Many studies have addressed the frequency of drug allergy (DA) in systemic lupus erythematosus (SLE) patients. Some studies suggest that there may be an increased risk in these patients1–4 while others do not confirm this.5–7 The conflicting results may be due to differences in definition of drug allergy; in the means of ascertaining allergic manifestations; and in the choice of controls. SLE patients are more frequently exposed to drugs, which, “per se”, represent a risk factor for drug allergy. Prompt recognition of DA, particularly anaphylaxis, is crucial, as it may be life-threatening and the appropriate treatment cannot be delayed. In a patient with SLE, the differential diagnosis of drug allergy is challenging. Features such as rash, fever and cytopenia may result from drug allergy but may also be part of SLE manifestations. Furthermore, drugs like sulphonamides are well known to exacerbate, or even induce SLE.8,9
The authors report a case of a 32-year-old Caucasian female housewife who presented at the emergency room with rash, diffuse myalgias, dry mouth, paraesthesia and dizziness. The symptoms started 90 minutes after taking a 250mg tablet of mefenamic acid. She was thought to have an allergic drug reaction and was treated with intramuscular clemastin and IV methylprednisolone 250mg. She was alert, her blood pressure was 100/57mmHg, her heart rate was 124 per minute and her temperature was normal. Despite receiving clemastin and methylprednisolone, two hours later she had a clinical deterioration starting with generalised pruritus and worsening of generalised exanthema, myalgias and arthralgias. She rapidly developed angio-oedema and anaphylactic shock. Her temperature was 37.4°C, her blood pressure dropped to 73/45mmHg and her heart rate was 120 per min. She was given dopamine, epinephrine and 250mg of IV methylprednisolone and intravenous fluids. Blood cell count revealed a haemoglobin level of 7.9g/dl [11.5–16.5], a white blood cell count of 5.4×103/μL [4–11], prothrombin time 22s [13], d-dimers of 7.17μg/L [0–0.6], fibrinogen of 2.6g/L [2–5.0], SGPT 219U/l [5−55]; SGOT 337U/l [5–34]; SGGT 15U/l [9–36], total bilirubin 0.8mg/dl [0.2–1.2], LDH 628U/l [125–243], albumin 2.8g/dl [3.5–5], CRP 6.31mg/dl [0–0.8] and ESR 69mm [0–25]. Chest x-ray and ECG were normal. She was diagnosed with DA to mefenamic acid and acute hepatic failure and was admitted into the Intensive Care Unit.
In the Intensive Care Unit, life support care was maintained and she started prednisolone 1mg/Kg/day and topical silver sulphadiazine for skin lesions. By the third day she was haemodynamically stable, her liver function was almost normal and the skin lesions were resolving.
This patient had a history of new onset of polyarthralgia three weeks previous to this episode which was treated by her general practitioner with mefenamic acid 250mg tablets bid. One week later, she had not improved and cyclobenzaprine 10mg qd was added to her treatment. She stopped both drugs the following day because of malaise and facial exanthema that resolved completely. She did not take any other medications except for chronic treatment with thyroxine for hypotiroidism. Laboratory tests done by her general practitioner 11 days before this episode showed an anti-nuclear antibody (ANA) of 1/2500 [<1/40] with homogenous pattern, anti-dsDNA of 1/80 [<1/10], Rheumatoid Factor of 28 IU/ml [<14], negative Waaler-Rose test, white blood cell count 3.96×103/μL [4–11×103/μL], lymphocytes of 1.0×103/μL [1.5–3.5], CPR 3.94 [<6.0] and ESR of 90mm [<20].
Repeat testing at the hospital confirmed ANA positivity, anti-dsDNA of 40.0IU/ml [<4.2], anti-histone 200U/ml [<40], positive anti-SSA and anti-SSB, negative anticardiolipin and anti-beta2GPI antibodies, low complement levels with C3 0.78g/L [0.9–1.8] and C4 0.15g/L [0.1–0.4], haptoglobin 3.14g/L [0,3–2], positive Coombs test, free T3 was 71pg/% [230–530], free T4 1.2ng/% [0.78–1.94] and TSH 6.5μU/ml [0.4–4]. As her d-dimer was above normal level, a peripheral limbs Doppler test was done but it showed no vascular occlusions.
