Experimental animal models constitute a useful tool to deepen our knowledge of central nervous system disorders. In the case of multiple sclerosis, however, there is no such specific model able to provide an overview of the disease; multiple models covering the different pathophysiological features of the disease are therefore necessary.
DevelopmentWe reviewed the different in vitro and in vivo experimental models used in multiple sclerosis research. Concerning in vitro models, we analysed cell cultures and slice models. As for in vivo models, we examined such models of autoimmunity and inflammation as experimental allergic encephalitis in different animals and virus-induced demyelinating diseases. Furthermore, we analysed models of demyelination and remyelination, including chemical lesions caused by cuprizone, lysolecithin, and ethidium bromide; zebrafish; and transgenic models.
ConclusionsExperimental models provide a deeper understanding of the different pathogenic mechanisms involved in multiple sclerosis. Choosing one model or another depends on the specific aims of the study.
El uso de modelos experimentales en animales permite aumentar el conocimiento sobre la patología del sistema nervioso central. Sin embargo, en la esclerosis múltiple, no existe un modelo que permita una visión general de la enfermedad, de forma que es necesario utilizar una variedad de modelos que abarquen los distintos cambios que se producen.
DesarrolloSe revisan los distintos modelos experimentales que pueden ser utilizados en la investigación en la esclerosis múltiple, tanto in vitro como in vivo. En relación a los modelos in vitro se analizan los distintos cultivos celulares y sus potenciales modificaciones así como los modelos en rodajas. En los modelos in vivo, se analizan los modelos de base inmune-inflamatoria como la encefalitis alérgica experimental en los distintos animales, además de las enfermedades desmielinizantes por virus. Por otro lado, se analizan los modelos de desmielinización-remielinización incluyéndose las lesiones químicas por cuprizona, lisolecitina, bromuro de etidio, así como el modelo de zebrafish y los modelos transgénicos.
ConclusionesLos modelos experimentales nos permiten acercarnos al conocimiento de los diversos mecanismos que ocurren en la esclerosis múltiple. La utilización de cada uno de ellos depende de los objetivos de investigación que planteen.
Experimental models are essential to advancing our understanding of disease and in the design of specific and effective treatments. The development of experimental models of multiple sclerosis (MS) is particularly challenging given that the condition only affects humans and that demyelinating diseases in animals are considerably different from human central nervous system (CNS) diseases.1–3 The search for animal models of demyelination has led to the development of both in vitro and in vivo models; the latter include transgenic mice as well as chemically-induced, viral, and autoimmune models.1,4–6 As no such model is comparable to MS, multiple models are needed to cover the different pathophysiological features of the disease.7
While MS is an inflammatory disease, it is remyelination failure, incomplete remyelination, and neurodegeneration that lead to disease progression and disability. This is relevant to MS treatment: current treatments only control immune mechanisms and are therefore effective in the early stages of the disease, but have no effect on remyelination and consequently on sequelae.8,9 Remyelination is therefore the main focus of new treatment options for MS.10 This study reviews the currently available experimental models providing information on this stage of the disease and enabling testing of different drugs.
