Cochlear implants have been able to treat some types of hearing loss, but those related to cochlear nerve impairment made it necessary to find new ways to manage these deficits; leading to auditory brainstem implants (ABI).
AimOur objective is to present the clinical profile of patients treated through an ABI and the results obtained from 1997 to 2017.
Material and methodsOn the one hand, patients with statoacoustic nerve tumours (VIII cranial nerve) were selected, and on the other hand, patients without VIII tumours with congenital malformations of the inner ear. Before and after the placement of the ABI, hearing was assessed through tonal audiometry, from which the PTA (Pure Tone Average) and the CAP (Categories of Auditory Performance) scale were obtained.
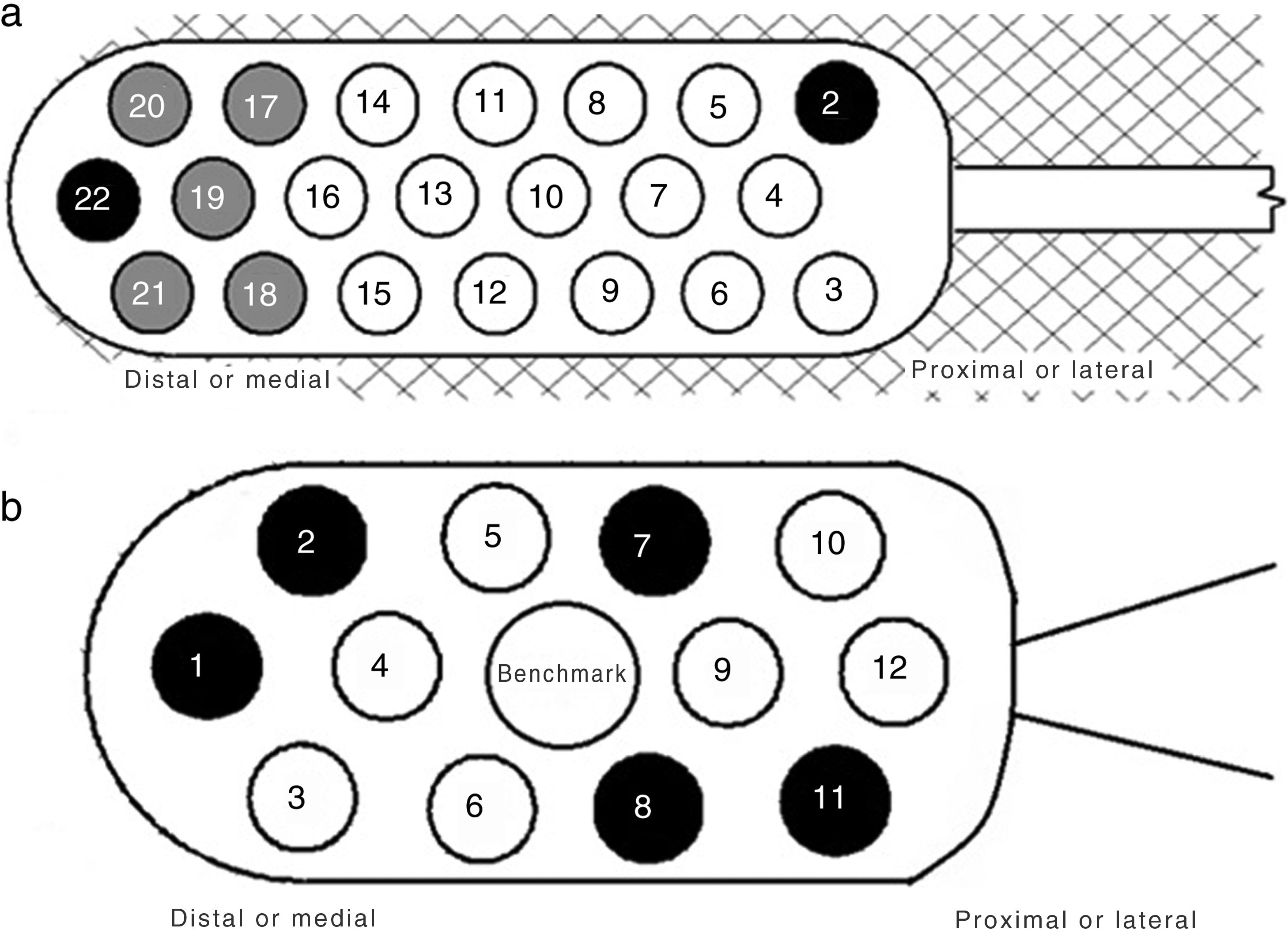
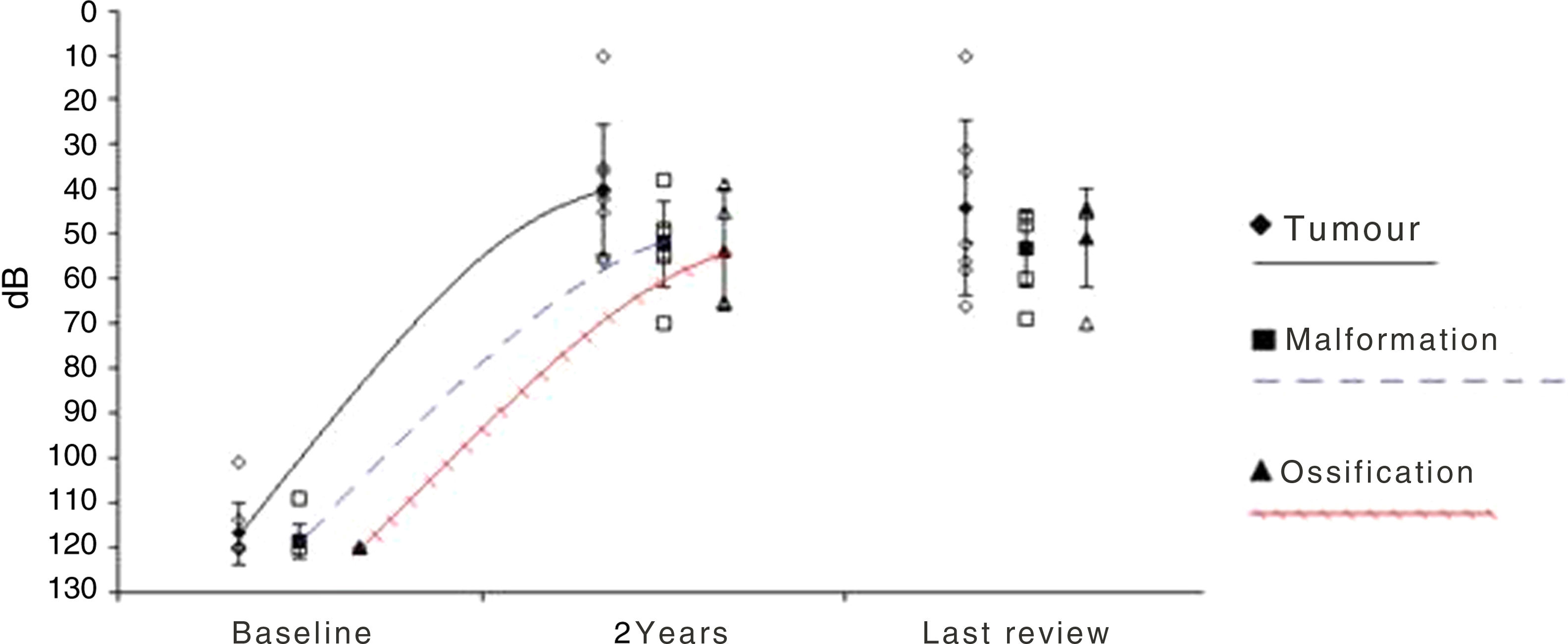
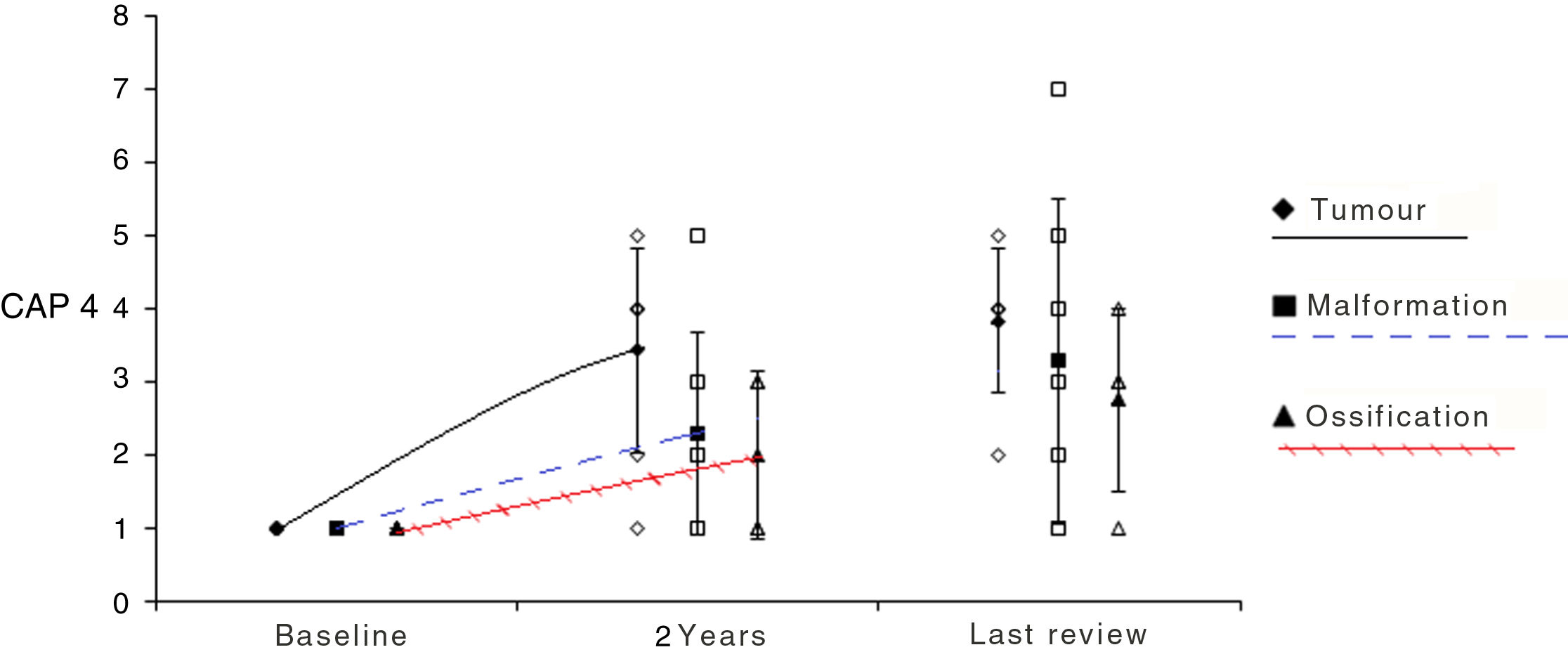
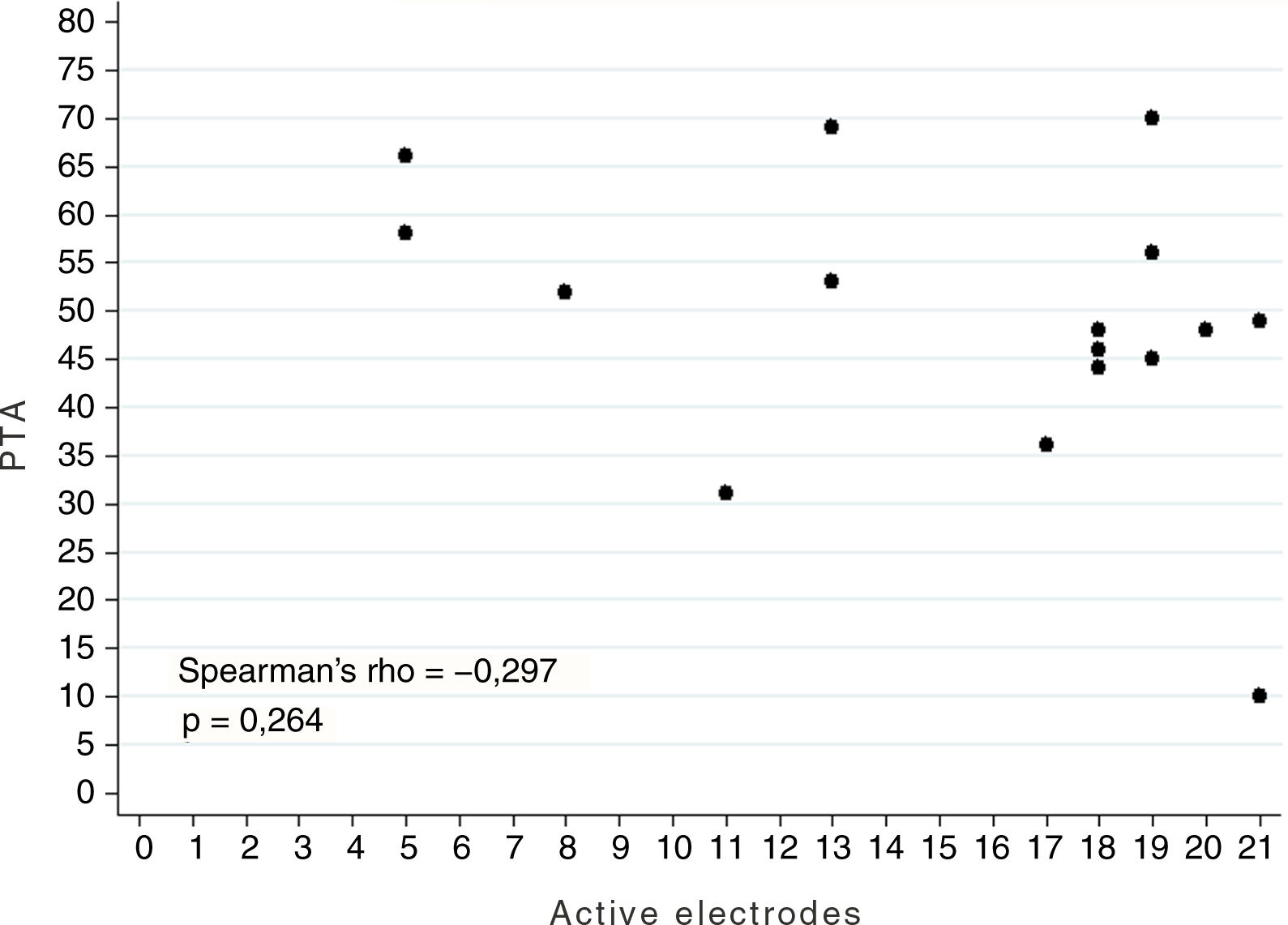
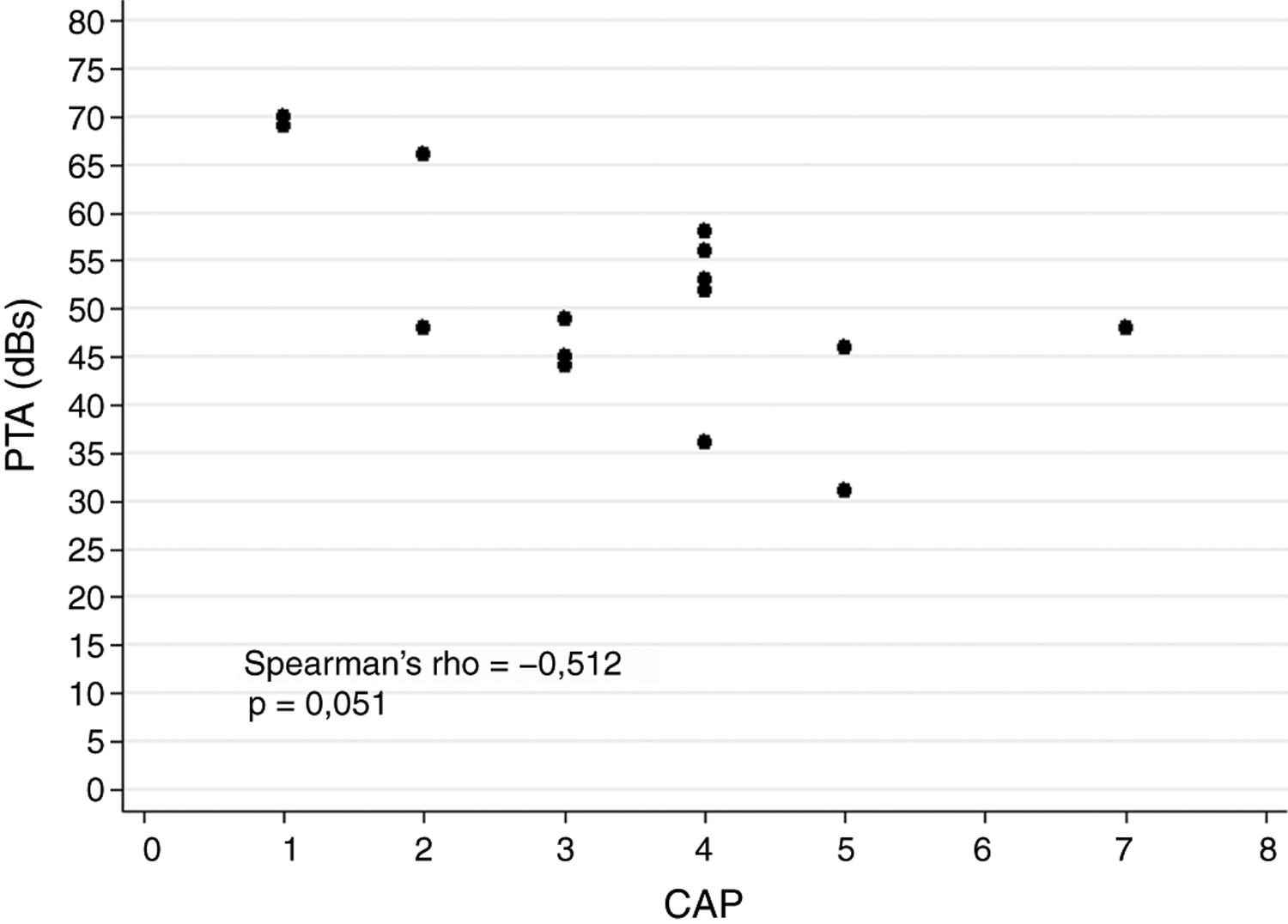
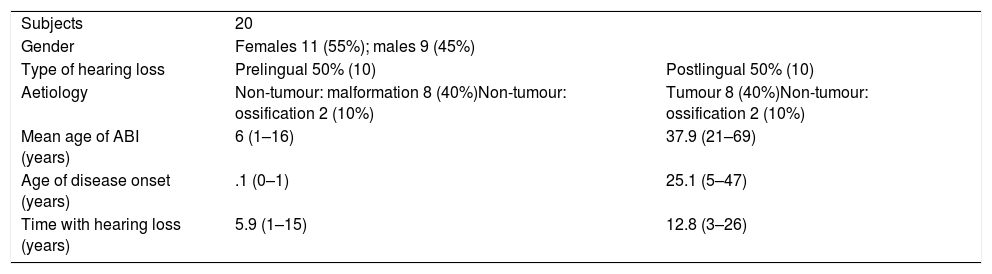
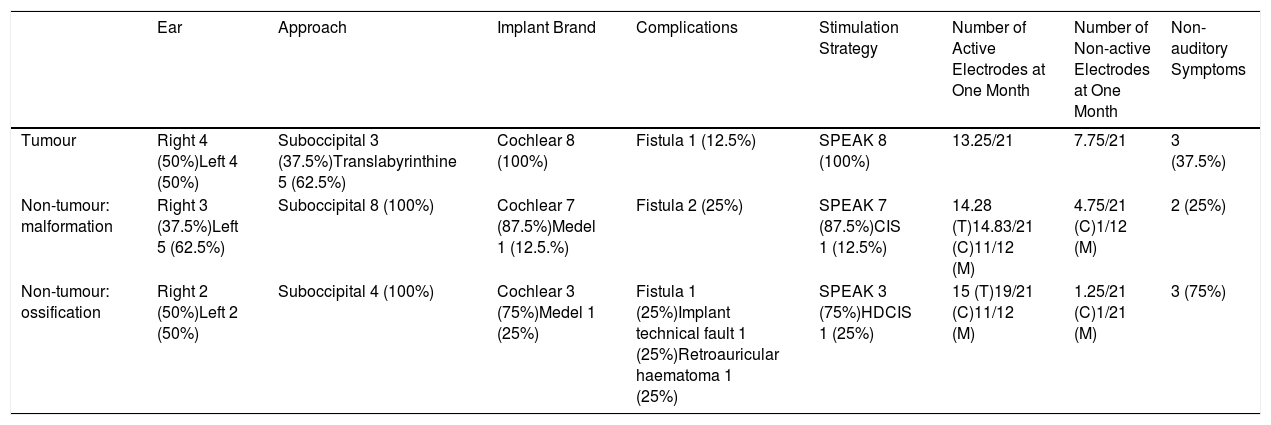
