There are few atopic dermatitis (AD) incidence cohort studies in young adults, the etiology of this disease remains obscure, and AD risk factors in adults are not well understood. The objective of this study was to estimate AD ten-year incidence and prevalence in a cohort of adolescent aged 14–16 at inception in Castellon province in Valencia Region, Spain and describe related risk factors.
Material and methodsFrom 2002 to 2012, a population-based prospective cohort study was carried out. Questionnaires from the International Study of Asthma and Allergies in Childhood (ISAAC) were used with an additional questionnaire for related factors completed by participants and their parents, respectively, in 2002. In 2012 the same questionnaires were completed by the participants’ through a telephone interview, and incidence and prevalence of AD were estimated. Directed acyclic graphs, Poisson regression and inverse probability weighted regression adjustment were used.
ResultsThe participation rate was 79.5% (1435/1805) with AD lifetime prevalence of 34.9% and AD incidence of 13.5 per 1000 person years. Females presented higher prevalence and incidence than males. After adjustment significant risk factors were being female, history of asthma or allergic rhinitis, family history of AD, history of respiratory infections, history of bronchitis, history of pneumonia, history of sinusitis, and birthplace outside Castellon province. The highest AD population attributable risks were female, 30.3%, and history of respiratory infections 15.3%. Differences with AD childhood risk factors were found.
ConclusionsAD incidence in our cohort was high and several risks factors were related to AD.
Atopic dermatitis (AD) is a chronic inflammatory skin disease with a high prevalence in children, adolescents and frequent in adults. A laboratory measure to confirm the diagnostic is lacking and AD clinical picture of adults has some diagnostic difficulties and may be laborious to distinguish from other skin diseases.1,2
Several studies of AD prevalence have indicated an increase in European countries with a stability of AD incidence.3 However, there are few cohort studies of AD incidence in adolescents and young adults.4–7 AD etiology remains obscure, it is considered a multifactorial disease,8,9 but AD adult risk factors are not well established.10,11
The objective of this study was to estimate AD incidence and prevalence in a cohort of adolescents aged 14–16 at inception followed until they were 24–26 years old in Castellon province and describe related risk factors related to new cases.
Material and methodsPhase I of the International Asthma and Allergies in Childhood (ISAAC) first survey was carried out during winter and spring in 1994, with the participation of schoolchildren aged 6–7 and 14–15. In 2002, Phase III of ISAAC, a second survey, was carried out in Castellon with the same groups. In 2012, the former participants, now aged 24–26 years completed a survey implemented by telephone; more details of this cohort study have been reported by Segura et al.12 The three surveys were carried out by staff of the epidemiology division of the public health center, and other health centers in Castellon, Spain.
The ISAAC questionnaire was used to estimate AD with an additional risk factors questionnaire; parents of the 6–7 years old group completed the two questionnaires in 1994 and an extensive additional questionnaire in 2002. The same cohort of schoolchildren who participated in the survey in 1994 was followed up to 2012, and a population-based prospective cohort study was implemented considering the period 2002–2012, from 14–16 to 24–26 years old. The three surveys were carried out in the same seasons of the year. AD incidence was estimated including only those participants free of AD at the beginning of the first and second surveys.
The questions used to estimate the AD incident cases were a positive answer to at least one of these two questions in the 2002 and 2012 surveys, respectively: Have you ever had eczema or atopic dermatitis? Do you take any medication for atopic dermatitis? The AD lifetime prevalence was estimated by a positive answer to the first question in the 1994 survey, and a positive answer to at least one of the two questions in the 2002 and 2012 surveys.
Risk factors for AD incidence were estimated from the second to third survey with an extended additional questionnaire. These risk factors were family history of AD, history of asthma or/and allergic rhinitis, history of respiratory infections (bronchitis, pneumonia, sinusitis, otitis), exposure of smoking at home, animals at home (dog, cat, others), history of breastfeeding, history of day care, frequency of truck traffic in home street, birthplace outside of Castellon province, age of mother at participant's birth, presence of older siblings, occupational social class base on parents’ occupation, town of residence with 50,000 inhabitants or more, and presence of ceramic industry in the town of residence.