DevelopmentIn vitro modelsIn vitro models of MS consist of cultures of immortalised cell lines or isolated mammalian brain cells, allowing the study of cell-cell interactions. These cell lines may be transformed genetically; they are stable and may be subjected to a wide range of experimental diseases.6,11 Studies of CNS cell cultures use microglial cells (mesoderm), neurons, astrocytes, and oligodendrocytes, and also their precursor cells (ectoderm). Studies of microglial cell cultures enable us to extrapolate the role of these cells to CNS immune response in MS, enabling the analysis of microglial response to certain signals, such as biochemical signals, and the potential mechanisms of activation of these cells.6,12,13 Ectodermal-lineage cell cultures allow us to study the cascade of events triggered by demyelination, astrocytic metabolic support in demyelinating lesions, activation and signalling pathways involved in oligodendrocyte replacement, and remyelination of demyelinated axons. Cultures of oligodendrocyte precursor cells or undifferentiated neural stem cells are of particular interest since they allow us to study differentiation and the effect of inflammatory factors, test new chemical compounds aimed at promoting oligodendrocyte proliferation,6 and develop strategies that may promote remyelination.14 Our research group has used these cells to introduce mutations associated with Alexander disease and to study myelination mechanisms in the condition.15 Primary astrocyte cultures are also useful since these cells are involved in the local environment, which is essential in remyelination. Astrocytes express growth factors, such as platelet-derived growth factors and insulin-like growth factor 1, which have been found to promote remyelination. The main disadvantage of in vitro models is that the response of individual cell types may not be extrapolated to tissues. Slice cultures are in vitro models that enable the study of cell-cell interactions14 and provide a more reliable picture of in vivo conditions.16 They also allow us to visualise the interaction between the immune system and CNS damage and repair mechanisms,1 and enable researchers to regulate alterations in oligodendrocyte maturation and remyelination failure due to chronic CNS astrogliosis.5 Slice cultures have some limitations, particularly the slicing procedure: slices must be 200-500μm thick, obtained from young or even neonatal animals, and manipulated under special conditions with carbogen gas.17,18