Lymphoblastic proliferation test to mefenamic acid was negative. Drug challenge test was not performed as it might be hazardous in this patient.
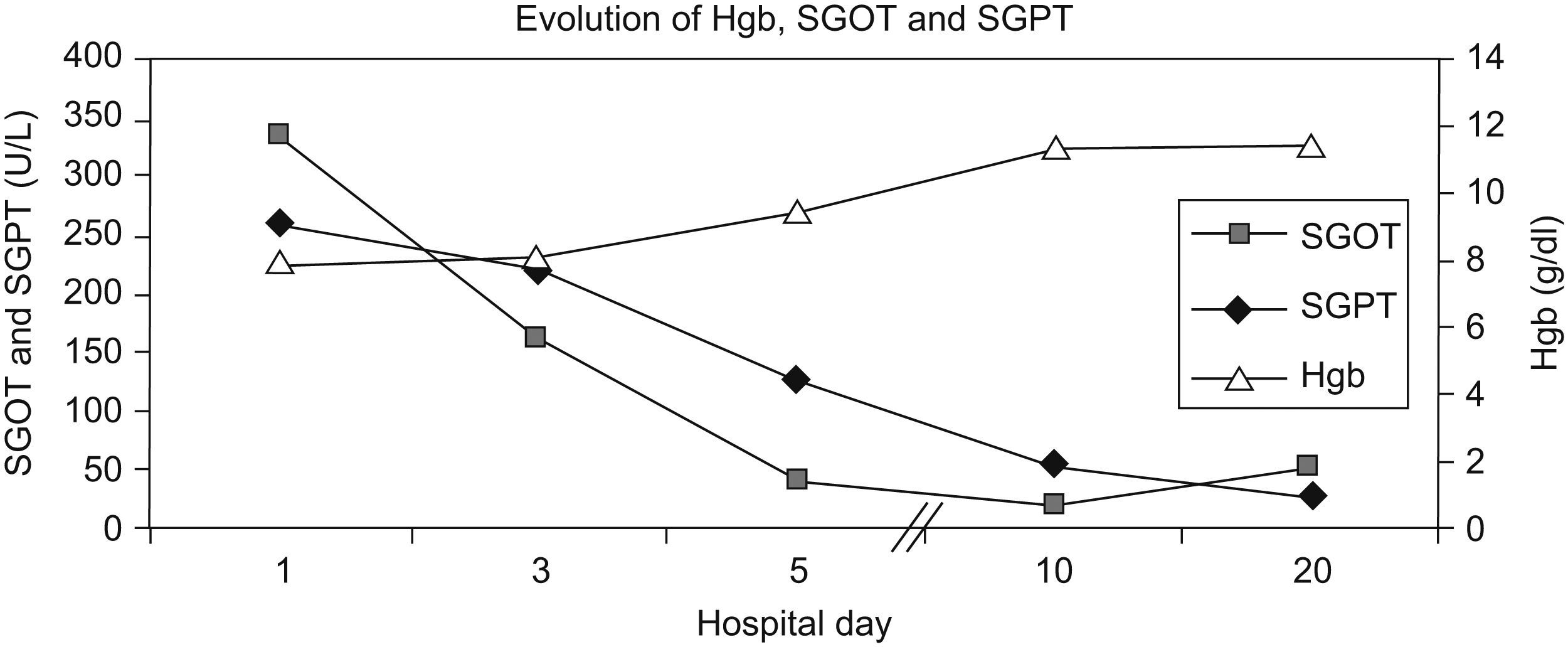
SLE was diagnosed on the basis of a positive ANA and anti-dsDNA, Coombs positive haemolytic anaemia, leucopenia, lymphopenia, arthritis and low C3 and C4 levels. Prednisone was tapered to 20mg by the time of hospital discharge and added hydroxychloroquine 200mg qd. Her clinical and laboratory (Figure 1) evolution were favourable and she was discharged on the 21st hospital day in a healthy condition.