Statistical analysisPerson years were calculated to estimate AD incidence during eight years from 1994 to 2002 and 10 years from 2002 to 2012; AD new cases were divided by the population free of AD in 1994 and 2002, respectively. AD lifetime prevalence was estimated with the accumulation of AD cases of the three described surveys. Chi2 and Fisher exact tests were used for comparison of categorical variables, and Kruskal–Wallis test for continuous variables. Univariate Poisson regression analysis was used to estimate associations between AD incidence and independent variables from 2002 to 2012. DAGitty version 3.013 was used to implement the Directed Acyclic Graphs (DAGs)14 approach which was employed to study the relationship between AD incidence (outcome) and potential confounders. Multivariate inverse probability weighted regression adjustment (IPWRA)15 was used to model AD incidence and potential risk factors adjusting for confounders and AD incidence. Age and gender were included in all models. Population attributable risks were estimated following Rokchill et al.16 Statistical analysis was performed using Stata®14 program.
The Ethic Committee of the Hospital General de Castellon approved the study and informed consent was obtained from each participant or their parents.
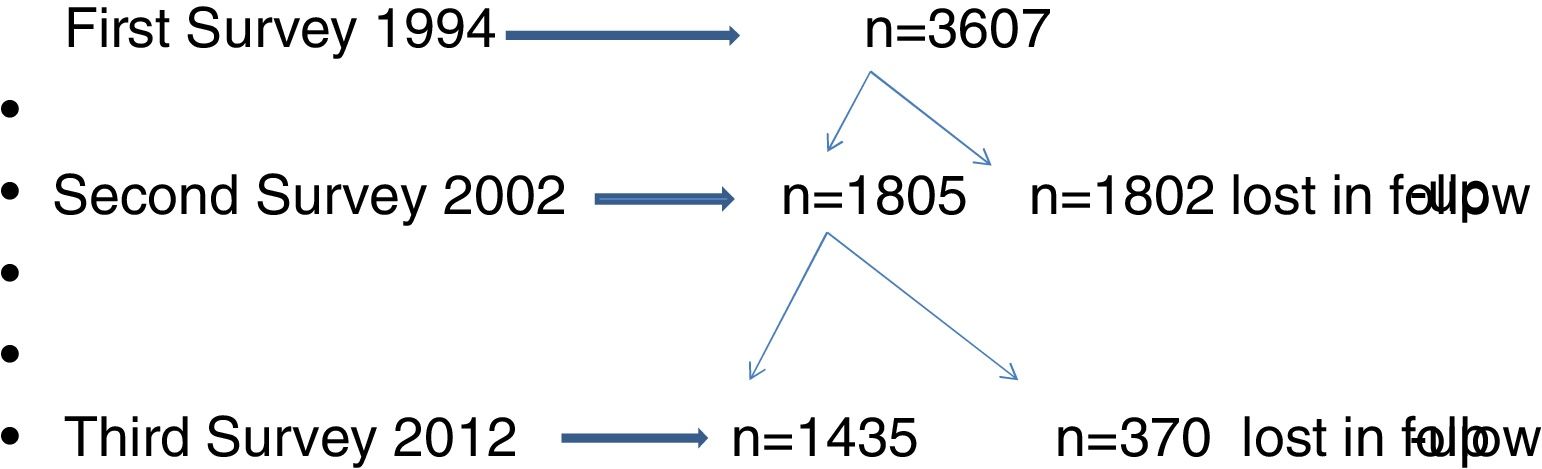
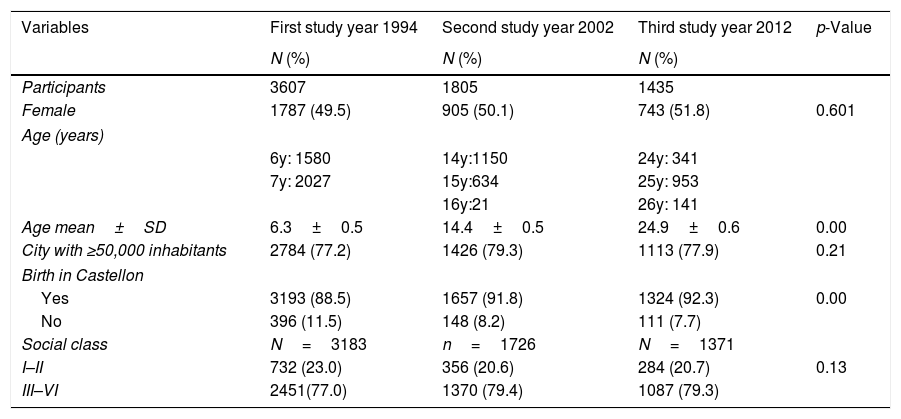
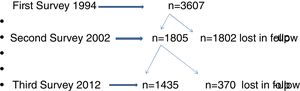
ResultsThe flow chart of participants of Castellon's cohort is shown in Fig. 1. In 1994, the first survey included 3607 participants and the second survey 1807 participants (50%). In the third survey, in 2012, the participation rate was 79.5% (1435/1807). Characteristics of the three surveys are presented in Table 1. No differences in gender, size of the residence town and social class were observed in the three surveys, although the percentages of participants born in Castellon had decreased.
