Eosinophilic oesophagitis (EoE) is a chronic clinical-pathological disorder with an immunological basis characterised by symptoms of oesophageal dysfunction and, histologically, eosinophilic inflammation.
ObjectiveTo evaluate the clinical characteristics and differences in children and adults diagnosed with EoE in a tertiary level hospital.
MethodDescriptive, retrospective and cross-sectional study. We randomly selected 40 children and 40 adults diagnosed with EoE between 2009 and 2016. The patient characteristics were analysed by means of epidemiological, clinical, diagnostic and therapeutic variables.
ResultsThe average age at diagnosis was 10 years (children) and 34 years (adults), with a higher frequency in males. The majority were sensitised to aeroallergens (77.5% children vs. 82.5% adults) and foods (75% children vs. 82.5% adults). Statistically significant differences were detected in sensitisation to fruits (p=0.007) and grains (p<0.001). Differences were observed in impaction (22.5% children vs. 82.5% adults), dysphagia (42.5% children vs. 77.5% adults) and abdominal pain (25% children vs. 7.5% adults). Endoscopy showed that children had a higher frequency of exudates (92.5%) and adults, trachealisation (50% vs. 5%) and stenosis (17.5% vs. 2.5%). Statistically significant differences were found in treatment with topical corticosteroids (30% children vs. 77.5% adults), with a variable positive response. 77.5% of the patients received elimination diets.
ConclusionsStatistically significant differences were observed between the paediatric and adult populations in the food sensitisation profiles, clinical manifestations, endoscopic findings and treatments received.
This is a complex pathology that calls for a multidisciplinary team and would require new non-invasive techniques to facilitate its management.
Eosinophilic oesophagitis (EoE) is a chronic clinical-pathological disorder with an immunological basis in which diagnosis is based on the presence of symptoms related to oesophageal dysfunction and a predominant infiltration of eosinophils on the wall of the oesophagus.1 Nowadays it is considered the most common cause of solid-food dysphagia and spontaneous perforation of the oesophagus.
EoE is more frequent in males (3:1), with an average age at diagnosis of between 30 and 50 years, although it can occur at any age.2 The majority present a personal and/or family history of atopy. Seasonal variations are also observed, with it being more frequent in the pollen season than in winter.3
EoE is predominantly but not exclusively an allergic disease triggered by food allergens. Its pathogenesis seems to depend to a great extent on cell-mediated hypersensitivity.4,5 These characteristics make EoE a unique and distinct form of food allergy in which the current tests for diagnosing food allergies (skin prick test and specific IgE) are suboptimal for predicting the foods that cause EoE, especially in adult patients.6
It is believed that EoE is caused by an immune response mediated by Th2 cells (involving IL-4, IL-5 and IL-13) to food and/or environmental allergens. IL-5 promotes selective expansion of eosinophils in bone marrow and their release into the circulating blood, whilst IL-13 stimulates the oesophageal epithelium to produce eotaxin-3 (a powerful chemokine that recruits eosinophils in the oesophagus).5,6 The activated eosinophils release multiple factors that promote local inflammation and tissue damage. In addition to the eosinophils, other inflammatory cells, such as T-cells, mast cells, basophils and NK cells, are also involved.5,7
There are differences in the pathogenesis and clinical presentation between EoE cases in children and adults, which has raised the question of whether they represent different entities of the disease.8,9
Infants and small children frequently present feeding difficulties (rejection of food, weight loss, growth retardation, etc.), while school-age children have a higher probability of presenting vomiting or abdominal pain.10 Dysphagia is the predominant symptom in adolescents.
The symptoms in adults with EoE are somewhat stereotyped and include dysphagia, thoracic pain, upper abdominal pain and food impaction (requiring endoscopic extraction of the bolus in 33–54% of cases11). Solid-food dysphagia continues to be the most common presenting symptom.12 The most frequent complications are nutritional deficiencies in children and oesophageal stenosis and perforation in adults.
