The management of patients with prostate cancer (PCa) is established in clinical practice guidelines, which are based on randomized studies according to the level of evidence. In Spain, the degree of compliance with these guidelines in clinical practice is unknown.
ObjectivesTo describe the profiles of PCa patients at the time of diagnosis and the management of patients with localized PCa and those with BCR in Spain.
Materials & methodsA medical survey was conducted in specialized care (85 urologists [UROs], 64 radiation oncologists [ROs], and 21 medical oncologists [MOs]). Three questionnaires were developed for this study with 22 (UROs and ROs) or 21 questions (MOs).
ResultsThe annual incidence of PCa was 24,057 in participating hospitals (N = 131). The extrapolated annual incidence in Spain is 40,531 cases. The estimated prevalence of PCa in Spain is 221,689. Of note, 79% and 80% of patients seen by UROs and ROs, respectively had localized PCa at diagnosis. Biopsy was the most used diagnostic test among the three specialties, followed by abdominopelvic computer tomography. More than 90% of patients with BCR underwent standard tests. Next generation imaging tests and PET-choline/PSMA are still used residually. Most patients with localized PCa are currently treated with either surgery or radiotherapy, while for BCR patients, UROs and ROs prefer radiotherapy and MOs androgen deprivation therapy alone or in combination.
ConclusionThis study describes patient profiles at the time of diagnosis and provides an overview of the current therapeutic management of localized PCa and BCR in clinical practice in Spain.
El tratamiento de los pacientes con cáncer de próstata (CaP) está establecido en las guías de práctica clínica, las cuales se basan en estudios aleatorizados según el nivel de evidencia. En España se desconoce el grado de cumplimiento de estas guías en la práctica clínica.
ObjetivosDescribir los perfiles de los pacientes con CaP en el momento del diagnóstico y el manejo de los pacientes con CaP localizado y con RBQ en España.
Materiales y métodosSe realizó una encuesta médica en tres especialidades médicas (85 urólogos [URO], 64 oncólogos radioterapeutas [OR] y 21 oncólogos médicos [OM]). Para este estudio se elaboraron tres cuestionarios, dos con 22 preguntas (URO y OR) y uno con 21 preguntas (OM).
ResultadosLa incidencia anual de CaP en los hospitales participantes (N = 131) fue de 24.057 casos. La incidencia anual extrapolada a España fue de 40.531 casos. La prevalencia estimada de CaP en España es de 221.689. Cabe destacar que el 79% y el 80% de los pacientes atendidos por URO y OR, respectivamente, presentaban CaP localizado en el momento del diagnóstico. La biopsia fue la prueba diagnóstica más utilizada en las tres especialidades, seguida de la tomografía computarizada de abdomen pelvis. Más del 90% de los pacientes con RBQ se sometieron a estas pruebas. Las técnicas de imagen de nueva generación y la PET con colina/PSMA se siguen utilizando en menor medida. Actualmente, la mayoría de los pacientes con CaP localizado reciben tratamiento con cirugía o radioterapia, pero en el caso de los pacientes con RBQ, los URO y OR prefieren la radioterapia y los OM la terapia de deprivación androgénica exclusiva o combinada.
ConclusiónEste estudio describe los perfiles de los pacientes en el momento del diagnóstico y proporciona una visión general del manejo terapéutico actual del CaP localizado y con RBQ en la práctica clínica en España.