ResultsA total of 20 patients undergoing ABI surgery were included. Eight were of tumour cause (40%) and 12 non-tumour (60%). In 15 subjects (75%) a suboccipital approach was performed and in 5 (25%) translabyrinthine. The mean of active electrodes before the implantation of Cochlear® (Nucleus ABI24) was 13/21 (61.90%) versus 8.5/12 (70.83%) of the Med-el® (ABI Med-el). An improvement in the mean PTA of 118.49dB was found against 46.55dB at 2 years. On the CAP scale, values of 1 were obtained in the preimplantation and of 2.57 (1–5) in the 2-year revision.
ConclusionThe ABI is a safe option, and with good hearing results when the indication is made correctly.
Los implantes cocleares han paliado algunas hipoacusias, pero las relacionadas con alteraciones del nervio coclear obligaron a buscar nuevas formas de tratamiento, dando lugar a los implantes auditivos del tronco cerebral (IATC).
ObjetivosExponer el perfil clínico de los pacientes tratados mediante un IATC y los resultados entre los años 1997 y 2017.
Material y métodosSe seleccionaron por un lado pacientes con tumores del nervio estatoacústico (VIII par craneal) y por otro lado pacientes sin tumores del VIII con malformaciones congénitas del oído interno. Previa y posteriormente a la colocación del IATC se evaluó la audición a través de audiometría tonal liminar, de la que se obtuvo el umbral tonal medio (UTM) y de la escala de rendimiento auditivo Categories Auditory Performance (CAP).
ResultadosSe incluyeron un total de 20 pacientes sometidos a una cirugía de IATC. Ocho de los casos fueron de causa tumoral (40%) y 12 no tumorales (60%). En 15 sujetos (75%) se realizó abordaje suboccipital y en 5 (25%) translaberíntico. La media de electrodos activos al inicio en los implantes de la casa comercial Cochlear® (Nucleus ABI24), la cual tiene un total de 21 electrodos, fue de 13 (61,90%) frente a 8,5 (70,83%) de los 12 electrodos que presenta el implante de la casa Med-el® (ABI Med-el). Se comprobó una mejora en el UTM medio de 118,49dB basal frente a 46,55dB a los 2 años. En la escala CAP se parte en todos los casos de un valor de 1, y en la revisión a los 2 años, de 2,57 (1-5).
ConclusiónConcluimos que el IATC es una opción segura y con buenos resultados auditivos cuando la indicación se hace de manera correcta.