Any patient with symptoms suggestive of EoE should have a detailed medical history taken, with particular attention to eating and swallowing habits. The additional tests to be performed for correct diagnosis are the following: (1) upper endoscopy: typical endoscopic alterations with EoE include fixed oesophageal rings (trachealisation) or transient oesophageal rings (felinisation), whitish exudates, reduction of oesophageal calibre, focal oesophageal strictures, linear furrows, reduced vascularisation and “crepe paper” mucosa (a sign of severe mucosal fragility),13,14 although none of them can be considered pathognomonic for EoE12; (2) biopsy: endoscopy with oesophageal biopsy is the only reliable diagnostic test for EoE. However, the finding of isolated EoE without associating compatible symptoms and ruling out other causes of EoE is insufficient for making the diagnosis.12 One study identified a diagnostic sensitivity of 84%, 97% and 100% with two, three and six biological samples, respectively15; (3) allergy testing: it is important to perform directed anamnesis to assess the possible involvement of foods in the disease. Skin prick tests with aeroallergens and foods, total and specific serum IgE determination and epicutaneous tests with foods (NPV 90%) should be performed, although the latter are not standardised. It is important to interpret the results accurately to avoid unnecessary avoidance diets; (4) biological markers: to date, there is no biological marker available that has demonstrated its utility in monitoring EoE activity. The markers studied include peripheral eosinophilia, total serum IgE and eosinophil mediators, but none of them has demonstrated sufficient specificity and sensitivity and, therefore, are not recommended as a tool for monitoring disease activity.12
The diagnostic criteria for EoE are as follows: (a) presence of symptoms related to oesophageal dysfunction; (b) inflammation with predominance of eosinophils in the oesophageal biopsy (>15 intraepithelial eosinophils/high-power field); (c) limited effect on the oesophagus; (d) exclusion of other causes of EoE; (e) response to treatment with elimination diets and/or corticosteroids (not essential).12
Among the diverse options for treatment of EoE, we find pharmacological therapy, food avoidance diets and oesophageal dilation. Within pharmacological treatment, we find proton pump inhibitors (PPIs) and corticosteroids (CS). PPIs are important for the differential diagnosis between gastro-oesophageal reflux disease (GORD) and PPI-responsive oesophageal eosinophilia (PPI-ROE). Whenever there is any suspicion of EoE, treatment should be started with PPIs at high doses and endoscopy repeated after 6-8 weeks to observe the response.12 Corticosteroids are powerful anti-inflammatory drugs that help to reduce EoE symptoms, and they can be administered topically (swallowed) or systemically.
There are currently three main modalities of dietary therapy for EoE: the elemental diet (consisting of feeding with an elemental formula in which all proteins have been eliminated and nitrogen is supplied exclusively by individual amino acids, making it devoid of antigenic capacity);4,16 the elimination diet, guided by food allergy testing (eliminating from the diet foods with positive results in cutaneous and epicutaneous testing);4,17 and empirical elimination diets (consisting of eliminating from the diet the six [cow's milk, wheat, egg, soy, peanut/tree nuts, fish, and seafood], four [milk, gluten-containing cereals, egg, legumes] or two [milk and gluten-containing cereals] most common trigger foods in EoE and re-evaluating after the re-introduction of each group after objectifying the histological remission). Elemental diet and empiric six-food elimination diet have consistently shown the best cure rates but their high level of restriction and need for multiple endoscopies have been an obstacle for both patients and physicians. Less restrictive empiric schemes (like four-food or two-food elimination diets) have lately shown encouraging results. Therefore, a novel step-up strategy (2-4-6) can improve patient uptake and promptly identify most responders to few food triggers, besides saving unnecessary dietary restrictions and endoscopic procedures.4,18–22
All dietary treatment strategies are aimed at inducing remission of EoE as a starting point for subsequent identification of possible food triggers. The ultimate objective is to exclude from the diet only the foods responsible for triggering and maintaining the disease in each patient. Therefore, once the biopsy shows remission of eosinophilia, the foods should be reintroduced one by one over a minimum of six weeks,4 with an endoscopy following the reintroduction of each food. Once all the food groups have been reintroduced individually, the triggers identified should be eliminated from the diet indefinitely, whilst the foods that are well tolerated can be eaten regularly. The disassociation between clinical symptoms and histology in EoE has been documented repeatedly in children and adults,23 which implies that the absence of symptoms following the reintroduction of the foods does not necessarily signify remission of the disease.13,23 Due to the lack of non-invasive biomarkers that can adequately predict the presence or absence of eosinophils in the oesophagus, multiple endoscopies with systematic biopsies are currently required to precisely identify the trigger foods.24
Our objective is to describe the differences in the epidemiological characteristics, sensitisation profiles, clinical manifestations, endoscopic findings and therapeutic management between a group of paediatric patients and a group of adult patients diagnosed with EoE.