Prostate cancer (PCa) is the most frequent neoplasm in Spanish men and the third leading cause of cancer-related mortality.1 In a recently published report by Spanish Society of Medical Oncology (SEOM), the incidence of PCa in Spain in 2023 was estimated at 29,002 new cases, and the prevalence was estimated at 259,788 patients in 2020.1 Most patients are diagnosed at 65 years of age or older, as it is a relatively slow-growing disease.2 In its early stages, PCa is asymptomatic in most cases. In more advanced stages, symptoms may include fatigue, anemia, bone events such as pain, fractures, or spinal cord compression, and renal failure due to bilateral ureteral obstruction.3 Despite controversies, the diagnosis is mainly based on prostate-specific antigen (PSA) testing and biopsies.3 When a high PSA level is detected, magnetic resonance imaging (MRI) is recommended prior to a biopsy. When the MRI is positive, a biopsy should be performed.4
The stages of disease in PCa are characterized by the state of the primary tumor, the presence of metastases, treatment, and testosterone levels.5 Localized PCa is characterized by not spreading beyond the prostate gland, while locally advanced PCa can invade some of the periprostatic tissue and local lymph nodes.6 Biochemical recurrence (BCR) is defined by an increase in PSA after receiving primary treatment with curative intention, depending on whether the primary treatment was done with surgery or radiation therapy.7 Although there is no consensus on the exact definition of BCR, the most widely used definition is having two consecutive values of PSA > 0.2 ng/mL, since these are associated with a higher rate of biochemical progression.8 After radiotherapy (RT), BCR is diagnosed after any PSA increase ≥2 ng/mL above the PSA nadir.9 Advanced prostate cancer includes a broad spectrum of diseases, ranging from hormone sensitive to castration resistant (including metastatic and non-metastatic states).10 Stages of disease preceding metastatic castration-resistant prostate cancer (mCRPC) are metastatic hormone-sensitive PCa (mHSPC), characterized by development of metastases while the patient still responds to medical or surgical castration, and non-metastatic castration-resistant PCa (nmCRPC), characterized by increased PSA levels despite testosterone castration levels with no metastases detected by conventional imaging.11,12 It has been determined that approximately half of patients who die from PCa have metastases at the time of diagnosis.13
Treatment for PCa at each stage is described in clinical practice guidelines. In recent years, there have been crucial therapeutic changes in the therapeutic management of metastatic PCa that significantly improve survival.14 Treatment of localized PCa, including active surveillance (AS) for patients with low-risk disease, RP, external radiotherapy, and brachytherapy, should be considered for men with a life expectancy > 10 years as the established treatments with curative intent. In some cases, local treatment is combined with hormone therapy (HT).15,16 The group risk, tumor stage, and comorbidities should be considered while selecting treatment for patients with localized PCa, considering shared decision-making based on patient preferences. Despite local therapy being curative in many patients, it is estimated that 27%–53% of patients will experience biochemical recurrence (BCR).9 The clinical guidelines recommend performing PSMA-PET/CT (if available), fluciclovine-PET/CT, or choline-PET/CT in patients suitable for curative salvage treatment. Salvage radiotherapy would be indicated for patients with BCR without evidence of metastatic disease after RP.15,17 In the presence of risk factors, hormonal therapy may be offered in addition to salvage radiotherapy to men with BCR. After radiotherapy, salvage RP, re-irradiation with brachytherapy, protons or IG-IMRT (image-guided intensity-modulated radiotherapy), high-intensity focused ultrasound, or cryosurgical ablation may be offered to selected patients.15 For systemic salvage treatment, Androgen Deprivation Therapy (ADT) should not be offered to M0 patients with a PSA doubling time >12 months.15,18 Early HT in BCR should be reserved to patients who have a high risk of disease progression, as defined by a short PSA-DT at relapse (<6–12 months) or a high initial ISUP grade (>2/3) and a long-life expectancy.4,15 Immediate systemic treatment with ADT should be offered to metastatic prostate cancer patients. Combination therapy, including ADT plus systemic therapy such as docetaxel with or without abiraterone acetate or darolutamide, abiraterone acetate plus prednisone, apalutamide, or enzalutamide, should be considered in all patients. Furthermore, prostate RT should be offered to patients with low-volume disease.
This study aimed to describe the profiles of PCa patients at the time of diagnosis and the management of patients with localized PCa and those with BCR in Spain, as well as contextualizing the results with recommendations from clinical practice guidelines.