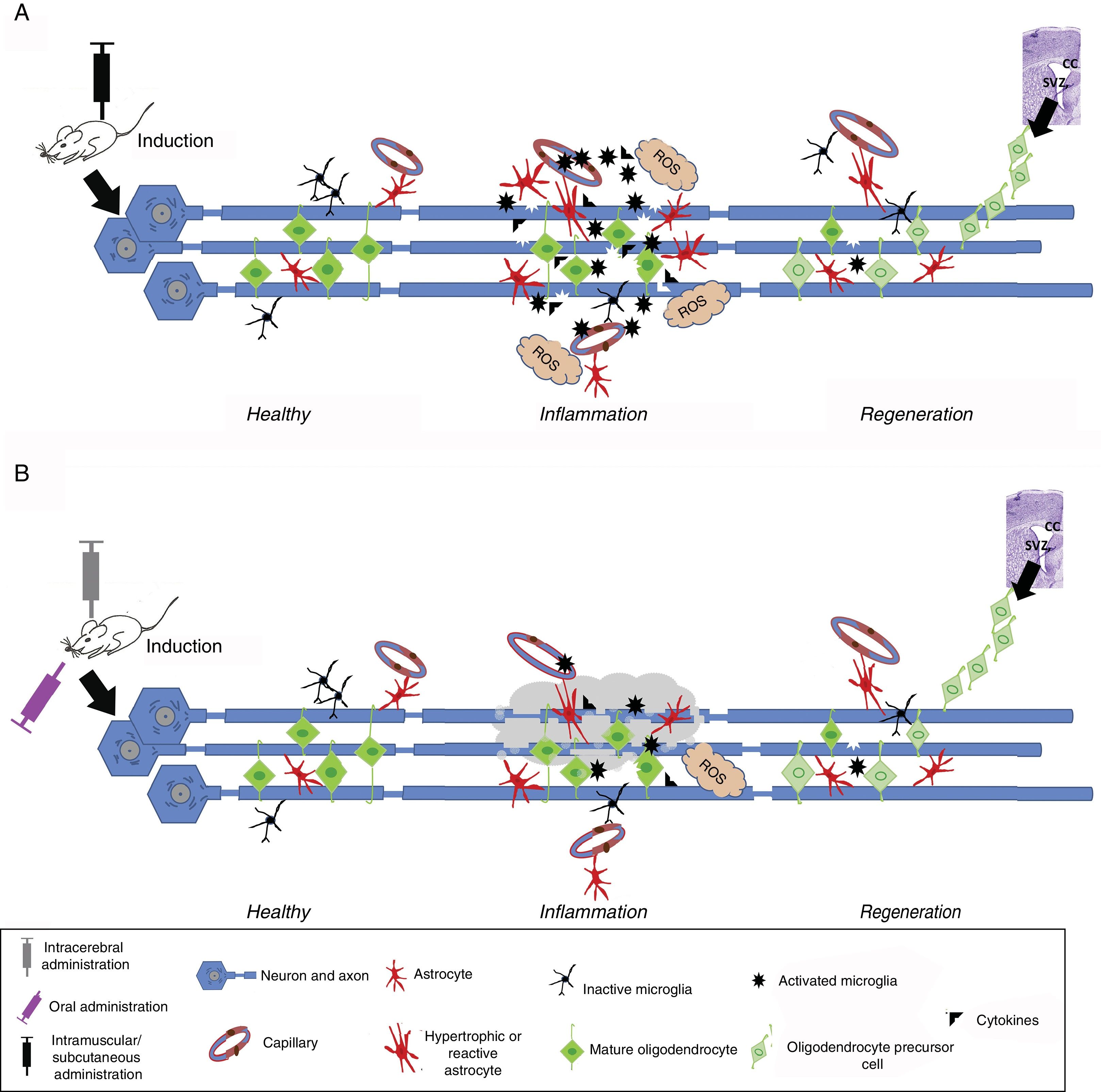
In vivo modelsIn vivo experimental models of MS may be classified into 3 categories: (1) autoimmune and/or inflammatory models (Fig. 1A), such as models of experimental allergic encephalitis (EAE) and viral models; (2) models of demyelination/remyelination (Fig. 1B), including models of chemical lesions induced with cuprizone, lysolecithin, and ethidium bromide, and the zebrafish model; and (3) transgenic models, which aim to more accurately replicate the key pathological features of the disease (Table 1).14
Induced inflammation and demyelination in in vivo models. (A) Induction in autoimmune and/or inflammatory models. An immunostimulator is administered intramuscularly or subcutaneously. Induced inflammation is associated with increased levels of proinflammatory cytokines, T cells, and activated microglia, leading to the formation of reactive oxygen species (ROS), oligodendrocyte and myelin damage, and reactive gliosis and vessel wall alterations, resulting in oedema. This stage is followed by regeneration, where the number of activated cells (astrocytes and microglia) decreases and oligodendrocyte precursor cells migrate mainly from the corpus callosum (CC) or subventricular zone (SVZ) to replace damaged cells and promote remyelination. (B) Induction in demyelination models. Demyelination is induced with chemicals or toxic agents, which are administered orally (diet), locally (intracerebral), intramuscularly, or subcutaneously. After entering the bloodstream, the chemical or toxic agent enters into contact with axons, causing myelin degeneration; this microenvironment induces microglial activation, ROS formation, inflammatory cytokine release, and reactive gliosis. This ultimately leads to the loss of myelin sheaths and myelin-producing cells (oligodendrocytes). This stage is followed by regeneration, where the number of activated cells (astrocytes and microglia) decreases and oligodendrocyte precursor cells migrate mainly from the CC or SVZ to replace damaged cells and promote remyelination.