Materials and methodsPatientsThe inclusion criteria were patients (adults and children) diagnosed with EoE from January 2009 to December 2016, meeting the criteria proposed in the 2011 consensus document by Liacouras et al.12 EoE diagnosis was understood to mean patients who presented symptoms of oesophageal dysfunction and had >15 eosinophils/high-power field in the oesophageal biopsy.
The existence of other allergic and/or digestive pathologies was not a reason for exclusion.
This is a descriptive, retrospective, cross-sectional study. A total of 80 patients were selected randomly: 40 paediatric patients from Hospital Universitario Miguel Servet (Zaragoza) and 40 adult patients from Hospital Clínico Universitario Lozano Blesa (Zaragoza).
A database of the patients diagnosed with EoE at both hospitals from 2009 to 2016, both inclusive, was available. Using the randomisation function of the software application Excel 2003 (Microsoft, Redmond, WA, USA), 40 patients from each group were selected. After selecting the 80 patients for the study, work began on a database with the included variables (see Fig. 1). Once the data from the clinical histories were obtained, the variables were included in an Excel 2003 database, without any data that would identify the patients, for subsequent analysis using the statistics program SPSS 15.0 (Chicago, IL, USA).
Statistical analysisA preliminary descriptive analysis was conducted in which the qualitative data were measured using percentages to express the proportions and frequencies observed.
Next, a comparative analysis between the two patient groups (children vs. adults) was conducted. A univariate analysis was conducted using the specific tests for each variable (Student's t-test for quantitative variables, chi-squared test for qualitative variables), considering a significance level with p<0.05.
ResultsEpidemiologyA higher proportion of males was observed in both groups, with a ratio of 3:1.
The average age at diagnosis was 10 years in the paediatric group [3–16] and 34 years in the adults [7–62].
There was a predominance of urban population with respect to rural population in both groups.
Noteworthy family histories included three children with a family history of EoE (two brothers and one father). As regards personal histories, 90% of the children and 77.5% of the adults were atopic. Two children had been treated with milk immunotherapy and five adults with subcutaneous immunotherapy with aeroallergens (pollen).
The characteristics of both groups are shown in Table 1.
Characteristics of the two groups.