MethodsA medical survey was conducted in specialized care (urologists [UROs], radiation oncologists [ROs], and medical oncologists [MOs]) to describe the management of patients with localized PCa and patients with BCR.
The study was conducted using structured interviews guided by questionnaires among a sample of UROs (N = 85), ROs (N = 64), and MOs (N = 21) representatively distributed throughout Spain, based on existing populations in autonomous communities. The interviews were carried out between July and August 2022. The sample size calculation was based on a universe of 2676 UROs, 2502 ROs, and 1066 MOs identified in public hospitals. The sample error was smaller than 10.46% for UROs, 21.30% for ROs, and 11.88% for MOs. To be eligible for the interview, the physicians must have had at least five years of experience after completing their training period (MIR), worked in a public hospital either full time or part time, and treated at least 15 PCa patients in the previous 12 months.
Three questionnaires were developed for this study: one for UROs(Q1) and one for ROs (Q2), which differed slightly from Q1, both of which consisted of 22 questions; and one for MOs (Q3), who see patients in later stages of the disease. Q3 consisted of 21 questions. The three questionnaires had three sections: a) a screening section to act as a filter to ensure that the interviewees met the required profile according to the filtering criteria (10 equal questions in all questionnaires collecting information about specialty, hospital, experience, and responsibility for prescribing PCa treatment); b) a patient management section to estimate the number of patients seen per specialty by stage of disease and their management (11 questions in Q1 and Q2 and 10 in Q3 about how many physicians work in their wards, the number of patients diagnosed in the last 12 months, and tests to define the treatment and profiles of newly diagnosed patients); and c) a coordination section (1 question in Q1, Q2, and Q3) to understand how the follow-up of PCa patients is shared between specialties. Of note, the UROs and ROs were also asked about the profiles and treatment of patients with localized PCa, how many patients had BCR, the complementary tests used in these patients, and about differences in the percentage of patients with localized PCa who end up having BCR according to the first line of treatment. On the other hand, MOs were asked about more advanced stages of the disease, i.e., patients who presented to the multidisciplinary committee on tumors.
A descriptive analysis of the variables of the study was performed using absolute and relative frequencies. The number of prevalent and incident patients, with BCR or with localized BCR was obtained through the medical survey from the hospitals included in the study sample. The percentage of the hospital population collected in the medical survey in each question was determined based on IQVIA's Sanibrick structure, which is equivalent to health care areas. To extrapolate the medical survey results to the total national population, the number of patients obtained in the medical survey was divided by the percentage of the Spanish population covered in each question. Finally, when the analysis required it, the percentage of patients who were being shared by multiple specialties was considered to avoid overestimation.
ResultsSample characterizationA total of 170 doctors were interviewed (85 UROs, 64 ROs, and 21 MOs), from 131 hospitals throughout Spain. Interviews were distributed according to the population, so that the autonomous communities with more hospitals presented a larger sample (Fig. S1, Supplementary Material).
The interviewed physicians had an average of 17 years (range 5–40) of experience. Most of the interviewed ROs and MOs (80% and 76% respectively) worked only in the public sector, compared with 32% of the interviewed UROs. On average, the UROs had visited 400 patients in the last 12 months (27% were new patients), RO, 175 patients (42% were new patients), and MOs, 58 new patients with PCa. The interviewed UROs were responsible for 38% of new PCa diagnoses of all patients arriving at their ward, while the corresponding figures were 43% for the ROs and 78% for the UROs. Of note, RO and MO hospital wards had, on average, ten physicians. In these wards, 50% of UROs and 40% of ROs initiated treatment for PCa patients, while only 19% of MOs were responsible for prescribing or following up on the treatment of PCa patients. More than half of the hospitals had monographic PCa consultations, mostly belonging to the Urology ward, although this percentage varied from 55% according to the answers of the ROs, 73% according to UROs, and 81% according to MOs answers. More than 90% of the hospitals had a multidisciplinary tumor committee.