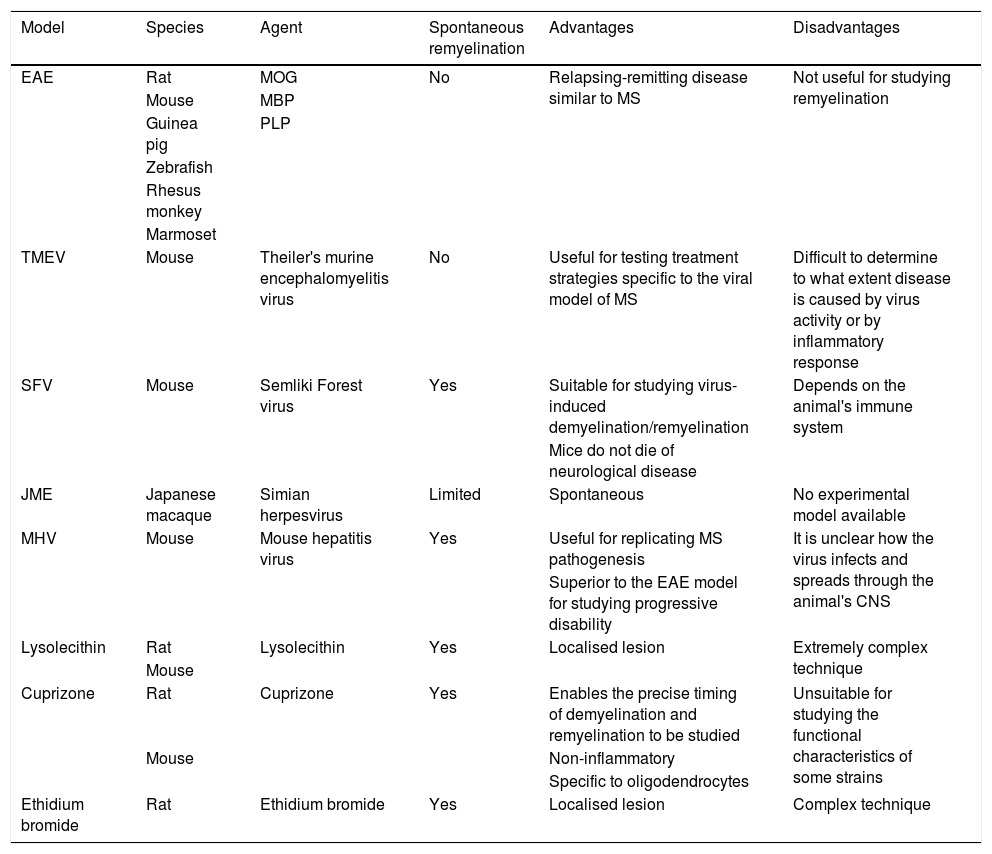
Main characteristics, strengths, and limitations of the different in vivo models.
| Model | Species | Agent | Spontaneous remyelination | Advantages | Disadvantages |
|---|---|---|---|---|---|
| EAE | Rat | MOG | No | Relapsing-remitting disease similar to MS | Not useful for studying remyelination |
| Mouse | MBP | ||||
| Guinea pig | PLP | ||||
| Zebrafish | |||||
| Rhesus monkey | |||||
| Marmoset | |||||
| TMEV | Mouse | Theiler's murine encephalomyelitis virus | No | Useful for testing treatment strategies specific to the viral model of MS | Difficult to determine to what extent disease is caused by virus activity or by inflammatory response |
| SFV | Mouse | Semliki Forest virus | Yes | Suitable for studying virus-induced demyelination/remyelination | Depends on the animal's immune system |
| Mice do not die of neurological disease | |||||
| JME | Japanese macaque | Simian herpesvirus | Limited | Spontaneous | No experimental model available |
| MHV | Mouse | Mouse hepatitis virus | Yes | Useful for replicating MS pathogenesis | It is unclear how the virus infects and spreads through the animal's CNS |
| Superior to the EAE model for studying progressive disability | |||||
| Lysolecithin | Rat | Lysolecithin | Yes | Localised lesion | Extremely complex technique |
| Mouse | |||||
| Cuprizone | Rat | Cuprizone | Yes | Enables the precise timing of demyelination and remyelination to be studied | Unsuitable for studying the functional characteristics of some strains |
| Mouse | Non-inflammatory | ||||
| Specific to oligodendrocytes | |||||
| Ethidium bromide | Rat | Ethidium bromide | Yes | Localised lesion | Complex technique |
CNS: central nervous system; EAE: experimental allergic encephalitis; JME: Japanese macaque encephalomyelitis; MBP: myelin basic protein; MHV: mouse hepatitis virus; MOG: myelin oligodendrocyte glycoprotein; MS: multiple sclerosis; PLP: proteolipid protein; SFV: Semliki Forest virus; TMEV: Theiler's murine encephalomyelitis virus.