| Children | Adults | ||||
|---|---|---|---|---|---|
| n=40 | n=40 | ||||
| Sex | |||||
| Male | 31 | 77.5% | 29 | 72.5% | |
| Female | 9 | 22.5% | 11 | 27.5% | |
| Origin | |||||
| Rural | 15 | 37.5% | 9 | 22.5% | |
| Urban | 25 | 62.5% | 31 | 77.5% | |
| Age at diagnosis | 10 | 34 | |||
| Family history of EoE | 3 | 7.5% | 0 | 0% | |
| Personal history of atopy | 36 | 90% | 31 | 77.5% | |
| Rhinitis/asthma | 28 | 70% | 28 | 70% | |
| Food allergy | 28 | 70% | 12 | 30% | |
| Atopic dermatitis | 20 | 50% | 2 | 5% | |
| Symptoms | |||||
| Dysphagia | 17 | 42.5% | 31 | 77.5% | p=0.001 |
| Impaction | 9 | 22.5% | 33 | 82.5% | p<0.001 |
| Abdominal pain | 10 | 25% | 3 | 7.5% | p=0.034 |
| Thoracic pain | 2 | 5% | 5 | 12.5% | |
| GOR | 5 | 12.5% | 7 | 17.5% | |
| Other | 3 | 7.5% | 0 | 0% | |
| Aeroallergen prick test | |||||
| Pollens | 25 | 62.5% | 30 | 75% | |
| Moulds | 8 | 20% | 16 | 40% | |
| Dust mites | 3 | 7.5% | 9 | 22.5% | |
| Epithelia | 5 | 12.5% | 15 | 37.5% | |
| Food prick test | |||||
| Milk | 7 | 17.5% | 4 | 10% | |
| Eggs | 11 | 27.5% | 6 | 15% | |
| Grains | 2 | 5% | 19 | 47.5% | p<0.001 |
| Nuts | 18 | 45% | 18 | 45% | |
| Fish/shellfish | 8 | 20% | 7 | 17.5% | |
| Fruits | 14 | 35% | 23 | 57.5% | p=0.007 |
| Legumes | 11 | 27.5% | 9 | 22.5% | |
| LTP | 13 | 32.5% | 15 | 37.5% | |
| Meats | 0 | 0% | 3 | 7.5% | |
| Soy | 0 | 0% | 1 | 2.5% | |
| Food-specific IgE | |||||
| Milk | 14 | 35% | 2 | 5% | |
| Eggs | 10 | 25% | 2 | 5% | |
| Grains | 12 | 30% | 18 | 45% | |
| Nuts | 23 | 57.5% | 16 | 40% | |
| Fish/shellfish | 8 | 20% | 3 | 7.5% | |
| Fruits | 34 | 72% | 15 | 37.5% | |
| Legumes | 11 | 27.5% | 5 | 12.5% | |
| LTP | – | – | 8 | 20% | |
| Meats | – | – | – | – | |
| Soy | 8 | 20% | 3 | 7.5% | |
| Upper endoscopy | |||||
| Normal | 1 | 2.5% | 10 | 25% | p=0.003 |
| Erosions | 3 | 7.5% | 2 | 5% | |
| Exudates | 37 | 92.5% | 14 | 35% | p<0.001 |
| Trachealisation | 2 | 5% | 20 | 50% | p<0.001 |
| Stenosis | 1 | 2.5% | 7 | 17.5% | p=0.025 |
| Treatment with PPIs | 25 | 62.5% | 31 | 77.5% | |
| Response to PPIs | 7 | 28% | 10 | 32% | |
| Treatment with CS | 12 | 30% | 36 | 90% | p<0.001 |
| Response to CS | 4 | 33.3% | – | – | |
| Elimination diets | 31 | 77.5% | 30 | 75% | |
| 1 food | 4 | 13% | 3 | 10% | |
| 2 foods | 4 | 13% | 7 | 24% | |
| 3 foods | 6 | 19% | 10 | 33% | |
| 4 or more foods | 17 | 55% | 10 | 33% | |
| Response to diet | 12 | 39% | 11 | 36.6% | |
72.5% of the children and 82.5% of the adults were sensitised to one or more aeroallergens, with a predominance of pollens in both groups.