Characteristics of Castellon's cohort in the three surveys 1994, 2002, 2012.
| Variables | First study year 1994 | Second study year 2002 | Third study year 2012 | p-Value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Participants | 3607 | 1805 | 1435 | |
| Female | 1787 (49.5) | 905 (50.1) | 743 (51.8) | 0.601 |
| Age (years) | ||||
| 6y: 1580 | 14y:1150 | 24y: 341 | ||
| 7y: 2027 | 15y:634 | 25y: 953 | ||
| 16y:21 | 26y: 141 | |||
| Age mean±SD | 6.3±0.5 | 14.4±0.5 | 24.9±0.6 | 0.00 |
| City with ≥50,000 inhabitants | 2784 (77.2) | 1426 (79.3) | 1113 (77.9) | 0.21 |
| Birth in Castellon | ||||
| Yes | 3193 (88.5) | 1657 (91.8) | 1324 (92.3) | 0.00 |
| No | 396 (11.5) | 148 (8.2) | 111 (7.7) | |
| Social class | N=3183 | n=1726 | N=1371 | |
| I–II | 732 (23.0) | 356 (20.6) | 284 (20.7) | 0.13 |
| III–VI | 2451(77.0) | 1370 (79.4) | 1087 (79.3) | |
Social class: I=professional, II=managerial and technical, III=skilled non-manual, IV=skilled manual, V–VI=partially skilled and unskilled.
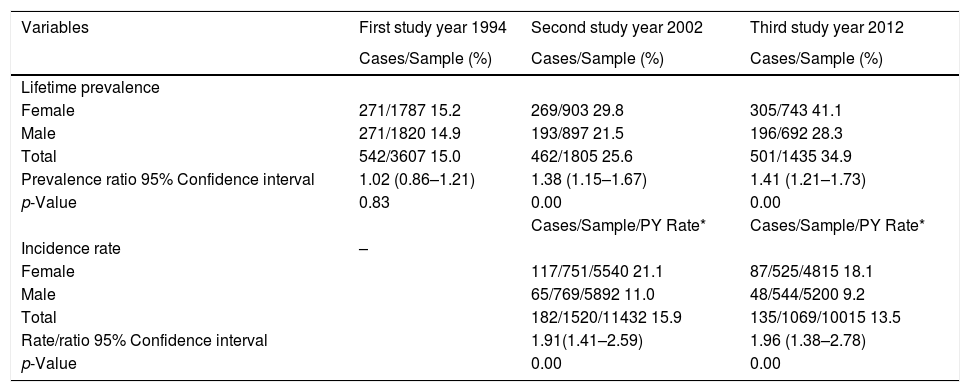
Lifetime prevalence of AD in the three surveys was 15.5%, 25.7%, and 34.9%, respectively (Table 2). Females presented significant higher prevalence than males in 2002 and 2012, and this difference increased with age. In 2002, 182 new cases of AD were found with an incidence of 15.9 per 1000 person years, 1.4% per year (182/1520x8). In 2012, 135 new cases of AD were found with an incidence of 13.5 per 1000 person years, and 1.3% per year (135/1069x10). A decrease rate ratio of 0.85 (95%CI 0.68–1.06) was observed between 2002 and 2012. Females had significantly higher incidences than males in the two surveys, 21.1 versus 11.0 and 18.1 versus 9.2 per 1000 person years.