Experimental allergic encephalitis shares some characteristics with MS: inflammation, demyelination, axonal loss, and gliosis. The main difference between the 2 entities is that EAE is not spontaneous, but rather must be induced.3,19 Different protocols have been developed for inducing EAE; the most widely used employ an autoantigen to induce reactive T cell activation (active EAE) or transfer autoreactive T cells to naïve animals (passive EAE).6,20 Active induction of EAE requires an adjuvant (most frequently Freund's Complete Adjuvant),21 which slowly releases the antigen from the inoculum2 and contains a mycobacterium (most commonly Bordetella pertussis). The bacterium causes clonal expansion of autoreactive T cells,6 triggering the manifestations typical of MS by promoting humoural immune response.22 While EAE has been used to study neuroinflammatory and immune response mechanisms, it is also useful for the study of demyelination and remyelination.19 As in MS, age, sex, and environmental factors have a major impact on EAE susceptibility, severity, and progression.14 This model has been tested in several species in search for the species most accurately simulating human MS. It has been tested in primates, such as rhesus monkeys, which displayed disseminated encephalomyelitis and paralysis associated with perivascular infiltrates and demyelination in the brain and spinal cord.23–25 This model resembles the Marburg variant of MS due to the severity and the hyperacute nature of symptoms. EAE has also been induced in marmosets with different procedures: (1) with a white matter homogenate, which causes lesions resembling those seen in autopsies of patients with MS26; (2) with recombinant human myelin oligodendrocyte glycoprotein (MOG), which causes smaller but longer-lasting demyelinating lesions26,27; and (3) with a subclinical dose of recombinant rat MOG followed by an injection of TNFα and interferon.27,28 Although primate EAE models bear a greater similarity to human MS than rodent models, the use of primates in research has ethical and economic limitations.27
The rodent immune system is considerably different to that of humans but does enable us to assess the chronic, progressive nature of the disease through immunogenetic, histopathological, and treatment studies. In mice, EAE is induced by an immune response triggered by injection of such myelin antigens as myelin proteolipid protein (PLP), MOG,29 and myelin basic protein (MBP); the latter 2 activate microglia and induce perivascular infiltration of T and B cells and even myelin damage, which usually coincides with relapses.30 Mouse models of neuromyelitis optica have also been developed.31,32 The rat model of EAE consists of inflammatory infiltration of mononuclear cells of the spinal cord, cerebellum, and medulla oblongata.33 EAE has also been induced in guinea pigs (Hartley and strain 13). Strain 13 guinea pigs are regarded as one of the animal models best replicating MS since the animals experience chronic relapses.34 EAE has also been induced in zebrafish models.35
Viral models of demyelinating diseasesCNS demyelination has also been induced with viruses. Theiler's murine encephalomyelitis virus is a murine virus capable of inducing a neurological disease characterised by demyelination due to neuronal infection and mainly manifesting as subclinical encephalitis.36,37 The acute stage is followed by a chronic, progressive stage characterised by spinal cord demyelination and remyelination, resulting in damage to macrophages, microglia, oligodendrocytes, and astrocytes.2,37–39 Spinal cord lesions are characterised by chronic inflammation, confluent demyelinating plaques from the first episode, axonal damage of varying severity, and remyelination. Active demyelination occurs at sites of activated microglia and macrophage infiltration; these lesions therefore present the typical features of MS lesions.2
The avirulent A7 strain of Semliki Forest virus is a neuroinvasive virus that induces CNS myelin damage when injected into susceptible mice. This strain induces viraemia lasting 3-4 days, after which the virus is cleared from the blood. In subsequent infections, the virus crosses the blood-brain barrier, infecting neurons and oligodendrocytes; viral replication peaks on days 5 to 7, and demyelination is observed between days 13 and 17. Myelin damage depends on the virus strain, the age of the carrier, and the developmental stage of the animal's immune system.20
Japanese macaque encephalomyelitis (JME) is a demyelinating disease associated with a simian herpesvirus. It usually appears in young adult animals, independently of sex. It causes multiple foci of demyelination, with varying degrees of oligodendrocyte and axonal loss in the white matter of the brain, cerebellum, medulla oblongata, and spinal cord; these lesions are associated with macrophage and lymphocyte infiltration. Although JME and MS share certain clinical, imaging, and pathological features, they also have some differences, such as the numbers of neutrophils and lymphocytes in the CSF, and the presence of necrosis and haemorrhages in JME. Lesions in animals with JME may be acute or chronic.40,41