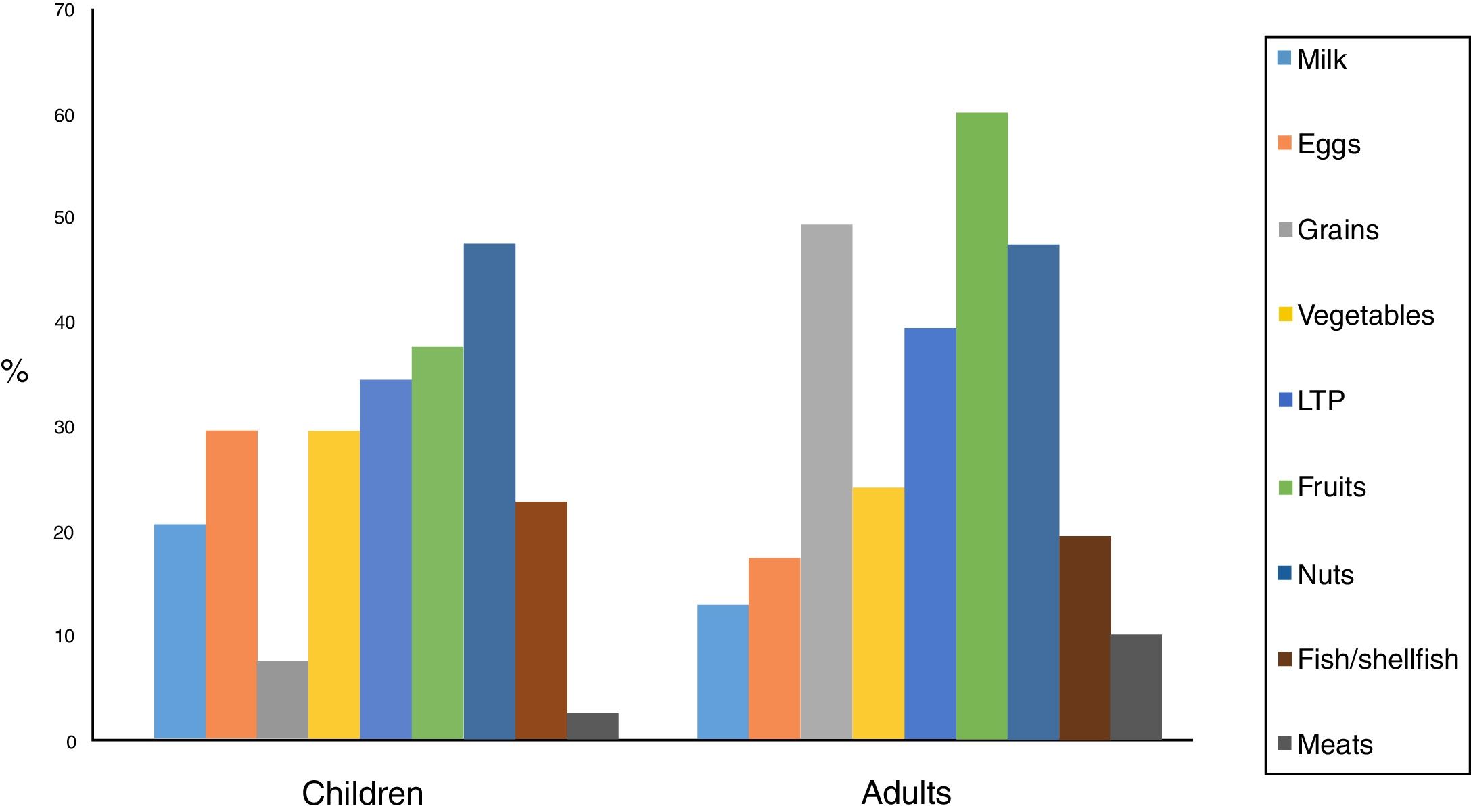
70% of the children were sensitised to foods, with the most frequent being nuts, fruits and LTP (lipid transfer protein). In a lower proportion were legumes and eggs, fish and shellfish, and milk.
Considering positivity to be specific IgE >0.35 KU/L, fruits and nuts predominated, followed by milk, grains, eggs and fish/shellfish, with sensitisation percentages that were slightly higher than for the skin prick tests.