Atopic dermatitis lifetime prevalence and incidence rates from 1994 to 2002 and from 2002 to 2012 for Castellon's cohort.
| Variables | First study year 1994 | Second study year 2002 | Third study year 2012 |
|---|---|---|---|
| Cases/Sample (%) | Cases/Sample (%) | Cases/Sample (%) | |
| Lifetime prevalence | |||
| Female | 271/1787 15.2 | 269/903 29.8 | 305/743 41.1 |
| Male | 271/1820 14.9 | 193/897 21.5 | 196/692 28.3 |
| Total | 542/3607 15.0 | 462/1805 25.6 | 501/1435 34.9 |
| Prevalence ratio 95% Confidence interval | 1.02 (0.86–1.21) | 1.38 (1.15–1.67) | 1.41 (1.21–1.73) |
| p-Value | 0.83 | 0.00 | 0.00 |
| Cases/Sample/PY Rate* | Cases/Sample/PY Rate* | ||
| Incidence rate | – | ||
| Female | 117/751/5540 21.1 | 87/525/4815 18.1 | |
| Male | 65/769/5892 11.0 | 48/544/5200 9.2 | |
| Total | 182/1520/11432 15.9 | 135/1069/10015 13.5 | |
| Rate/ratio 95% Confidence interval | 1.91(1.41–2.59) | 1.96 (1.38–2.78) | |
| p-Value | 0.00 | 0.00 |
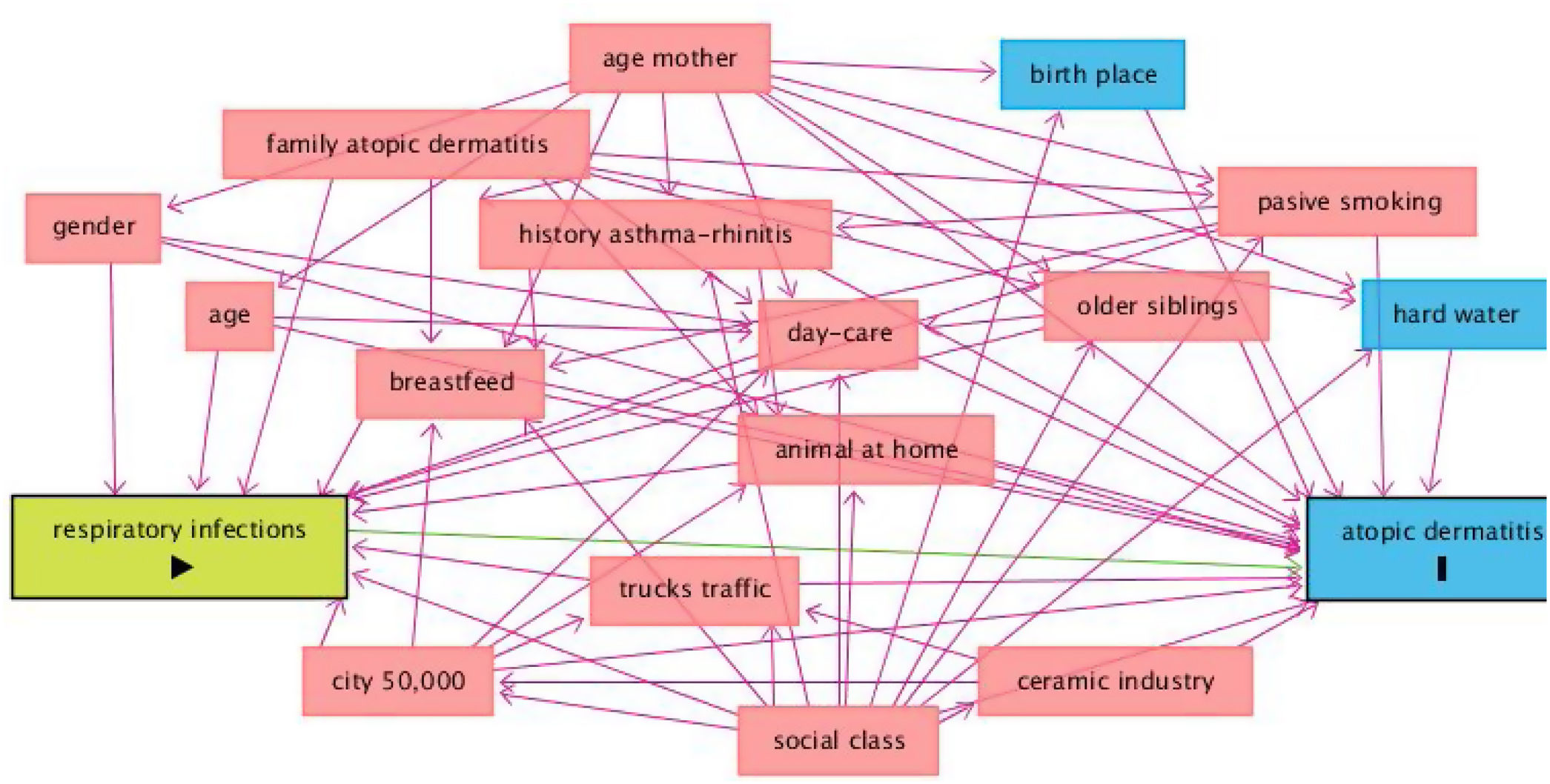
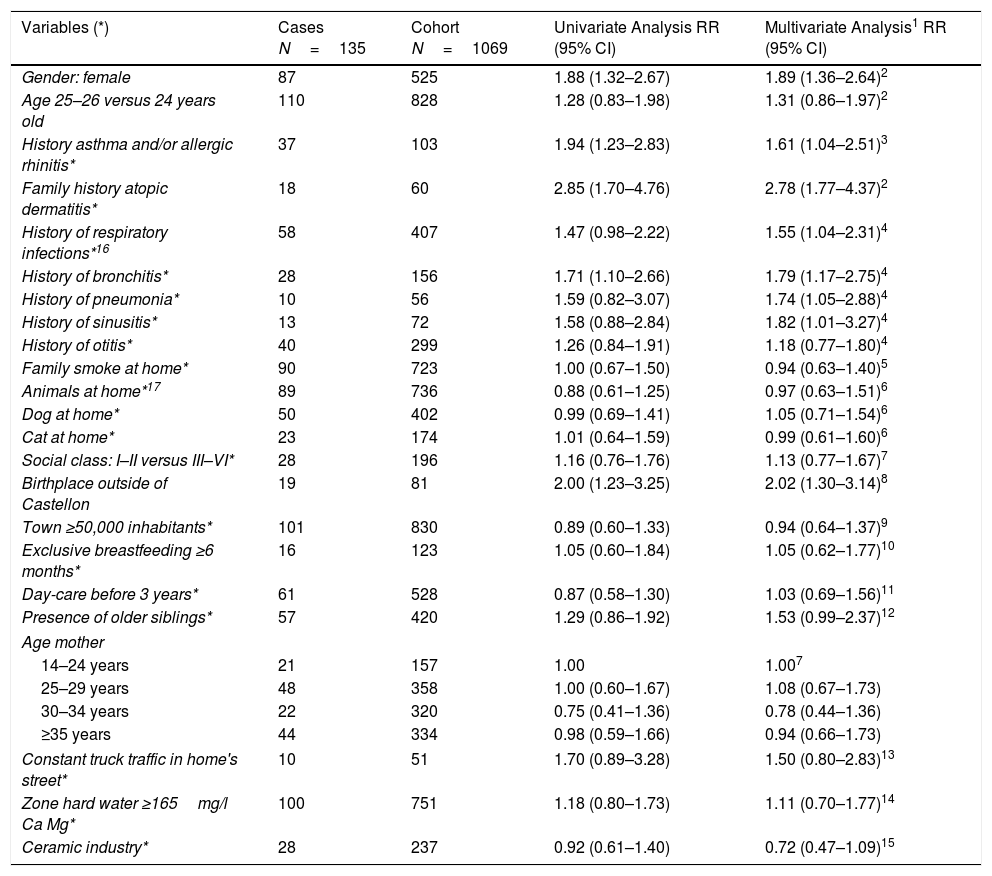
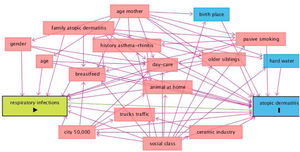
DAGs approach is presented in Fig. 2, and univariate and multivariate analysis of variables related to AD incidence are shown in Table 3. In the period 2002–2012, AD incidence rate in females was 16.6% (87/525) and 8.8% (48/544) in males (p=0.000). The mean age was 24.9±0.5 in cases and 24.9±0.6 in no-cases (p=0.307). In the Poisson analysis, the significant risk factors were: being female, history of asthma and/or rhinitis, family history of atopic dermatitis, history of respiratory infections, history of bronchitis, and birthplace outside of Castellon province. In the IPWRA analysis, significant risk factors were: female (RR=1.89; 95% CI 1.36–2.64), history of asthma and/or allergic rhinitis (RR=1.61; 95% CI 1.04–2.51), family history of AD (RR=2.78; 95% CI 1.77–4.37), history of respiratory infections (RR=1.55;95% CI 1.04–2.31), history of bronchitis (RR=1.79; 95% CI 1.17–2.75), history of pneumonia (RR=1.74;95% CI 1.05–2.88), history of sinusitis (RR=1.82;95% CI 1.01–3.27) and birthplace outside of Castellon province (RR=2.02; 95% CI 1.30–3.14).
