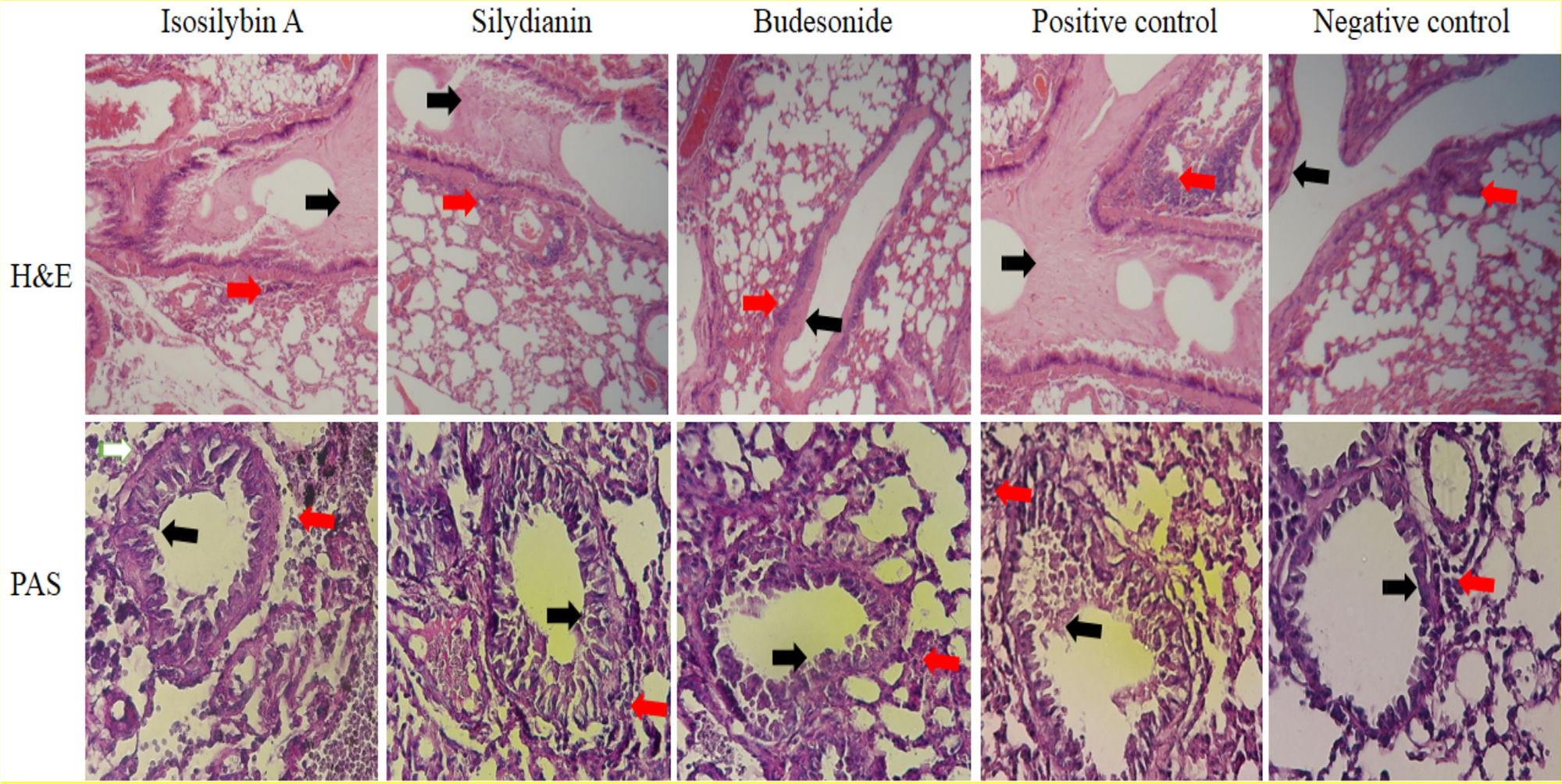
Effects of silymarin isomers on lung histologyIn the positive control group, mucus hyper-secretion (3.7 ± 0.2-fold) and goblet cell hyperplasia (score: 3.3) were significantly higher compared with negative control group (P < 0.05, Fig. 6). Mucus hyper-secretion was not significantly different between mice treated with either isosilybin A (3.7 ± 0.0-fold) or silydianin (3.6 ± 0.6-fold) in comparison with the positive control group (P > 0.05). The scores of eosinophilic infiltrations significantly decreased in mice treated with isosilybin A (perivascular: 0.7 ± 0.2, peribronchial: 0.8 ± 0.2) and silydianin (perivascular: 0.6 ± 0.4, peribronchial: 0.7 ± 0.01) compared with animals that received budesonide (perivascular: 1.8 ± 0.2, peribronchial: 1 ± 0.4). Moreover, the budesonide, silymarin and silymarin treated groups showed a lower number of eosinophils (Fig. 6) compared to positive control group (3.2 ± 0.1).
Histopathology of lung sections in animal models of allergic asthma. Tissues were stained with Hematoxylin & Eosin, and peribronchial inflammation, goblet cell hyperplasia (within the airway epithelium) and mucus hyper-secretion were also assessed. PAS staining was used to assess mucus production and Goblet cells. Mucus secretion and peribronchial inflammation have been indicated with black and red arrows, respectively.