82.5% of the adults were sensitised to foods, with the most frequent ones being fruits, grains, nuts and LTP. In a lower proportion were legumes, fish/shellfish, eggs, milk and meats. The high percentage of adults sensitised to grains should be noted, 57.5% of these being to corn, 53% to wheat and barley, 26% to rice, and 21% to oats and rye. Statistically significant differences were found in the sensitisation to fruits (p=0.007) and to grains (p<0.001).
Considering positivity to be specific IgE >0.35kU/L, grains, nuts, fruits, LTP, fish/shellfish and milk and egg predominated, with sensitisation percentages that were slightly lower than for the skin prick tests, contrary to the case of children.
Food sensitisation differences between the two groups are shown in Fig. 2.
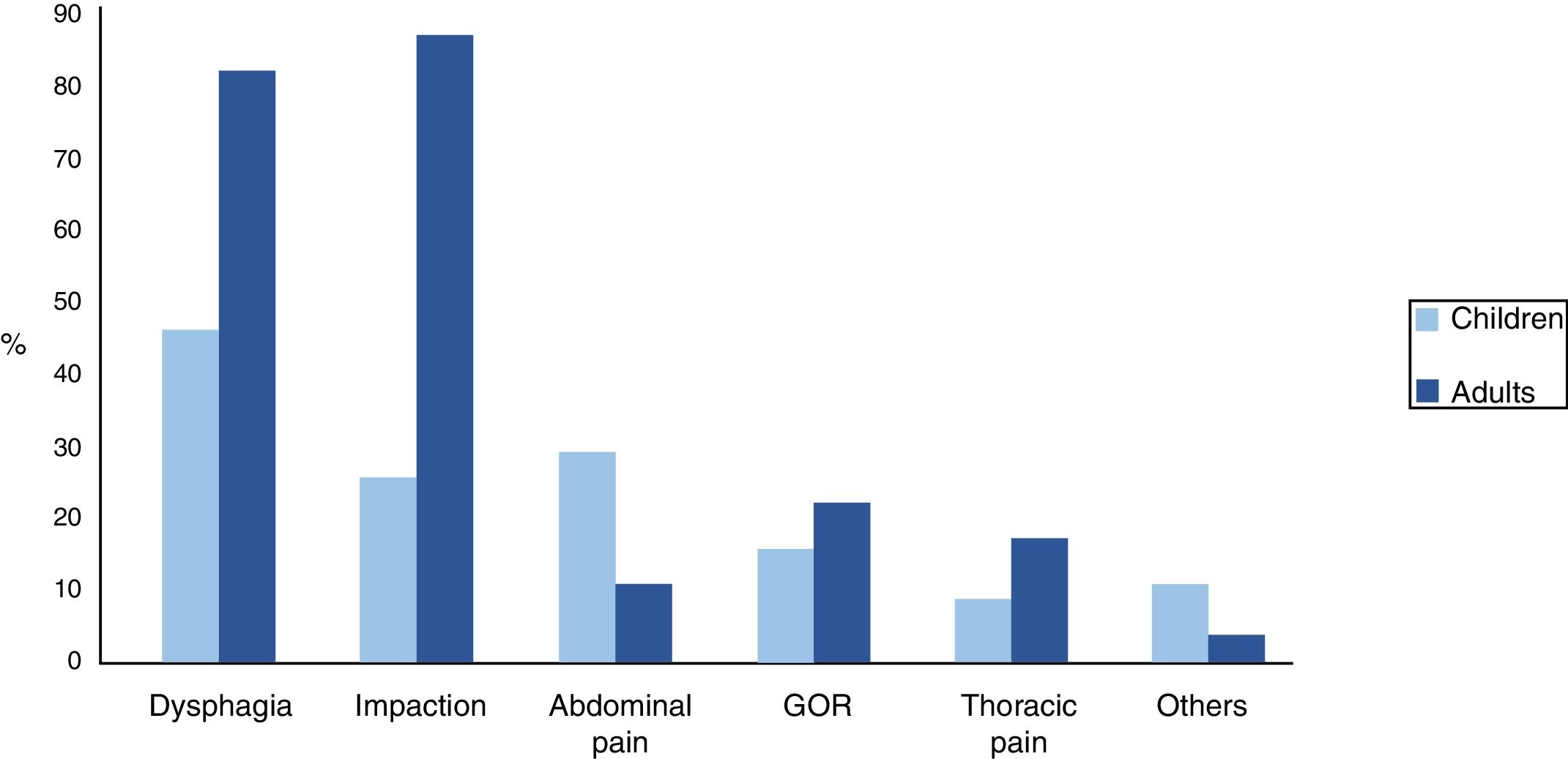
SymptomsDysphagia was the most frequent symptom in children, whilst in adults it was impaction. We observed statistically significant differences between the two groups in the presentation of impaction (p<0.001), dysphagia (p=0.001) and abdominal pain (p=0.034). Other symptoms, such as gastro-oesophageal reflux (GOR) and thoracic pain, were less frequent. In the paediatric group, there was a small group (7%) that had started with symptoms that were more unspecific, such as constipation, yellow faeces or blood in faeces. Fig. 3 shows clinical differences between the two groups
DiagnosisIn the endoscopies of the paediatric population, the most frequent finding was exudates, whilst in the adult population it was trachealisation. We observed statistically significant differences between the two groups in the endoscopic findings of exudates (p<0.001), trachealisation (p=0.001), stenosis (p=0.025), and absence of endoscopic alterations (p=0.003).
The oesophageal biopsies of all the patients in the two groups showed >15 eosinophils/high-power field at diagnosis.
TreatmentStatistically significant differences were observed in treatment with swallowed CS (p<0.001) but not in treatment with PPIs.
Both drugs had been administered at different points in time or, in some cases, simultaneously.
77.5% of the children had followed food elimination diets with one, two, three and four or more foods, and the percentage was observed to increase in proportion to the number of foods eliminated from the diet. The most frequently withdrawn foods were milk, eggs, wheat, nuts and fish.
75% of the adults followed food elimination diets with one, two, three and four or more foods. The most frequently withdrawn foods were nuts, followed by fruits and grains, mustard and milk, and eggs.