Risk and protective factors of new cases of atopic dermatitis incidence by univariate analysis (Poisson regression) and multivariate analysis (inverse probability weighted regression adjustment) (IPWRA). Castellon's cohort 2012.
| Variables (*) | Cases N=135 | Cohort N=1069 | Univariate Analysis RR (95% CI) | Multivariate Analysis1 RR (95% CI) |
|---|---|---|---|---|
| Gender: female | 87 | 525 | 1.88 (1.32–2.67) | 1.89 (1.36–2.64)2 |
| Age 25–26 versus 24 years old | 110 | 828 | 1.28 (0.83–1.98) | 1.31 (0.86–1.97)2 |
| History asthma and/or allergic rhinitis* | 37 | 103 | 1.94 (1.23–2.83) | 1.61 (1.04–2.51)3 |
| Family history atopic dermatitis* | 18 | 60 | 2.85 (1.70–4.76) | 2.78 (1.77–4.37)2 |
| History of respiratory infections*16 | 58 | 407 | 1.47 (0.98–2.22) | 1.55 (1.04–2.31)4 |
| History of bronchitis* | 28 | 156 | 1.71 (1.10–2.66) | 1.79 (1.17–2.75)4 |
| History of pneumonia* | 10 | 56 | 1.59 (0.82–3.07) | 1.74 (1.05–2.88)4 |
| History of sinusitis* | 13 | 72 | 1.58 (0.88–2.84) | 1.82 (1.01–3.27)4 |
| History of otitis* | 40 | 299 | 1.26 (0.84–1.91) | 1.18 (0.77–1.80)4 |
| Family smoke at home* | 90 | 723 | 1.00 (0.67–1.50) | 0.94 (0.63–1.40)5 |
| Animals at home*17 | 89 | 736 | 0.88 (0.61–1.25) | 0.97 (0.63–1.51)6 |
| Dog at home* | 50 | 402 | 0.99 (0.69–1.41) | 1.05 (0.71–1.54)6 |
| Cat at home* | 23 | 174 | 1.01 (0.64–1.59) | 0.99 (0.61–1.60)6 |
| Social class: I–II versus III–VI* | 28 | 196 | 1.16 (0.76–1.76) | 1.13 (0.77–1.67)7 |
| Birthplace outside of Castellon | 19 | 81 | 2.00 (1.23–3.25) | 2.02 (1.30–3.14)8 |
| Town ≥50,000 inhabitants* | 101 | 830 | 0.89 (0.60–1.33) | 0.94 (0.64–1.37)9 |
| Exclusive breastfeeding ≥6 months* | 16 | 123 | 1.05 (0.60–1.84) | 1.05 (0.62–1.77)10 |
| Day-care before 3 years* | 61 | 528 | 0.87 (0.58–1.30) | 1.03 (0.69–1.56)11 |
| Presence of older siblings* | 57 | 420 | 1.29 (0.86–1.92) | 1.53 (0.99–2.37)12 |
| Age mother | ||||
| 14–24 years | 21 | 157 | 1.00 | 1.007 |
| 25–29 years | 48 | 358 | 1.00 (0.60–1.67) | 1.08 (0.67–1.73) |
| 30–34 years | 22 | 320 | 0.75 (0.41–1.36) | 0.78 (0.44–1.36) |
| ≥35 years | 44 | 334 | 0.98 (0.59–1.66) | 0.94 (0.66–1.73) |
| Constant truck traffic in home's street* | 10 | 51 | 1.70 (0.89–3.28) | 1.50 (0.80–2.83)13 |
| Zone hard water ≥165mg/l Ca Mg* | 100 | 751 | 1.18 (0.80–1.73) | 1.11 (0.70–1.77)14 |
| Ceramic industry* | 28 | 237 | 0.92 (0.61–1.40) | 0.72 (0.47–1.09)15 |
* Variables with lost information in some participants. 1: In the IPWRA analysis, all variables were adjusted for gender and age. 2: Age of mother. 3: Family history AD, family smoke at home, age of mother, social class. 4: Exclusive breastfeeding ≥6 months, town ≥50,000 inhabitants, day-care before 3 years, family history AD, family smoke at home, social class, older siblings, constant trucks in home's street, animals at home. 5: Family history AD, age of mother, social class. 6: history of asthma or allergic rhinitis, town ≥50,000 inhabitants, social class, family history AD. 7: Gender, age. 8. Social class, age of mother 9: ceramic industry, social class. 10. History of asthma or allergic rhinitis, town ≥50,000 inhabitants, family history AD, age of mother, family smoke at home, social class. 11: town ≥50,000 inhabitants, family history AD, presence older siblings, social class, age of mother. 12: Family history AD, social class, age of mother. 13: ceramic industry, town ≥50,000 inhabitants, social class. 14: Family history AD, age of mother, social class. 15: Social class. 16: Respiratory infections: positive bronchitis, pneumonia, sinusitis, or otitis. 17: Animals at home; positive dog, cat, or other animal at home.