Estimation of incidence and prevalence of prostate cancer based on data from medical surveyBased on data from the interviews, 24,057 new patients were diagnosed with PCa during the last 12 months in participating hospitals (N = 131), covering 59% of the total Spanish population. Extrapolating the sample to the whole of Spain, the annual incidence can be estimated at 40,531 newly diagnosed patients.
Regarding the prevalence of patients with PCa, 97,167 patients visited UROs, 64,476 ROs, and 45,405 MOs in the hospitals included in the sample. Applying the above rationale and correcting the bias caused by some patients being visited by multiple specialists, the estimated prevalence of PCa in Spain is 221,689 patients.
Diagnosis and profiles of PCa patients at time of diagnosis and at referralRegarding the stage of disease at diagnosis, 79% of patients seen by UROs and 80% of patients seen by ROs had localized PCa at diagnosis (21% and 20%, respectively, corresponded to metastatic disease). MOs were asked about the PCa stage at the time when patients were referred to them; accordingly, the following figures were obtained: localized PCa (29%), non-metastatic CRPC (10%), non-metastatic BCR PCa (10%), metastatic CRPC (22%), synchronous metastatic HSPC (15%), and metachronous HSPC (14%).
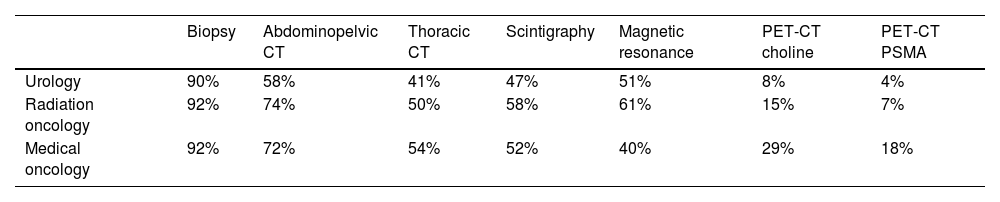
Regarding the diagnostic tests used to diagnose PCa, biopsy was the most used test among the three specialties (90% UROs and 92% ROs and MOs), followed by abdominopelvic computer tomography (CT) (58% URO, 74% RO, and 72% MOs), multiparametric magnetic resonance (mpMRI) (51% UROs and 61% ROs), and thoracic CT (54% MOs) (Table 1). Biopsy was done in most patients regardless of their age, health condition, and PCa risk, while the PCa risk was the main deciding factor when choosing between the other tests, especially next-generation imaging tests (PET/CT-PSMA/choline) (Table S1, Supplementary Material).
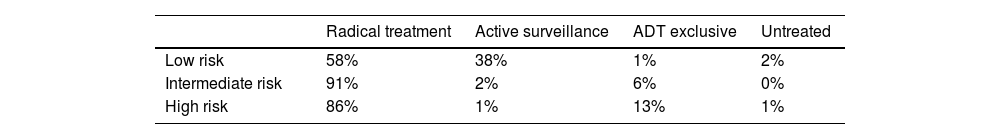
Treatment of localized PCa patientsAccording to the survey, most patients diagnosed with localized PCa were treated with radical treatment in both the URO (61%) and RO (57%) wards (Table S2, Supplementary Material). Untreated patients and patients under active surveillance typically had low-risk PCa. Considering all the patients diagnosed in the last 12 months by risk group (Table 2), 58% of low-risk patients received radical treatment and 38% were on active surveillance, while these percentages were 91% and 2% for intermediate-risk patients and 86% and 1% for high-risk patients, respectively. Patients on ADT exclusive treatment accounted for 1% of low-risk patients, 6% of intermediate-risk patients, and 13% of high-risk patients, while untreated patients accounted for 2% of low-risk patients, 0% of medium-risk patients, and 1% of high-risk patients. Extrapolating the number of incident patients diagnosed with localized PCa in the last year, according to the treatment they were receiving, 17,582 patients were treated with radical treatment, 3753 with active surveillance, 1422 with ADT exclusive, and 683 remained untreated. Of all the patients diagnosed with incident localized PCa, 2590 experienced BCR post-surgery: 1601 post-RT, 1286 locoregional recurrence, and 1288 metastatic recurrence.