EvolutionOf the 25 children treated with PPIs, 28% showed good clinical-pathological response; of the 12 treated with CS, 33.3% responded.
39% of the children who followed a diet free of one or more foods responded to it. Of the nine children not following a diet free of certain foods, three improved following treatment with PPIs.
Of the 31 adults treated with PPIs, 32% showed good response, 19% did not respond, and in 49% the response was unknown because the protocol of periodic endoscopies could not be followed exactly. Of the patients treated with swallowed CS, the same results were obtained, as many of them were in treatment with both drugs when the biopsy was performed or the response to the other drug had not been ascertained prior to adding or replacing it with the current one, making interpretation of these results invalid.
36.6% of the adults who followed a diet free of one or more foods responded, with the result being unknown in 33% of the cases (the endoscopy was not performed at the indicated time).
DiscussionEoE is a disease of growing interest in which the real prevalence is unknown, but advances in its current recognition have resulted in greater detection of it in patients with high clinical suspicion. It predominantly affects children and young adults of the male sex. In our series, a high predominance of males was observed (3:1) in both groups, which coincides with the findings described in earlier studies.8,17 As regards age, EoE can occur at any age, but it is diagnosed predominantly in the paediatric age group and in adults under the age of 45 years. In our sample, the average age at diagnosis was 10 years in the paediatric group and 34 years in the adults.
In the pathogenesis of EoE, an aberrant response to allergens seems to exist,25,26 which explains why 90% of the children and 77.5% of the adults in our series present a history of atopy. 72.5% of the children and 82.5% of the adults in our sample were sensitised to one or more aeroallergens. 70% of the children and 82.5% of the adults were sensitised to some food. These data are similar to other Spanish series.3,27–29 This shows the benefits of allergy testing in these patients, as indicated by other studies.12,26 Skin prick tests for foods like milk, eggs, soy, wheat, meat or nuts were disproportionately represented in our series compared to others.17 Nuts and fruits predominated in the children, whilst in the adults fruits and grains predominated, unlike other studies that indicate milk, eggs and wheat as the most frequently implicated foods.17,26 In our sample, we found a lower proportion of patients sensitised to milk (17% children and 10% adults), eggs (27% children 15% adults), and wheat (5% children and 47% adults).