In this study, the therapeutic effects of two purified silymarin isomers, isosilybin A and silydianin (Fig. 7), were investigated in a Balb/c mouse model of allergic asthma. The isosilybins A and silydianin were isolated using the powerful technique of HSCCC.18 These two isomers have shown anti-inflammatory, anti-spasmodic, and immunomodulatory effects delivering them as beneficial therapeutic agents to control the allergic mechanisms and immunological pathways that contribute to allergic asthma. Nevertheless, silymarin isomers are unable to control mucus production, which limits their therapeutic application.
In the present study, we demonstrated that isosilybin A and silydianin reduced AHR in a way that was comparable with budesonide (commercial anti-asthmatic drug). It has been shown that silymarin has a protective effect against bronchospasm in allergic asthma.14 This effect can be mediated through membrane-stabilizing and the anti-inflammatory activities of silymarin, as well as its inhibitory effects on arachidonic acid pathway.14 Furthermore, silymarin can inhibit acute phase response through suppressing inflammatory factors. Based on these, it has been suggested that silymarin can be used as a protective agent to manage asthma attacks and histaminic disorders.14
Isosilybin A and silydianin isomers also reduced the number of eosinophils in BAL fluid which can inhibit eosinophilic attacks in asthma. Eosinophils are important in asthma pathogenesis and can be key targets for treatment of the disease.1 In this regard, silymarin and its isomers (isosilybin A and silydianin) may be useful to manage complications of asthma triggered by eosinophils.
Cytokines, in particular Th2-derived cytokines, have significant roles in allergic responses, airways eosinophilia, and AHR in allergic asthma. IL-4 promotes IgE production and Th2 lymphocytes differentiation,25 and IL-5 orchestrates the proliferation, activation, migration, and infiltration of eosinophils into airways.26,27 IL-13, on the other hand, induces mucus hyper-secretion and airway obstruction in allergic asthma.28,29 In this study, we showed that isomers of silymarin, isosilybin A and silydianin, had regulatory effects on the balance of Th2 cytokines in mouse model of allergic asthma. Mice treated with isosilybin A and silydianin had reduced levels of IL-4 and IL-5; however, IL-13 level was not affected in the OVA-challenged groups. Reduced IL-4 level by these isomers can mitigate allergic reactions.
IgE is one of the important mediators of allergic reactions in asthma. In this study, we observed that silymarin isomers decreased serum levels of total and OVA-specific IgE. Low levels of IgE promoted by reduction in IL-4 can inhibit allergic processes in asthmatic animals.
As mentioned, isosilybin A and silydianin attenuated IgE production, eosinophilic inflammation, and AHR in the mouse model of allergic asthma. Despite that, these isomers had no effects on mucus hypersecretion. Assessments on the BAL fluid revealed that although isosilybin A and silydianin reduced IL-4 and IL-5 mRNA expressions, they had no effects on IL-13 and MUC5AC gene expressions. The gene regulatory effects of isosilybin A and silydianin can be mediated by modulating cell signaling pathways and transcription factors. In a study by Deep et al. in 2010, they reported that isosilybin A inhibited Akt phosphorylation and cellular proliferation. Furthermore, isosilybin A treatment modulated the level of NF-κB in the nucleus influencing cellular proliferation and activation.24
In our study, isosilybin A and silydianin isomers of silymarin effectively inhibited perivascular and peribronchial eosinophilic infiltrations in the lungs of mouse models of allergic asthma. These isomers also prevented goblet cell hyperplasia and AHR. Nevertheless, no significant effects were observed on mucus hyper-secretion. Jang et al. in 2012 showed that administration of skullcapflavone II, a flavonoid derived from Scutellaria baicalensis, reduced eosinophilic infiltration and inflammation, as well as AHR, but not mucus production.30 In this study, the two studied isomers reduced the Penh value which is an important indicator for predicting acute asthma attacks. The balance of Th1/Th2 immune response is another key point in the management of atopic diseases.31,32 We observed that isosilybin A and silydianin modulated the levels of IL-4 and IL-5 as Th2 cytokines.
The main asthma symptoms (i.e. inflammation, AHR, airflow obstruction, and mucus hyper-secretion)33,34 sustained in the current study; however, remodeling was not studied. Considering all of these parameters, the effects of the two studied isomers (isosilybin A and silydianin) were similar with no significant differences were observed between them.
ConclusionAccording to our study, isosilybin A and silydianin can control the main symptoms of asthma through modulating immune system responses, reducing eosinophilic inflammation as well as perivascular and peribronchial eosinophilic infiltrations, modulating IL-4 and IL-5 gene expressions, and reducing goblet cell hyperplasia, bronchospasm and AHR in animal models of allergic asthma. Nevertheless, no significant effects were observed on IL-13 production and mucus hyper-secretion. Considering their long-lasting effects and excellent safety profile, these natural isomers can be used as anti-inflammatory and anti-asthma drugs; however, they may be more efficient in combination with anti-mucus agents to prevent asthma attacks.
Conflicts of interestThere are no conflicts of interest.
The authors would like to acknowledge Research Center for Food Hygiene and Safety, Shahid Sadoughi University of Medical Sciences, Yazd, Iran and School of Traditional Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The authors also thanks Dr. Yari, Dr. Hasanzade, Mrs Dargahi, Mrs Kashafrudi and Mrs Jafari.