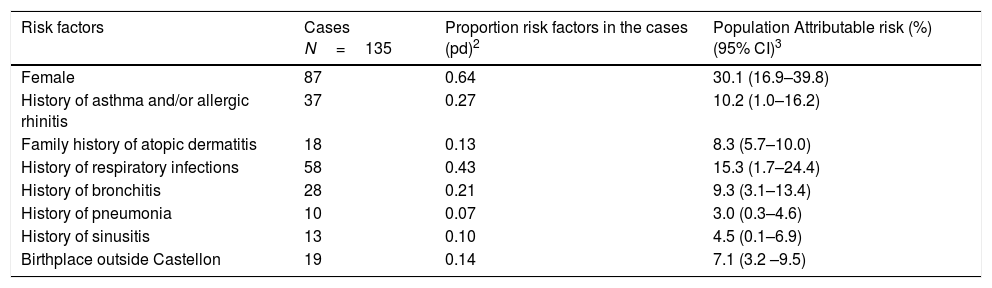
Population attributable risk of AD related factors in the adjusted analysis are shown in Table 4. Being female represents a 30.1% AD risk, followed by history of respiratory infections 15.3%, and history of bronchitis 9.3%. Fewer effects were observed with history of asthma and/or allergic rhinitis, history of pneumonia, history of sinusitis, family history of AD, and birthplace outside of Castellon province.
Population attributable risk1 of atopic dermatitis of Castellon's cohort through relative risks (RR) adjusted for confounding factors from inverse probability weighting regression adjusted (IPWRA). Castellon's cohort 2012.
| Risk factors | Cases N=135 | Proportion risk factors in the cases (pd)2 | Population Attributable risk (%) (95% CI)3 |
|---|---|---|---|
| Female | 87 | 0.64 | 30.1 (16.9–39.8) |
| History of asthma and/or allergic rhinitis | 37 | 0.27 | 10.2 (1.0–16.2) |
| Family history of atopic dermatitis | 18 | 0.13 | 8.3 (5.7–10.0) |
| History of respiratory infections | 58 | 0.43 | 15.3 (1.7–24.4) |
| History of bronchitis | 28 | 0.21 | 9.3 (3.1–13.4) |
| History of pneumonia | 10 | 0.07 | 3.0 (0.3–4.6) |
| History of sinusitis | 13 | 0.10 | 4.5 (0.1–6.9) |
| Birthplace outside Castellon | 19 | 0.14 | 7.1 (3.2 –9.5) |
1: Population attributable risk=pd (RR-1)/RR. 2: pd=cases with a risk factor/total of cases 3: CI=Confidence interval.
In this cohort of young adults, AD incidence and lifetime prevalence were estimated. Risk factors ranged between RR of 1.55 (respiratory infections) and RR of 2.78 (family history of AD) which indicate moderate associations with AD and this variability suggests multifactorial genesis. Some of these risk factors may be modifiable, such as asthma, allergic rhinitis, and respiratory infections.