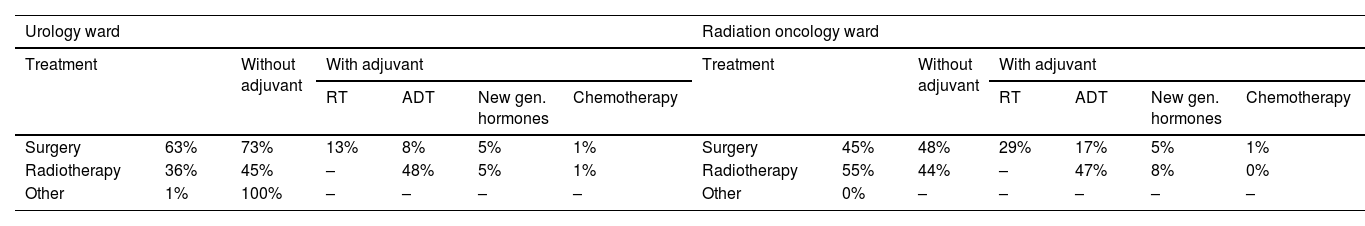
Most localized PCa patients undergoing radical treatment were treated with surgery (63% for UROs and 45% for ROs). In terms of patient profiles, surgery was performed on younger patients (<70 years old), those with ECOG scores 0–1 (good health status), and those with intermediate risk; RT was performed on patients between 70 and 85 years old who had good health status and intermediate risk; and ADT was prescribed to older patients (>85 years old), regardless of ECOG score or high risk. Adjuvant treatments were prescribed by UROs and ROs to patients at high risk of PCa (Table 3).
Radical treatment on localized PC in urology and radiation oncologist ward (patients diagnosed in last 12 months).
| Urology ward | Radiation oncology ward | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Without adjuvant | With adjuvant | Treatment | Without adjuvant | With adjuvant | ||||||||
| RT | ADT | New gen. hormones | Chemotherapy | RT | ADT | New gen. hormones | Chemotherapy | ||||||
| Surgery | 63% | 73% | 13% | 8% | 5% | 1% | Surgery | 45% | 48% | 29% | 17% | 5% | 1% |
| Radiotherapy | 36% | 45% | – | 48% | 5% | 1% | Radiotherapy | 55% | 44% | – | 47% | 8% | 0% |
| Other | 1% | 100% | – | – | – | – | Other | 0% | – | – | – | – | – |
| Patient main profile per type of treatment (Age, health condition, PC risk) | Patient main profile per type of treatment (Age, health condition, PC risk) | ||||||
|---|---|---|---|---|---|---|---|
| Surgery | <70; Good; Mid | Radiotherapy | 70−85; Good; Mid | Surgery | <70; Good; Mid | Radiotherapy | 70−85; Good; Mid |
| Surgery + ADT | Indif.; Good; High | Radiotherapy + ADT | 70−85; Good; High | Surgery + ADT | <70; Good; Mid | Radiotherapy + ADT | 70−85; Good; High |
| Surgery + adjuvant RT | <70; Good; High | ADT | >85; Indif.; High | Surgery + adjuvant RT | <70; Good; High | ADT | >85; Indif.; High |
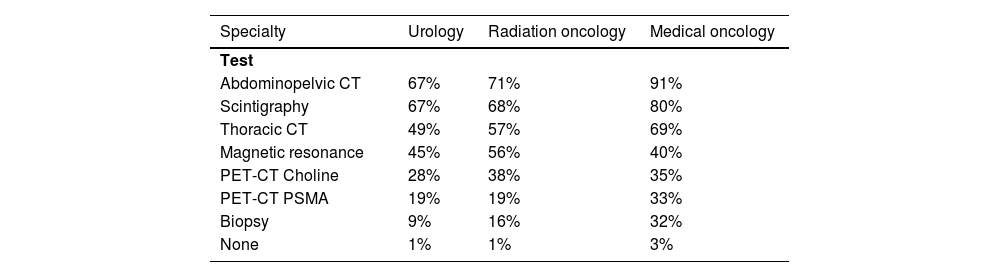
Extrapolating the figures, a total of 6144 prevalent patients are estimated to have non-metastatic BCR nowadays, regardless of when they were diagnosed with PCa. In the last 12 months, 5063 incident patients had BCR (metastatic or non-metastatic). More than 90% of patients with BCR underwent standard tests. Abdominopelvic CT and scintigraphy were the most commonly used tests in BCR patients by all specialties, followed by thoracic CT (in URO and RO specialties) and MRI (in MO specialty). PET-CT choline was performed in 28%–38% of the patients, while PET-CT PSMA was performed in 19% (UROs and RO) and 33% (MOs) of the patients. Biopsy, on the other hand, had limited use (Table 4). According to most specialists, the selection of tests was not related to the patient's age, health condition, or BCR risk.