The symptoms varied by age, from difficult feeding in infants, vomiting and abdominal pain in school-age children, to dysphagia in adolescents and adults. Significant differences were also observed between the two groups in the forms of clinical presentation. Coinciding with earlier studies,8 dysphagia is the main symptom of EoE, but our series showed different proportions in children (42%) and adults (78%).
Diagnosis requires a high degree of suspicion by the doctor, who needs to be attentive to both the gastrointestinal symptoms of EoE and the accompanying symptoms (growth retardation, allergic rhinitis, asthma, etc.). An upper digestive endoscopy should be requested, indicating the diagnostic suspicion for adequate visualisation and biopsy and referral to a specialist for an assessment and proper management of the disease. The endoscopic findings of EoE can vary greatly,14 ranging from normal-looking mucosa to significant changes such as whitish exudates, oesophageal rings or oesophageal stenosis. In our sample, we observed different endoscopic findings in the two groups, with exudates being more frequent in children (92.5% vs. 35%) and trachealisation more frequent in adults (50% vs. 5%), as observed in other series but with a smaller magnitude of the differences (36% vs. 19% and 57% vs. 11% respectively).30 We also found stenosis to be more frequent in adults (17.5% vs. 2.5%), a fact that was not observed in other series (9% vs. 11%).30 2% of the paediatric endoscopies and 25% of the endoscopies of adults in our series were macroscopically normal, which contrasts with other series (21% vs. 15%),30 but also justifies the use of oesophageal biopsy in all cases of diagnostic suspicion, even in healthy mucosa. The use of biopsies in patients with suspected EoE has been the subject of extensive studies; as regards the location and number of these, it seems reasonable to say that at least four biopsies, in all the oesophageal segments (proximal and distal), establish the diagnosis with a high degree of sensitivity and specificity whenever the morphological-histological study demonstrates the presence of intraepithelial eosinophils, thickening of the basal layer and a count of >15 eosinophils per 40x field. In our sample, the presence of these histological findings was demonstrated in all cases.
One of the advantages of this study is the inclusion of children and adults in our analysis, whilst the majority of studies have focused on a single age group. Hence the main characteristics of the two groups can be compared to observe whether there are differences between them.
The main limitations of our study include its retrospective nature, the small sample size and the variation in the tests of sensitisation to food and environmental allergens among our patients (the same skin prick test and specific IgE determinations were not performed for all of them, as they came from two different hospitals and no established protocol previously existed for allergological assessment of this disease).
EoE is a complex disease that behaves differently according to the age of onset, calls for a multidisciplinary team for correct assessment and requires new non-invasive techniques to facilitate its management.
In conclusion, in accordance with the literature, our findings show a high prevalence of atopic diseases and sensitisation to food and environmental allergens in both children and adults, as well as differences in the food sensitisation profiles, clinical manifestations, endoscopic findings and treatments received. Knowledge of these differential data can help us to establish a suspected diagnosis of EoE based on the symptoms presented by the patient initially; this would avoid a delay in diagnosis, which is associated with oesophageal remodelling and the development of stenosis. Current data suggest that EoE is the same disease in children and adults taken from the same population, probably representing a different phenotype or spectrum, but more longitudinal studies are needed to show the evolution of the disease in paediatric patients and adults. It is important to clarify this point and try to elucidate whether there are differences in the characteristics, evolution or prognosis of the disease depending on the age at which the disease's debut occurs.
DisclaimerData of this study have not been presented in abstract or poster form at conferences.
Funding sourcesNil.
Author conflicts of interestThe authors have no conflict of interest to declare.