The aim of this study was to evaluate the impact of tumour size and rete testis invasion in progression free survival of our patients with stage I testicular seminoma. A literature review is also made.
Material and methodsA retrospective observational study was performed. We included patients with stage I seminoma between January 2010 and July 2022. Patients without factors of poor prognostic –Group A– were compared with patients with factors of poor prognostic –Group B–. Kaplan-Meier curves and log-rank testing were used to compare progression free survival (PFS) between these groups. Statistical significance was considered at P≤.05.
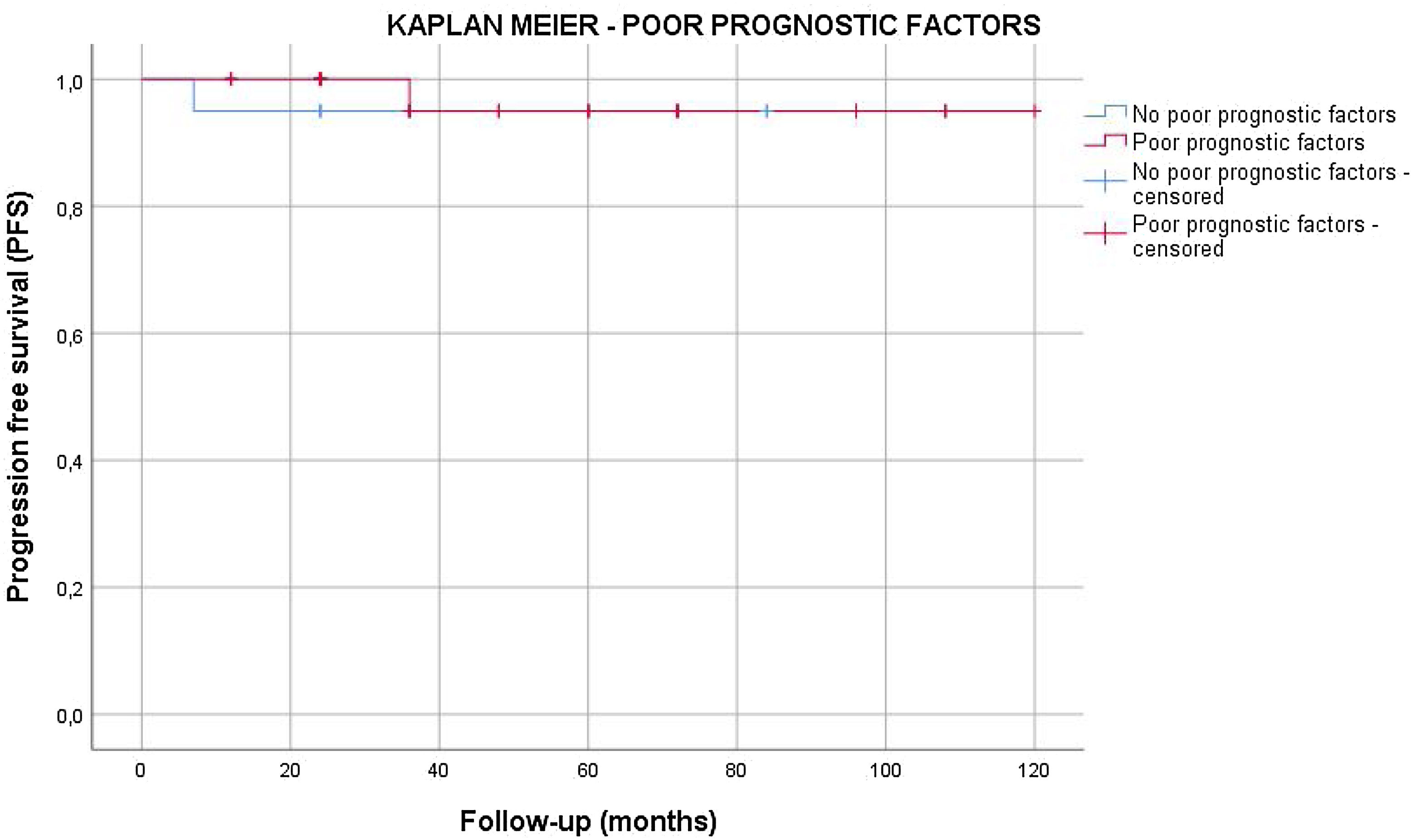
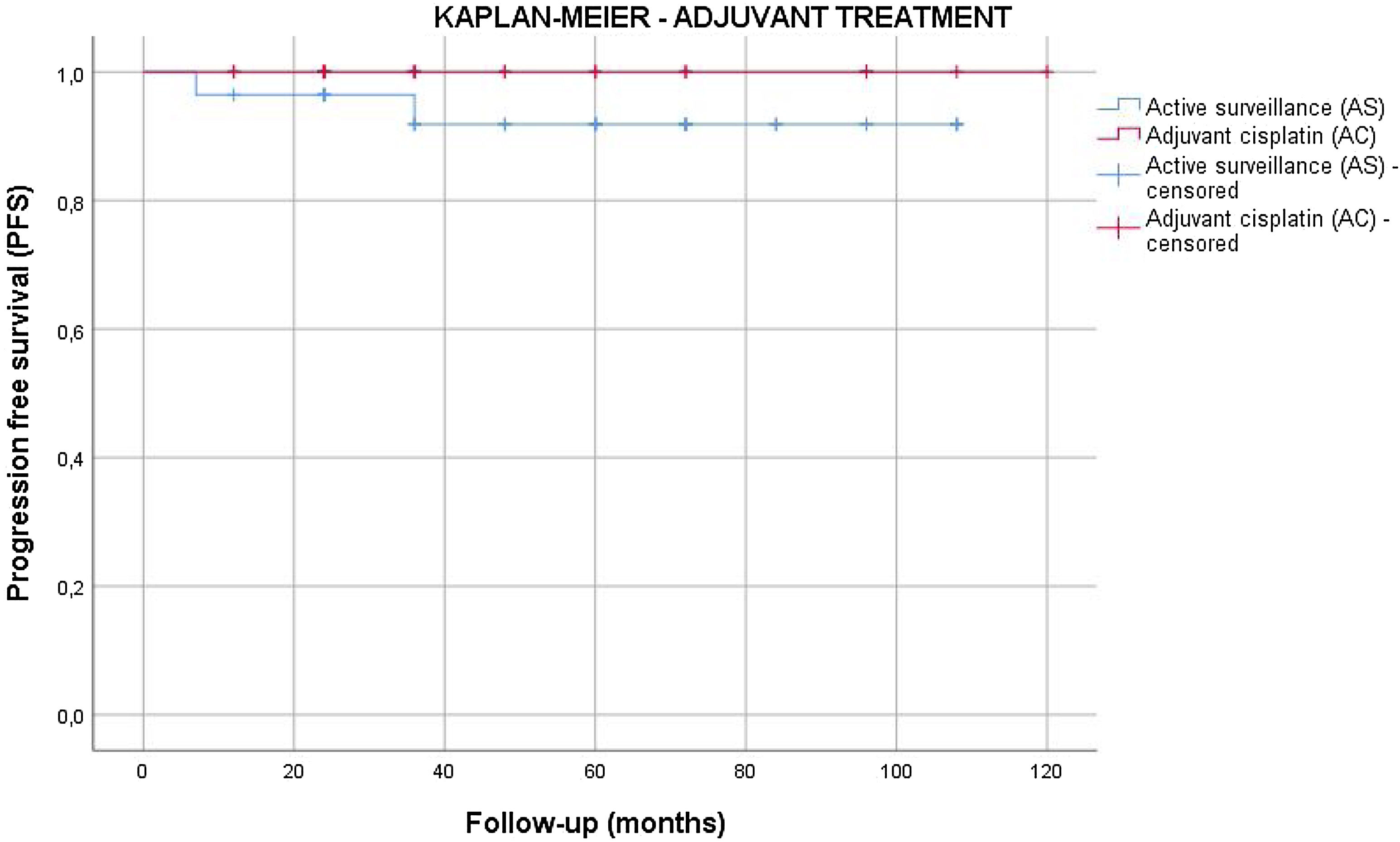
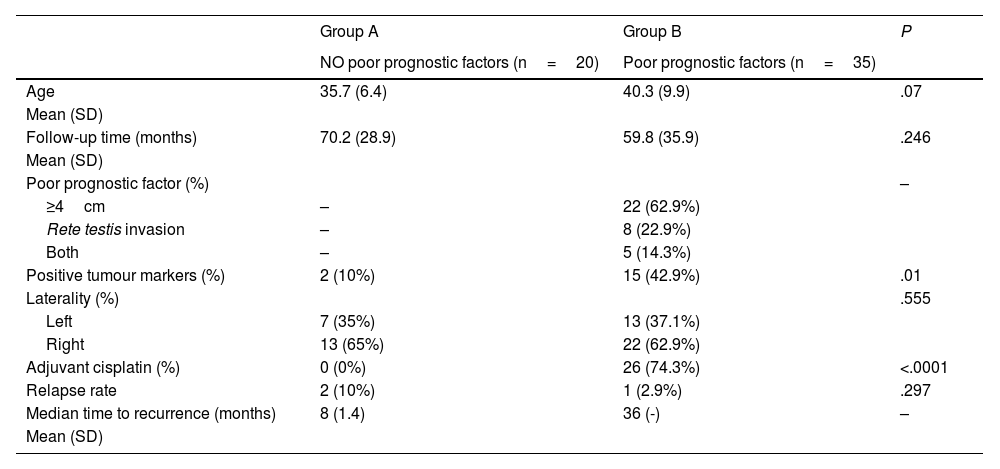
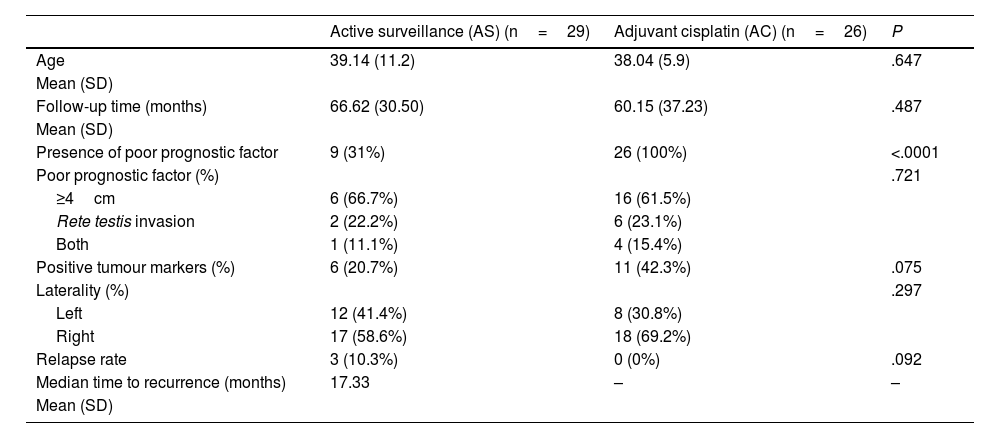
Results55 patients were included in this study. 20 patients (36.4%) were of good prognostic –Group A– and 35 (63.6%) had factors of poor prognostic –Group B–. The mean age was similar in both groups (mean±standard deviation), 38.62±9.04 years. The mean follow-up time was 63.5±33.6 months. All the patients in group A and 25.7% of the patients in group B underwent active surveillance (AS). 