Regarding BCR management in the MO ward, in 81% of the centers, all patients were presented to the tumor committee, while in the remaining 19%, only high-risk and bad-evolution patients were presented to the tumor committee.
Of all metastatic HSPC patients, 44% recurred after being treated for localized PCa (approximately 50% of them treated with RP), and 56% were synchronous mHSPC. Of all metastatic CRPC patients, 47% were metachronous metastatic PCa and 53% were synchronous metastatic PCa.
Around one-third of the UROs and ROs interviewed perceived differences in the rate of patients with localized PCa who would progress to BCR in five years, depending on the first line of treatment. Regarding patients presenting with BCR, the higher the PCa risk, the higher the probability of presenting with BCR (Table S3, Supplementary Material).
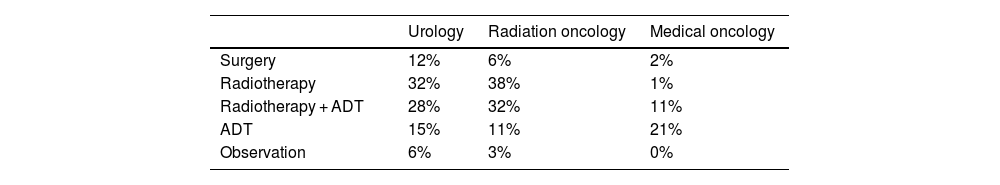
Treatment of patients with biochemical recurrenceRegarding treatment options prescribed to BCR patients, RT alone or with ADT was the preferred treatment for UROs (60%) and ROs (70%), while ADT alone was preferred by 15% of UROs and 11% of RO. Surgery was mainly preferred by UROs and RO, while surgery combined with ADT was used sporadically by UROs and MOs. The observation option was not contemplated by the MOs; it was only considered by the UROs (6%) and ROs (3%) (Table 5). ADT without imaging tests was prescribed by 10% of the UROs and 15% of the RO, on average, for 27%–29% of their patients. Of note, 63% of the UROs' patients receiving ADT without imaging tests had already exhausted all salvage treatment options, while the figure was 44% for the ROs' patients. On the other hand, around 30% of the MOs prescribed ADT treatments without imaging tests to around 36% of patients with BCR. When enquired about the patient profile in which to prescribe ADT without prior imaging, UROs, ROs and MOs mentioned elder patients, with many comorbidities and/or reduced life expectancy. Main cited reason by UROs and ROs was to improve quality of life while MOs cited willingness of patient to avoid complex treatments.