AD incidences of our study were 15.9 (1994–2002) and 13.5 per 1000 person years (2002–2012), lower than AD cumulative incidence in Danish adolescents, 21.5 per 1000 person years,7 but higher than another cohort followed from age 9–11 to 16–20 in Germany.4 However, our case definition was different. Peters et al.4 included only 12 months AD symptoms, with AD incidence 1.7%; this incidence is higher than the AD incidence annual rate in our study, 1.3%. Also, our AD incidence was higher than the incidence found in a Danish cohort age 14 to 29 years5 with medical examination and questionnaires, 8.9 per 1000 person years; in contrast, the AD lifetime prevalence in our study was 34.9%, which was similar to the 34.1% in the Danish study.5 In an Icelander cohort from birth to 21 years age, AD lifetime prevalence was 45%.17 In two British cohorts of 1958 and 1970, AD lifetime prevalence was inferior, 18% and 28%, suggesting a constant increase of AD.18
Some risk factors in our study, such as history of asthma or allergic rhinitis, and family history of AD are in line with the results of other cohort studies.4,6 The risk factor of birthplace outside of Castellon was observed in the first survey,19 and AD prevalence in the group age 6–7 years was lower than other zones; some studies found more AD prevalence in the migrant population than the native population considering the age of migration.20 In general, females have higher AD incidence than males, although this is not consistent in all studies.18,21
Other childhood AD risk factors were not related to AD incidence, including breastfeeding, pets, day care, siblings, and dog exposure in early life.21,22 In fact, some studies suggest that AD in children is associated with low rates of infection, absence of older siblings, and antibiotics exposure.23,24 Our results are opposed, considering that history of respiratory infections, history of bronchitis, pneumonia and sinusitis were risk factors. It has been suggested that some infections may protect against AD while others increase the risk of AD. Previous studies support our results, reporting AD increase with prenatal infections,25 early infections,26 enterovirus and other virus infections,27,28Staphylococcus aureus colonization before AD onset,29 whereas older siblings increased AD risk in two German birth cohort studies.30 In addition, AD patients suffer frequent infections,31 suggesting a high susceptibility to infection. It is plausible that respiratory infections may be contributing components to AD. On the other hand, AD risk factors may be differing from childhood to adulthood.32
Other risk factors such as social class, passive smoking, truck traffic exposure, and hard-water exposure were not related to AD incidence. High social class and passive smoking have been identified as risk factors in a cohort of AD incidence from adolescence to young adults.4 However, lower social class has been associated with AD lifetime prevalence in adults.18 Smoking was associated with AD in adults and adolescents and tobacco is considered a risk factor of eczema.33 Air pollution has increased AD prevalence in childhood and elderly.34 With respect to hard water, its actions may be more important in childhood than in adolescents and young adults. In the second survey, hard-water exposure was associated with AD in 6–7 years old group, but not adolescents 14–16 years old group,35 and this association has been found in two studies from Great Britain and Denmark in infants.36,37
Our study has a prospective design, acceptable participation, control of potential confounders by IPWRA, uses a validated ISAAC questionnaire, and all surveys were implemented in the same seasons of the year. A parallel question used in our study has been used by Stenberg et al.38 (have you had childhood eczema?) with sensitivity of 89.9% and specificity of 70.7%. However, Silverberg et al.39 used the question (have you ever been told by a doctor or other health professional that you had eczema or any kind of skin allergy?) for AD self-report history in adults with low sensitivity (43%) and high specificity (97%).
The limitations of this study include the following points: first, no medical examination was carried out to confirm the cases and some observational bias may have occurred. Second, in the follow-up of the cohort, the power to detect significant differences has decreased. Third, the AD incident definition presents high variability and there is not a general consensus.21 Fourth, some potential factors which have been related to AD incidence were not included in the questionnaires such as diet, food allergy, air pollution, or vaccinations.8,9
Considering AD complexity, a global approach has been proposed to study its prevention, diagnosis, and treatment, including genetics, immune and environmental factors.40 Cohort studies are well situated to better understand and to bring new knowledge to this frequent and chronic pathology.
In conclusion the AD incidence in our cohort was high and several risks factors were related to AD.
Conflict of interestThe authors have no conflict of interest to declare.
We thank all the participants in the Castellon cohort and their families for their cooperation and support in making this study possible. We appreciate the help of Ricardo Tosca-Segura, Miriam Ortuño-Forcada, Saul Fernández-González, Francisco Conde, and Lidon Museros-Recatalá in this study.