Mouse hepatitis virus is a coronavirus capable of inducing several diseases, depending on the strain. Intracranial or nasal infection with a neurovirulent strain causes neurological disease. The disease develops in 2 stages; the first of these starts several days after infection and causes virus-induced panencephalitis. Animals subsequently recover, and the second stage starts 4 weeks later, with neuroparalytic disease associated with inflammatory demyelinating lesions. The virus is cleared from the brain at the end of the first stage, but viral RNA remains in tissues throughout the course of the disease.6,42
A major difference between viral models and EAE models is that virus-induced chronic inflammatory demyelination is associated with significant microglial activation.6
Models of demyelination and remyelinationChemical lesion modelsThe agents most commonly used in these models are cuprizone, lysolecithin, and ethidium bromide, all of which cause focal demyelination.6 The first 2 chemicals are used most frequently due to their ability to induce extensive demyelination in such CNS areas as the striatum, hippocampus, spinal cord, and optic nerve.6,43 Cuprizone is a chelating agent that is widely used due to its ability to induce CNS demyelination following systemic administration.44 It causes acute demyelinating lesions that may become chronic while cuprizone administration is continued. This model has the advantage that discontinuing administration triggers spontaneous remyelination, enabling the study of both processes. Furthermore, cuprizone causes T cell suppression, which enables the effects induced by immune system activation to be separated from those caused by the chemical compound in the study of demyelination and remyelination.6 Injection of lysolecithin into the white matter causes focal demyelinating plaques, as the toxin damages the myelin sheath.2,45 The compound also acts as a chemoattractant to monocytes, triggering an inflammatory response; its effects can be observed in several experimental animals, including rats, mice, cats, and rabbits.46 As with other toxin-induced models of demyelination, the myelin damage stage is quickly followed by remyelination, although the speed and degree of remyelination are age-dependent.2 The toxicity of ethidium bromide is based on the compound's interaction with DNA and mainly affects astrocytes: lesions are induced due to the lack of support factors released by these cells. This model has revealed that oligodendrocyte-mediated remyelination requires the presence of astrocytes.2,47
Zebrafish model of myelinationThe zebrafish is a small, relatively simple animal, which is optically transparent in the embryonic stage. Zebrafish embryonic development is fast48: myelinated axons can be studied from the third day, enabling real-time, in vivo analysis of myelination and remyelination.49 Zebrafish reach sexual maturity very early, can produce hundreds of offspring, and are less costly to maintain than other experimental animals. Furthermore, they can be used to create transgenic lines expressing green fluorescent protein50,51 in oligodendrocytes and oligodendrocyte precursor cells, enabling the study of remyelination52,53 and testing of treatments promoting remyelination.54
Transgenic animal modelsTransgenic mice carrying insertions and deletions of genes coding for immune factors allow us to extrapolate the role of certain factors in the pathogenesis of CNS damage. Alterations similar to those observed in MS have been replicated in knockout animal models of several myelin proteins.5 Furthermore, myelination has been studied in various mutant mouse models of myelin deficiency, such as shiverer mice (in which the gene coding for MBP is duplicated and inverted), rumpshaker mice (mutation in the gene coding for PLP), and jimpy mice (point mutation in the gene coding for PLP); these mouse models all develop demyelination.6
ConclusionsThere is no specific model displaying the multiple factors involved in MS pathogenesis; researchers have therefore developed a wide range of models replicating various features of the disease. The development of new models or variations of existing models will contribute to a better understanding of the disease and its treatment. Animal models allow us to extrapolate information on treatment response. The development of models better able to reproduce the pathological changes of MS constitutes the first stage in the development of new treatments; however, we are most likely to gain true understanding of the disease through data from patients.
Chemical lesion models provide highly valuable information on demyelination and remyelination, but their results are not directly applicable. Selecting one model or another depends on a study's specific aims.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Torre-Fuentes L, Moreno-Jiménez L, Pytel V, Matías-Guiu JA, Gómez-Pinedo U, Matías-Guiu J. Modelos experimentales de desmielinización-remielinización. Neurología. 2020;35:32–39.