Coordination between wardsAccording to the UROs, 69% of the patients were treated exclusively in the urology ward, 20% in collaboration with radiation oncology, and 10% in the medical oncology ward. According to the ROs, they affirmed that 37% of the patients were shared with the urology ward, 52% were managed exclusively by them, and 11% were treated in collaboration with the medical oncology ward. According to the MOs, 47% of the patients were treated only in the medical oncology ward, 33% were shared with urology, and 19% were treated in coordination with the radiation oncology ward.
DiscussionPCa is the most prevalent neoplasm in Spanish men. This disease is considered a relevant public health problem due to the significant impact on patients' quality of life and the costs associated with the high number of consultations generated by patients, as well as the expenses in sick leave and complementary tests.11,12 The present study was pursued to better understand the profiles of PCa patients at the time of diagnosis and the current management of patients with localized PCa and BCR in Spain, from the point of view of the three specialties involved: URO, RO, and MO.
The extrapolated incidence of PCa reported in this study (40,551) is higher than that reported in the last SEOM report (29,002 patients for 2022).10 On the contrary, the prevalence calculated from the interviews carried out in the present study (221,689) is lower compared to that reported by SEOM (259,788 patients in 2020). Despite this being a perception study, the results are consistent between UROs and ROs regarding SEOM estimates, although the SEOM incidence and prevalence estimations do not consider the effects of the COVID-19 pandemic.
Regarding the question about the stage of patients at diagnosis during the last 12 months, it was estimated that 21% were synchronous metastatic PCa patients, considerably higher than figures previously reported. According to the SEOM, currently most cases of PCa are diagnosed in the early stages,19 with only 10% in advanced cases (metastatic) at diagnosis.20 This could be an underestimation, although with the COVID-19 pandemic, the number of synchronous metastatic patients could have increased due to a possible delay in diagnosis.21 Considering that synchronous metastatic patients have a more active follow-up and that the interviewees respond based on their recall, patients in this group could have been overestimated.
According to the results, around 30% of patients are referred to MOs at the localized stage, which is considerably higher than expected. This result could be explained if some centers with no radiotherapy MOs had to refer the patient to another hospital. Most UROs work closely with oncology departments in clinical practice. However, it is estimated that less than 5% of patients are referred to a MOs prior to the onset of hormone-refractory disease.22 According to the answers of the three specialties, more than 90% of patients undergo biopsy after a diagnosis of PCa to decide on treatment, which contrasts with the real-life studies that determine that around 20% of patients do not undergo histological diagnosis,23,24 followed by magnetic resonance for both UROs and ROs and thoracic CT for MOs. RADAR III and EAU guidelines point out that conventional imaging continues to have an important role in PCa staging, even though next-generation imaging (NGI) is more sensitive and robust.15,25 Recent studies such as the Pro-PSMA study show that PET-PSMA is more accurate in detecting metastasis at the beginning.26 However, according to the answers, PET choline/PSMA is the least commonly used test, although it is somewhat more used by MOs. At the time of this study, these tests were not routinely performed in the initial stages of PCa; they were only done in patients with BCR and patients in whom metastases were suspected. On the other hand, these tests could be performed in clinical trials, which would be carried out mainly by MOs.