26 patients (74.3%) of the patients in Group B were treated with one cycle of adyuvant carboplatin. Three patients suffered a relapse with retroperitoneal lymph nodes (10.3%), all of them were treated with three cycles of BEP, with a complete response of the disease. No statistical significant differences were found in PFS between Group A and B (log Rank P=.317).
ConclusionIndividualization of adjuvant treatment in stage I seminoma is important, avoiding the adverse effects derived from them.
El objetivo de este estudio fue evaluar el impacto del tamaño tumoral y la invasión de la rete testis en la supervivencia libre de progresión de nuestros pacientes con seminoma testicular en estadio I. También se llevó a cabo una revisión bibliográfica.
Material y métodosSe realizó un estudio observacional retrospectivo incluyendo a los pacientes con seminoma en estadio I entre enero de 2010 y julio de 2022. Se compararon los pacientes sin factores de pronóstico favorable -Grupo A- con pacientes que presentaban factores de pronóstico desfavorable -Grupo B-. Se utilizaron curvas de Kaplan-Meier y pruebas de log-rank para comparar la supervivencia libre de progresión (SLP) entre estos grupos. La significación estadística se consideró a P≤,05.
ResultadosSe incluyeron 55 pacientes en este estudio. Veinte pacientes (36,4%) tenían un pronóstico favorable -Grupo A- y 35 (63,6%) presentaban factores de pronóstico desfavorable -Grupo B-. La edad media fue similar en ambos grupos (media±desviación estándar), 38,62±9,04 años. El tiempo medio de seguimiento fue de 63,5±33,6 meses. Todos los pacientes del grupo A y el 25,7% de los pacientes del grupo B se sometieron a vigilancia activa (VA). Veintiséis pacientes (74,3%) del grupo B fueron tratados con un ciclo de carboplatino adyuvante. Tres pacientes sufrieron recidiva en ganglios retroperitoneales (10,3%), todos tratados con tres ciclos de BEP (bleomicina, etopósido, y cisplatino), presentando remisión completa de la enfermedad. No se encontraron diferencias estadísticamente significativas en la SLP entre los grupos A y B (log-rank P=,317).
ConclusionesLa individualización del tratamiento adyuvante en el seminoma estadio I es esencial para evitar los efectos adversos derivados del mismo.