She continues to be followed up as an outpatient for her SLE. One year after this episode, the ANA, anti-dsDNA, anti-SSA, anti-SSB and anti-histone autoantibodies remain positive at nearly identical levels.
Although pharmacological inhibition of cyclooxygenase with overproduction of cysteinyl leukotrienes is one of the most important mechanisms of the adverse mechanism of NSAIDs, in this patient the occurrence of a rapid onset of rash, generalised pruritus, angio-oedema and hypotension suggest an allergic-type response, presumably by immunological IgE-mediated mechanism.10 Previous contact with the culprit drug, corresponding to the sensitisation period, supports the diagnosis of DA.11 Skin testing to NSAIDs, prick and intradermal testing, are usually negative and correlate poorly with the clinical history of hypersensitivity reactions. Moreover, in vitro tests are not useful in establishing diagnosis.10 Controlled oral challenge test remains the gold standard but it was not performed because of its high risk of inducing a life threatening reaction.
In this case, a two-step reaction with rash and dizziness occurring 90min after the ingestion of mefenamic acid and then the worsening of exanthema, pruritus, angio-oedema and hypotension two hours after the first clinical manifestations that were treated with parental steroids and anti-histamines, support mefenamic acid as the most likely cause for anaphylaxis. Anaphylaxis usually begins within 5 to 30min after antigen exposure, but a delay of one hour or more can occur.11 Anaphylactic shock can be biphasic and, several hours after its initial presentation, a new episode may begin and it is not unusual for the second episode to be more severe.11 Causality assessment was done applying the Hutchinson et al. algorithm for Adverse Drug Reaction, and corroborates the association between the drug and DA (score 5=probable).12
Concerning laboratory results, the raise in hepatic enzymes, thrombocytopenia, lymphopenia and haemolytic anaemia may be due to drug toxicity or to concomitant complement and fibrinolytic cascade activation and immune complex activation. Renal and hepatic manifestations may also occur as a part of a generalised allergic reaction.11 Also, both anaphylaxis and mefenamic acid can lower C3 and C4 levels. The presence of haemolytic anaemia, lymphopenia, low C3 and C4 are features of SLE, but could not be definitively attributed in this context.
Another diagnostic hypothesis is a drug-induced lupus (DIL). This has been reported for more than 80 drugs. The strongest evidence of causality is for procainamide, hydralazine and isoniazid.8,13 Drugs such as anti-hypertensives, antibiotics and anti-convulsivants are of low risk.8 Among NSAID only celecoxib14 has been implicated in DIL and there are no published case reports for mefenamic acid. The typical features of systemic DIL are mild SLE manifestations with positive ANA and anti-histone antibodies temporally related to drug exposure which typically resolves after discontinuation of the offending drug.8,13 Despite the presence of anti-histones our patient had polyarthritis, anti-dsDNA and hypocomplementemia which play against the possibility of DIL. In addition, more than a year after the withdrawal of mefenamic acid the patient maintained these clinical and laboratory features.
However, drugs such as sulphonamides,9 tetracyclines,15 griseofulvin,16 piroxicam,17 ibuprofen18 and oestrogens19 have been reported to exacerbate SLE or to trigger new cases. Before this report, mefenamic acid has not been reported in any of the cases.
In this case, the most likely explanation is that anaphylaxis occurred in a patient with recent SLE. Whether mefenamic acid was implicated in exacerbation of the disease is difficult to discount or prove.
This case illustrates the difficulties and the wide differential diagnosis in an SLE patient who presents with a drug reaction. The attribution of the presenting symptoms to DA, DIL, drug triggering of SLE onset / exacerbation or to the underlying SLE and/or to the drugs used to treat SLE its coexisting conditions is a major challenge. We discuss some clues to approach this problem in the clinical setting.
We are extremely grateful to Dr Matthew Liang for his help in the manuscript preparation.