According to the specialists, most patients with localized prostate cancer receive radical treatment (either surgery or radiotherapy), while a low percentage receive ADT as the primary treatment, since the guidelines recommend offering these to high-risk patients and selected patients (PSADT < 12 months and PSA > 50 ng/mL or a poorly-differentiated tumor and unwilling to try curative treatment).15 This is in contrast with the results of real-life studies conducted in Spain, such as the AFRODITA study (n = 103), which showed that 75% of the included patients (CRPC-MX) did not receive treatment with curative intent at initial diagnosis, and the Spanish Prostate Cancer Registry, where 14% of patients received ADT as their first treatment.24,27 Moreover, most nmCRPC patients included in the SPARTAN clinical trial had not received treatment with curative intention either,28 and according to the GESCAP group, 15.66% of patients with localized PCa received HT as their first treatment.29 The current landscape is expected to change in the future with the introduction of new hormonal therapies (NHTs) as neoadjuvant and adjuvant treatments to radical prostatectomy or radiotherapy.30,31
In the case of PSA recurrence after RP, the EAU guidelines weakly recommend performing PSMA-PET/CT if the PSA level is >0.2 ng/mL and if the results would influence subsequent treatment decisions. In cases where PSMA-PET/CT is not available, and the PSA level is >1 ng/mL, it is recommended to perform fluciclovine-PET/CT or choline-PET/CT imaging if the results would influence subsequent treatment decisions. In the case of PSA recurrence after radiotherapy, the guidelines weakly recommend performing prostate MRI to localize abnormal areas and guide biopsies in patients fit for local salvage therapy and strongly recommend performing PSMA-PET/CT (if available) or fluciclovine-PET/CT or choline-PET/CT in patients fit for curative salvage treatment. Regarding the most used tests in BCR, biopsy would no longer be the most used, but rather abdominopelvic CT and scintigraphy. For RO, scintigraphy would be the main test, followed by abdominopelvic CT and magnetic resonance imaging, which seems coherent, since MRI has shown excellent results at detecting local recurrences after RT.14 PSMA-PET/CT is more accurate for staging than CT and bone scans for high-risk disease, but to date, no outcome data exist to inform subsequent management.15 The high rate of CT scans and scintigraphy used in patients with BCR (>60%) is remarkable. This could be because, in many sites, it is required to have a previous test to order PET choline/PSMA. The low use of NGI is alarming, given the low sensitivity of conventional tests and the current recommendation of guidelines endorsing NGIs in this scenario.14,25 Focusing on treatment with ADT only, most of the physicians interviewed stated that they did not prescribe ADT without prior imaging tests, which contrasts with previous real-world studies, where between 23% and 33% of patients treated with continuous ADT did not have baseline imaging tests.23,24 It must be noted that until recently, no sensitive imaging tests for low PSA values were available, and in the context of patients receiving salvage RT (i.e.: with PSAs below 1 ng/mL) were therefore not commonly requested.
This study has a few limitations inherent in the methodology; information was collected from interviews with physicians, and hence, a recall bias cannot be excluded. Another limitation is in relation to the panel, assumed to be representative of the whole of Spain, but these physicians may differ from those who did not contribute, resulting in potential selection bias. Despite these limitations, this study describes the patient profiles at the time of diagnosis and provides a vision of the current therapeutic management of localized PCa and BCR in clinical practice in Spain. An important strength of this study is that the three specialties that usually manage patients with PCa have been included in the survey in addition to different types of hospitals (with different size, population covered, with and without multidisciplinary committee, etc) throughout Spain, and therefore providing a very realistic view of real clinical practice in Spain.
ConclusionsTo conclude, this is the first study using physician surveys that provides insight into clinicians’ perceptions and outlines how these perceptions are similar and differentiated from clinical practice studies and guideline recommendations. In our study, carried out on a sample of the different specialists who currently treat prostate cancer, the number of patients with PCa obtained exceeds other estimations previously reported that might not take into account the delay in diagnosis during the pandemic. Despite the low sensitivity of conventional tests in some stages of PCa and the recommendations of the guidelines to use NGI tests, PET choline/PSMA is still used residually, although the age of patients, and not their health condition, appears to be the main driver when choosing between different diagnosis tests. Most patients with localized PCa are currently treated with surgery and RT, while for BCR patients, UROs and ROs prefer radiotherapy and MOs prefer ADT alone or in combination. Taking into account the current guidelines recommendations, our findings show a higher proportion of patients treated with ADT alone or without prior biopsy than expected.
Author contributionsAll authors contributed to the development of the concept, questionnaires, and provided expertise on Prostate cancer management. All authors have read and provided feedback on the manuscript draft, and approved the final draft of the manuscript for submission.
FundingThis work was supported by Janssen.